A broader understanding was sought regarding the influence of specific adipose tissue depots on the isolated adipose-derived stem cell (ASC) populations through a systematic comparison of donor-matched abdominal subcutaneous fat and omentum, and donor-matched pericardial adipose tissue and thymic remnant samples. Results suggest that depot selection is an important factor to consider when applying ASCs in tissue-specific cell-based regenerative therapies, and also highlight pericardial adipose tissue as a potential new ASC source.
Keywords: Adipose tissue, Adult stem cells, Cellular therapy, Matched-pair analysis, Differentiation, Hypoxia
Abstract
Adipose tissue is an abundant source of multipotent progenitor cells that have shown promise in regenerative medicine. In humans, fat is primarily distributed in the subcutaneous and visceral depots, which have varying biochemical and functional properties. In most studies to date, subcutaneous adipose tissue has been investigated as the adipose-derived stem cell (ASC) source. In this study, we sought to develop a broader understanding of the influence of specific adipose tissue depots on the isolated ASC populations through a systematic comparison of donor-matched abdominal subcutaneous fat and omentum, and donor-matched pericardial adipose tissue and thymic remnant samples. We found depot-dependent and donor-dependent variability in the yield, viability, immunophenotype, clonogenic potential, doubling time, and adipogenic and osteogenic differentiation capacities of the ASC populations. More specifically, ASCs isolated from both intrathoracic depots had a longer average doubling time and a significantly higher proportion of CD34+ cells at passage 2, as compared with cells isolated from subcutaneous fat or the omentum. Furthermore, ASCs from subcutaneous and pericardial adipose tissue demonstrated enhanced adipogenic differentiation capacity, whereas ASCs isolated from the omentum displayed the highest levels of osteogenic markers in culture. Through cell culture analysis under hypoxic (5% O2) conditions, oxygen tension was shown to be a key mediator of colony-forming unit-fibroblast number and osteogenesis for all depots. Overall, our results suggest that depot selection is an important factor to consider when applying ASCs in tissue-specific cell-based regenerative therapies, and also highlight pericardial adipose tissue as a potential new ASC source.
Introduction
Human adipose tissue is an abundant source of regenerative cells, termed adipose-derived stem cells (ASCs), which are being investigated in the laboratory and in preclinical trials for a broad range of cell-based therapies [1, 2]. An important regenerative mechanism for ASCs is the secretion of paracrine factors that modulate biological responses, including apoptosis, angiogenesis, inflammation, and matrix remodeling [3–5]. Furthermore, ASCs have been demonstrated to have an immune-privileged status, as well as an immunomodulatory capacity [6–8]. In terms of differentiation potential, ASCs are a heterogeneous population that includes multipotent stem cells that can differentiate into cells derived from the mesoderm, with most literature to date focused on the adipogenic, osteogenic, and chondrogenic lineages. However, studies have demonstrated plasticity toward other lineages, including endothelial [9], epithelial [10], hepatocyte [11], and neural [12, 13] populations.
In developing fat as a cell source for clinical applications, it is important to consider the effects of depot and donor variability. Adipose tissue is found throughout the human body and can generally be subdivided into the subcutaneous and visceral depots. The comparative studies to date have begun to explore the differences in ASC populations isolated from subcutaneous adipose tissue at varying anatomical locations, as well as from the omentum [14–17]. These studies have indicated that the specific tissue source can influence the characteristics of the extracted cell populations. In particular, Schipper et al. [17] isolated ASCs from subcutaneous adipose tissue at different sites and observed variations in proliferation that were dependent on donor age, as well as depot-dependent differences in apoptotic susceptibility and lipolytic function. Immunophenotype analysis by flow cytometry has also indicated that ASCs from different sites can have varying surface marker expression profiles [16]. Moreover, several studies have found that the adipogenic and osteogenic differentiation potential in culture varies for ASCs derived from subcutaneous fat versus the omentum [16, 18].
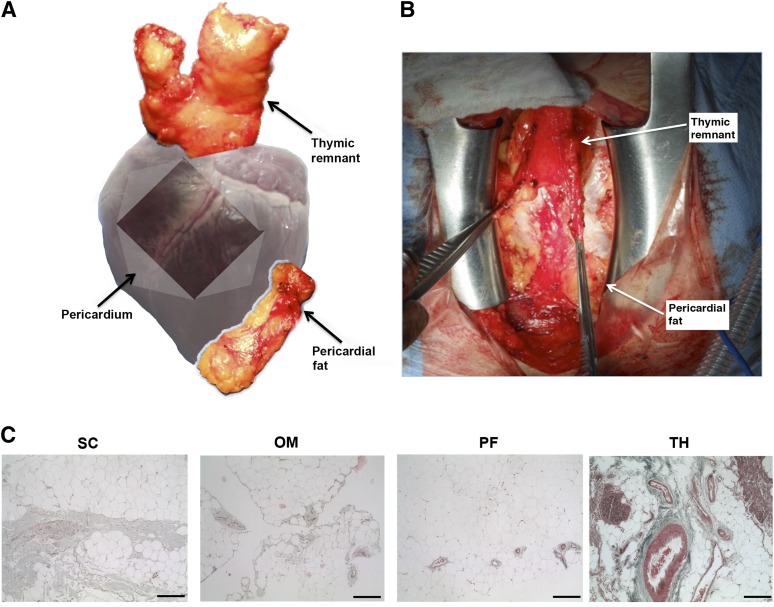
In addition to the omentum, visceral adipose tissue is found within the intrathoracic region. Recently, human epicardial adipose tissue was explored as a regenerative cell source in the treatment of myocardial infarction [19]. However, pericardial adipose tissue and the thymic remnant are intrathoracic depots that are anatomically distinct (Fig. 1), which have not yet been characterized as potential ASC sources. Whereas epicardial fat is continuous with the myocardium and shares the same microcirculation, the pericardial depot is more anteriorly located and anatomically separated from the heart muscle [20]. Similarly, the thymic remnant is derived from the thymus, which undergoes atrophy starting in the third year of life, culminating with its substitution with adipose tissue [21, 22]. In the context of cardiac surgery, pericardial adipose tissue and thymic remnant represent accessible and expendable fat sources for cell therapies to promote cardiovascular regeneration.
Figure 1.
Anatomic location of the intrathoracic adipose tissue depots and histological overview of the tissue ultrastructure. (A): Schematic showing the anatomical position of the pericardial and thymic remnant adipose tissue depots. (B): Intraoperative image of the intrathoracic depots exposed through median sternotomy. (C): Representative Masson’s trichrome staining of subcutaneous, omentum, pericardial, and thymic remnant adipose tissue. Scale bars represent 100 μm. Abbreviations: OM, omentum; PF, pericardial fat; SC, subcutaneous; TH, thymic remnant.
With a view toward optimizing depot selection in the development of ASC-based therapies, our primary objective was to conduct a broad range of in vitro characterization studies to assess depot-dependent variability in the ASC populations isolated from donor-matched subcutaneous adipose tissue and omentum, as well as donor-matched pericardial adipose tissue and thymic remnant. A secondary goal was to investigate the effects of oxygen tension during culture on ASC clonogenic potential, proliferation, and adipogenic/osteogenic differentiation by comparing the responses under normoxic (95% air/5% CO2) and hypoxic (5% O2/90% N2/5% CO2) conditions. Oxygen tension is emerging as a key mediator of mesenchymal stem cells (MSCs), and hypoxia can influence MSC proliferation [23, 24], apoptosis [25], and differentiation [26, 27] through hypoxia-inducible transcription factors. Preconditioning stem cells under hypoxic conditions has been investigated as a means of promoting a proangiogenic phenotype to enhance the regenerative potential of the cells for cardiovascular applications [28, 29]. Moreover, studies have indicated that hypoxia can impede the osteogenic differentiation of subcutaneous ASCs [26, 30], although the effects on adipogenesis are not well understood [31]. As such, we were interested in exploring whether hypoxia could be a key influencing factor on ASC proliferation and differentiation for all of the depot sources.
Materials and Methods
Materials
Unless otherwise stated, all chemicals were purchased from Sigma-Aldrich Canada (Oakville, Canada) and used as received. All human adipose tissue samples were collected en bloc at the Kingston General Hospital and Hotel Dieu Hospital in Kingston, Canada. Research ethics board approval was obtained from Queen’s University (REB CHEM-002-07). Matched abdominal subcutaneous and omentum adipose tissue samples were collected from single sites with informed consent from patients undergoing bowel surgery (N = 14 donors), and matched pericardial and thymic remnant adipose tissue samples were obtained during open-heart surgery in a second group of patients (N = 24 donors). The samples were transported to the laboratory on ice in sterile phosphate-buffered saline (PBS) supplemented with 2% bovine serum albumin (BSA) and processed within 2 h. The patient gender, age, and body mass index (BMI) were recorded (supplemental online Table 1). For all assays, the number of replicate samples for each donor (n) and the number of repeat trials with cells from different donors (N) are reported.
Histological Analysis
Tissue samples were fixed in 10% neutral buffered formalin, paraffin embedded, and sectioned for Masson’s trichrome staining.
Cell Isolation and Yield
Primary human ASCs were isolated using published protocols, with all tissue sources processed using identical methods [32]. The viable cell yield in the stromal vascular fraction (SVF) was estimated for all depots (n = 3, N = 7) using the Guava ViaCount assay (Millipore, Billerica, MA) with analysis with a Guava easyCyte 8HT flow cytometer. The yield for each sample was normalized to the digested tissue mass.
ASC Culture
The SVF was plated on tissue culture flasks (Corning 75 cm2; Fisher Scientific, Oakville, Canada) at 30,000 cells/cm2 in growth medium comprised of DMEM:Ham’s F12 supplemented with 10% fetal bovine serum (HyClone, Logan, UT; Fisher Scientific), 100 U/ml penicillin, and 0.1 mg/ml streptomycin (1% pen-strep; Life Technologies, Burlington, Canada). The cells were cultured in a standard CO2 incubator (37°C, 5% CO2). After 24 hours, nonadherent cells were removed through PBS rinsing. The growth medium was changed every 2–3 days, and the cells were passaged at 80% confluence. Passage 2 (P2) cells were used for all culture analyses.
Immunophenotype Characterization
Flow cytometry analysis was performed on P2 ASCs (n = 3, N = 6) using a Guava easyCyte 8HT flow cytometer. Single marker staining was performed with monoclonal, fluorochrome-conjugated antibodies from eBioscience (San Diego, CA), as follows: CD34-APC (catalogue 17-0349-41), CD31-phycoerythrin (PE; catalogue 12-0319-41), CD44-PE-Cy7 (catalogue 25-0441-81), CD90-fluorescein isothiocyanate (FITC; catalogue 11-0909-41), CD29-PE (catalogue 12-0299-71), CD73-FITC (catalogue 11-0739-41), CD4-PE (catalogue 12-0049-41), and CD166-PE-Cy7 (catalogue 46-1668-41). All samples were stained for 30 minutes at 4°C and protected from light. Following incubation, the cells were fixed in 0.5% paraformaldehyde for 15 minutes at 4°C. Unstained controls were included in every trial.
In Vitro Clonogenic Potential
Colony-forming unit-fibroblast (CFU-F) assays were performed on P2 ASCs (n = 3, N = 3) using established protocols [33]. Briefly, ASCs were plated at 100 cells per 100 mm diameter tissue culture dish in growth medium and cultured under normoxic (95% air/5% CO2) and hypoxic (5% O2/90% N2/5% CO2) conditions, using a ProOx 110 oxygen controller and subchamber system (Biospherix, Lacona, NY), with medium changes every 3 days. At 14 days, the cells were stained with 0.5% crystal violet in methanol. Stained colonies having a diameter ≥5 mm were counted, and the colony-forming efficiency (CFE) was calculated as the total number of colonies per 100 seeded cells.
ASC Proliferation
The proliferative capacity of P2 ASCs was assessed under normoxic (95% air/5% CO2) and hypoxic (5% O2/90% N2/5% CO2) culture conditions (n = 3, N = 6). The ASCs were plated at 2,600 cells/cm2 in 6-well plates in growth medium. Every 48 hours for 8 days, triplicate wells were trypsinized and counted using the ViaCount assay with the Guava easyCyte 8HT flow cytometer, and used to calculate the doubling time for each population.
Adipogenic Differentiation
P2 ASCs were plated at 50,000 cells/cm2 in 6-well plates and cultured in growth medium under normoxic (95% air/5% CO2) or hypoxic (5% O2/90% N2/5% CO2) conditions until confluent. The medium was changed to serum-free adipogenic differentiation medium (DMEM:Ham’s F12 supplemented with 33 μM biotin, 17 μM pantothenate, 66 nM human insulin, 1 nM triiodothyronine, 10 μg/ml transferrin, 100 nM hydrocortisone, and 1% pen-strep). For the first 72 hours, 1 μg/ml troglitazone and 0.25 mM isobutylmethylxanthine were added [34]. Adipogenesis was assayed at 7 days postinduction through glycerol-3-phosphate dehydrogenase (GPDH) enzyme activity (n = 3, N = 6), as well as oil red O staining of intracellular lipid (n = 3, N = 6), using published methods [32, 35, 36]. Negative controls maintained in growth medium were included in all assays.
Osteogenic Differentiation
P2 ASCs were plated at 20,000 cells/cm2 on laminin-coated 6-well plates (2 μg/cm2; Sigma-Aldrich L2020) and cultured in growth medium under normoxic (95% air/5% CO2) or hypoxic (5% O2/90% N2/5% CO2) conditions until 70% confluence. The medium was replaced with osteogenic differentiation medium comprised of growth medium supplemented with 50 μM ascorbate-2-phosphate, 10 mM β-glycerophosphate, 100 nM dexamethasone, and 0.01 μM 1,25-dihydroxyvitamin D3, with medium changes every 2–3 days [13]. Osteogenic differentiation was assessed at 28 days after induction in terms of alkaline phosphatase (ALP) enzyme activity (n = 3, N = 6) and von Kossa staining of matrix mineralization (n = 3, N = 6), using published methods [37, 38]. Negative control cultures maintained in growth medium were included in all assays.
Endpoint Reverse Transcription-Polymerase Chain Reaction Analysis
Endpoint reverse transcription-polymerase chain reaction (RT-PCR) analysis of lineage-specific gene expression was conducted for the induced adipogenic and osteogenic cultures under normoxic (95% air/5% CO2) and hypoxic (5% O2/90% N2/5% CO2) conditions (n = 3, N = 2) using published protocols [35]. Gene-specific primers (Life Technologies) were designed with Primer3 software and had a melting temperature of 60°C. Adipogenesis was assayed at 7 and 14 days post-induction in terms of the following: PPARγ (forward, 5′-TTCAGAAATGCCTTGCAGTG-3′, and reverse, 5′-CCAACAGCTTCTCCTTCTCG-3′); C/EBPα (forward, 5′-CAGAGGGACCGGAGTTATGA-3′, and reverse, 5′-TTCACATTGCACAAGGCACT-3′); and lipoprotein lipase (LPL) (forward, 5′-GTCCGTGGCTACCTGTCATT-3′, and reverse, 5′-TGGCACCCAACTCTCATACA-3′). Osteogenesis was analyzed at 21 and 28 days post-induction in terms of the following: RUNX2 (forward, 5′-CGGAATGCCTCTGCTGTTAT-3′, and reverse, 5′-TGGGGAGGATTTGTGAAGAC-3′); osteonectin (ON) (forward, 5′-GTGCAGAGGAAACCGAAGAG-3′, and reverse, 5′-AAGTGGCAGGAAGAGTCGAA-3′); and bone sialoprotein (BSP) (forward, 5′-AGAACCACTTCCCCACCTTT-3′, and reverse, 5′-AGGTTCCCCGTTCTCACTTT-3′), and GAPDH (forward, 5′-ACAGTCAGCCGCATCTTCTT-3′, and reverse, 5′-ACGACCAAATCCGTTGACTC-3′) was included as the housekeeping gene. Each PCR was carried out for 40 cycles (95°C for 30 s, 58°C for 30 s, and 72°C for 30 s) using a Bio-Rad C1000 thermal cycler. Expression was analyzed following agarose gel electrophoresis and ethidium bromide staining using a Syngene G:Box Chemi HR16 system.
Statistical Analysis
Statistical analyses were carried out using GraphPad Prism version 6 (San Diego, CA). Differences were considered significant at p < .05. To compare depot differences in the donor-matched sets, Wilcoxon matched-pairs signed rank t tests were used. Unpaired parametric two-tailed t tests with Welch’s correction were used to analyze the effect of oxygen tension or depot for individual donors. Friedman one-way analysis of variance (ANOVA) with a Dunn’s post hoc test was used for multiple comparisons in the matched donor sets. Kruskal-Wallis one-way ANOVA with a Dunn’s post hoc test was used for multiple comparisons between depots.
Results
Tissue Ultrastructure
There were differences in the tissue ultrastructure of each depot (Fig. 1). In the matched donor sets, the omentum was macroscopically less stiff and more vascularized than abdominal subcutaneous adipose tissue. Masson’s trichrome staining confirmed that subcutaneous fat contained more fibrous connective tissue, with fewer blood vessels distributed through the collagen (Fig. 1C). The pericardial and thymic remnant samples were macroscopically similar, although the thymic remnant appeared more fibrous in younger patients (<60 years). However, Masson’s trichrome staining revealed that the thymic remnant had much higher complexity, generally containing more fibrous collagen, large blood vessels, and regions enriched in immune cells (Fig. 1C).
Viable SVF Yield Is Depot Dependent
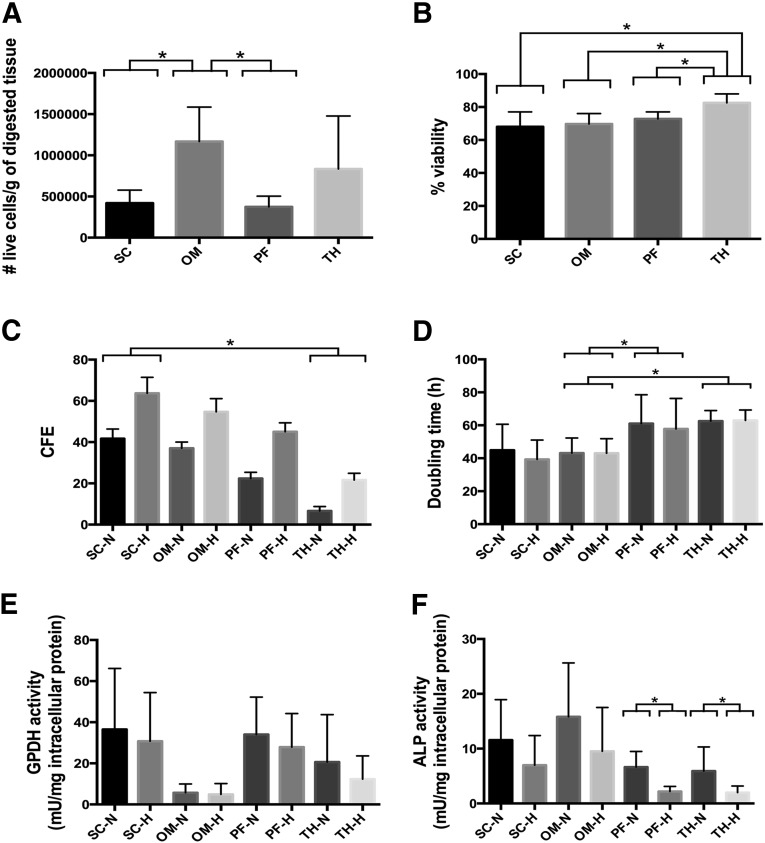
The average viable cell yield in the SVF was calculated per gram of digested tissue for all depots (Fig. 2A). The numerical values for all graphical data shown in Figure 2 are summarized in supplemental online Table 2. A significantly higher yield was obtained from the omentum as compared with subcutaneous fat for all of the donors. Similarly, the thymic remnant had a higher average yield than pericardial adipose tissue, but the values were more variable and not statistically different (Fig. 2A). Pericardial adipose tissue had a lower average percentage viability in the SVF than the thymic remnant (72.8% ± 4.2% versus 82.5% ± 5.4%) (Fig. 2B). In comparing the matched donor samples, this difference was statistically significant for all patients, with the exception of donor 4. No trends were noted in terms of the effect of donor age or BMI on the SVF yield or percent viability.
Figure 2.
Data summary for average values for all donors for each of the depots. All data are expressed as mean ± SD. ∗, Significantly different at (p < .05). (A): Viable cell yield per gram of digested tissue (n = 3, N = 7). (B): Percent viability in stromal vascular fraction (n = 3, N = 7). (C): In vitro clonogenic potential measured through the colony-forming unit-fibroblast assay at 14 days (n = 3, N = 3). (D): Doubling time of P2 ASCs (n = 3, N = 6). (E): GPDH enzyme activity at 7 days post-induction of adipogenic differentiation (n = 3, N = 6). (F): ALP enzyme activity at 28 days post-induction of osteogenic differentiation (n = 3, N = 6). Abbreviations: ALP, alkaline phosphatase; CFE, colony-forming efficiency; GPDH, glycerol-3-phosphate dehydrogenase; H, hypoxic (5% O2/90% N2/5% CO2); N, normoxic (95% air/5% CO2); OM, omentum; PF, pericardial fat; SC, subcutaneous; TH, thymic remnant.
CD34 and CD166 Expression in Cultured ASCs Varies Depending on the Adipose Tissue Source
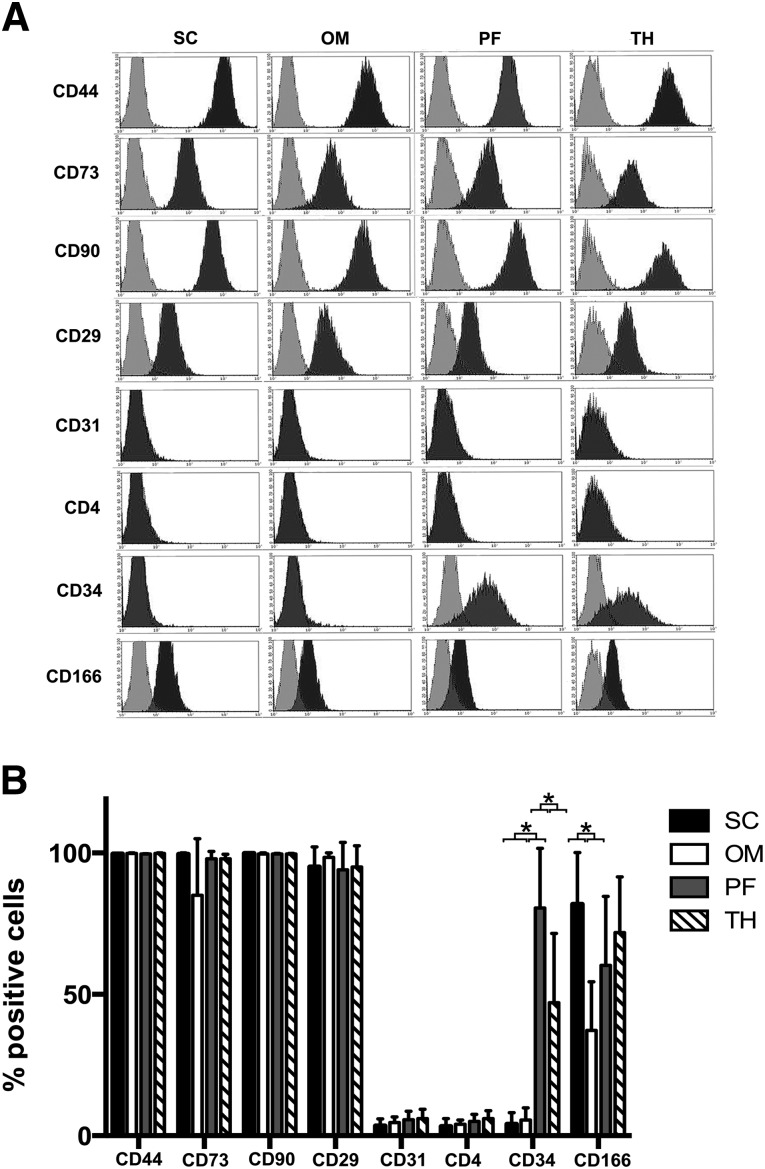
Representative surface marker expression profiles for P2 ASCs from each depot are shown in Figure 3. Subcutaneous and omentum samples were collected from four male and two female donors with an average age of 56 ± 22 years, and the pericardial adipose and thymic remnant samples were collected from five male donors and one female donor with an average age of 70 ± 12 years. All populations were strongly positive for CD44, CD73, CD90, and CD29, with very low expression of CD31 and CD4. Analysis of CD4 was included to confirm absence of expression in the populations from the thymic remnant.
Figure 3.
Immunophenotype of P2 ASCs isolated from each of the depots. (A): Representative histograms of donor-matched subcutaneous/omentum samples and donor-matched pericardial fat/thymic remnant samples. (B): Percentage of positive cells for each of the markers analyzed. Data are presented as the mean ± SD (n = 3, N = 6). Interdepot differences in the expression were observed for CD34 and CD166. ∗, Statistically significant (p < .05). Abbreviations: OM, omentum; PF, pericardial fat; SC, subcutaneous; TH, thymic remnant.
Whereas cultured ASCs from subcutaneous fat or the omentum did not express CD34, 81% ± 21% and 47% ± 25% of P2 ASCs from pericardial adipose tissue and thymic remnant, respectively, were CD34+ (Fig. 3B), with the pericardial depot having significantly higher expression than all other depots. A significantly higher percentage of P2 ASCs isolated from subcutaneous adipose tissue was CD166+, with an overall average of 82% ± 18%, as compared with 37% ± 17% for the omentum. CD166 expression was typically lower in the ASCs derived from pericardial adipose tissue (average 60% ± 25%) than from the thymic remnant (average 72% ± 20%), but the difference was not statistically significant.
In Vitro Clonogenic Potential Is Enhanced Under Hypoxic Conditions
In the matched donor sets, there was a trend for higher CFE in ASCs from subcutaneous fat versus the omentum, and also for ASCs from pericardial adipose tissue versus the thymic remnant (Fig. 2C). In comparing all depots, there was a significant difference in CFE between the subcutaneous and thymic remnant samples under both normoxic and hypoxic conditions. Furthermore, colony formation was enhanced for all depots under hypoxic culture conditions (Fig. 2C). Although the average CFE was lower for the ASCs from the intrathoracic depots, the pericardial and thymic remnant ASCs were more susceptible to the inductive effects of hypoxia, showing a higher percentage increase in colony formation under hypoxic conditions (supplemental online Fig. 1A).
ASCs Isolated From the Intrathoracic Depots Have a Longer Average Doubling Time
ASCs from the intrathoracic depots had a longer average doubling time than ASCs from the subcutaneous/omentum donors, although the difference was only statistically significant relative to the omentum (Fig. 2D). For the donors in our study, we did not observe any specific trends between donor age and proliferative capacity. Under the current study conditions, the proliferation rate was not affected by the oxygen tension in the culture environment.
Subcutaneous ASCs Demonstrate Enhanced Adipogenic Differentiation Relative to ASCs Derived From the Omentum
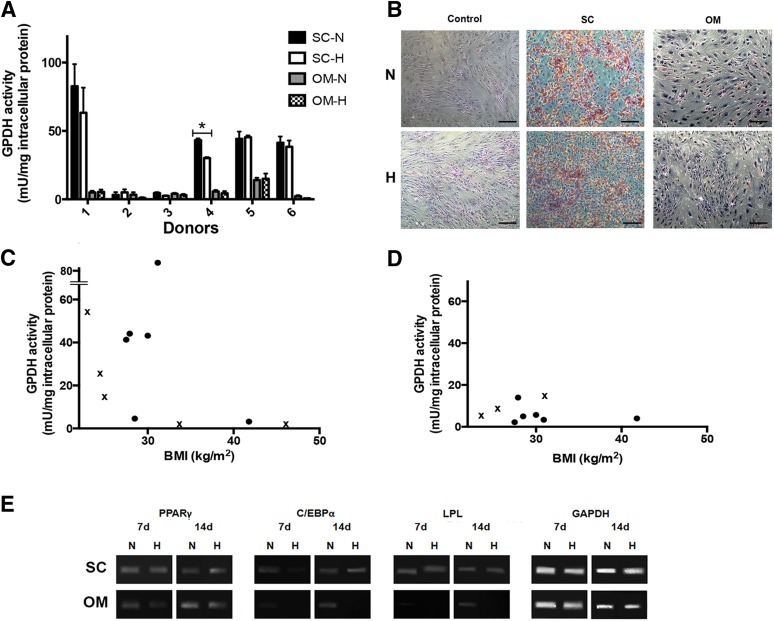
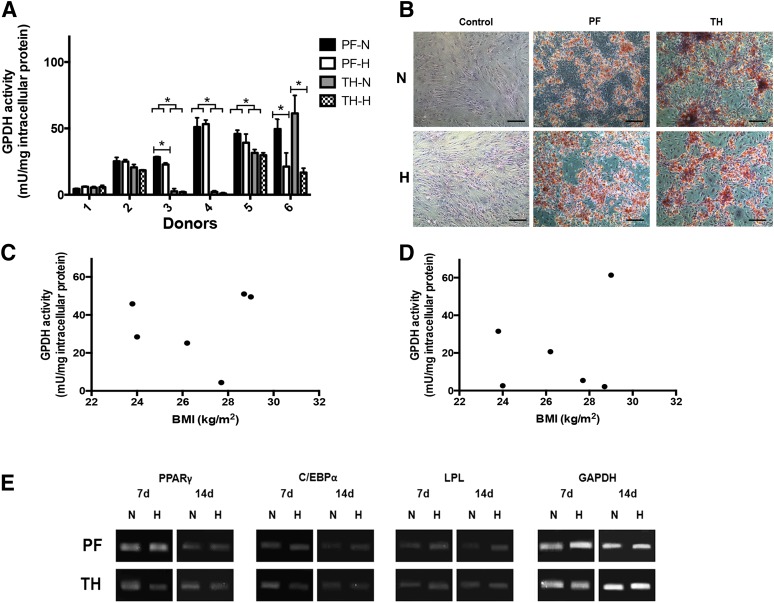
The P2 ASCs isolated from the subcutaneous depot had a significantly higher GPDH activity level than the ASCs isolated from the omentum for four of the six donors (Fig. 4A). Moreover, except for donor 5, the GPDH activity levels for the induced omental ASCs were the same as the non-induced controls, indicating that the cells were not undergoing adipogenesis. In the subcutaneous samples, lower GPDH levels were observed for more severely obese donors (BMI > 30) (Fig. 4C), whereas low levels were observed for all omentum samples regardless of BMI (Fig. 4D). Average GPDH activity levels for all depots are highlighted in Figure 2E. The GPDH results were confirmed with oil red O staining, which showed extensive intracellular lipid in the subcutaneous ASCs, which qualitatively correlated with the GPDH activity level (Fig. 4B). In contrast, no positive staining was observed in the omental ASCs at 7 days, with the exception of the donor 5 sample, which showed a small fraction of differentiating cells.
Figure 4.
Adipogenic differentiation of ASCs isolated from subcutaneous fat and the omentum. (A): GPDH enzyme activity of induced donor-matched P2 ASCs at 7 days. Data are expressed as mean ± SD (n = 3, N = 6). ∗, Significantly different (p < .05). (B): Representative oil red O staining at 7 days post-induction. Intracellular lipid accumulation was observed under both oxygen conditions for subcutaneous ASCs, but not ASCs derived from the omentum or the negative controls. Scale bars represent 100 μm. Relationship between GPDH activity and donor BMI for ASCs derived from subcutaneous adipose tissue (C) and omentum (D). Crossed values (x) indicate nonmatched donor samples not included in the comparative assessments. (E): Representative endpoint RT-PCR analysis of adipogenic gene expression at 7 and 14 days (n = 3, N = 2) with GAPDH as the housekeeping gene. Abbreviations: BMI, body mass index; GPDH, glycerol-3-phosphate dehydrogenase; H, hypoxic (5% O2/90% N2/5% CO2); N, normoxic (95% air/5% CO2); OM, omentum; SC, subcutaneous.
Based on the GPDH and oil red O data, oxygen tension did not have a major impact on adipogenic differentiation in the subcutaneous and omentum samples at 7 days, although GPDH activity was reduced in the subcutaneous ASCs from donors 1 and 4 under hypoxic conditions (statistically significant for donor 4) (Fig. 4A). In terms of gene expression (Fig. 4E), the master adipogenic regulator PPARγ and the late marker LPL were expressed at 7 and 14 days in all induced subcutaneous ASC samples. C/EBPα expression was lower at 7 days under hypoxic conditions in this group. In contrast, for the omentum, whereas PPARγ was expressed at both time points with qualitatively higher expression under normoxic conditions, C/EBPα was only detected under normoxic conditions and was expressed at very low levels at 7 days. Similarly, LPL was expressed strictly in the normoxic samples at 14 days. These patterns suggest a lower level of adipogenic differentiation in the hypoxic cultures.
Pericardial ASCs Demonstrate Enhanced Adipogenic Differentiation Relative to ASCs Derived From the Thymic Remnant
For the pericardial and thymic remnant samples, GPDH activity was observed in the pericardial ASCs but not in the donor-matched thymic remnant ASCs from donors 3 and 4 (Fig. 5A). Furthermore, for the ASCs derived from the thymic remnant from donors 1, 3, and 4, the GPDH activity was the same as the non-induced controls, indicating that the cells were not undergoing adipogenesis. For donor 1, no significant differentiation was also observed for the pericardial ASCs. The GPDH activity was not clearly correlated with the BMI for the intrathoracic depots, although all of the donors had a BMI < 30 (Fig. 5C, 5D). Intracellular lipid was observed for ASCs from both intrathoracic depots under normoxic and hypoxic conditions for all samples with a GPDH activity >5 mU/mg intracellular protein (Fig. 5B).
Figure 5.
Adipogenic differentiation of ASCs isolated from pericardial and thymic remnant adipose tissue. (A): GPDH enzyme activity of induced donor-matched P2 ASCs at 7 days. Data are expressed as mean ± SD (n = 3, N = 6). ∗, Significantly different (p < .05). (B): Representative oil red O staining at 7 days post-induction. Scale bars represent 100 μm. Relationship between GPDH activity and donor BMI for ASCs derived from pericardial adipose tissue (C) and thymic remnant (D). (E): Representative endpoint RT-PCR analysis of adipogenic gene expression at 7 and 14 days (n = 3, N = 2) with GAPDH as the housekeeping gene. Abbreviations: BMI, body mass index; GPDH, glycerol-3-phosphate dehydrogenase; H, hypoxic (5% O2/90% N2/5% CO2); N, normoxic (95% air/5% CO2); PF, pericardial fat; TH, thymic remnant.
In terms of oxygen tension, GPDH activity was reduced in the pericardial ASCs under hypoxic conditions for three of six of the donors (statistically significant for donors 3 and 6) (Fig. 5A). Similarly, the GPDH activity for the thymic remnant ASCs from the three donors that responded was negatively affected by hypoxic culturing, but this difference was only statistically significant in donor 6. In terms of adipogenic gene expression, all markers were detected under normoxic and hypoxic culture conditions for both intrathoracic depots (Fig. 5E).
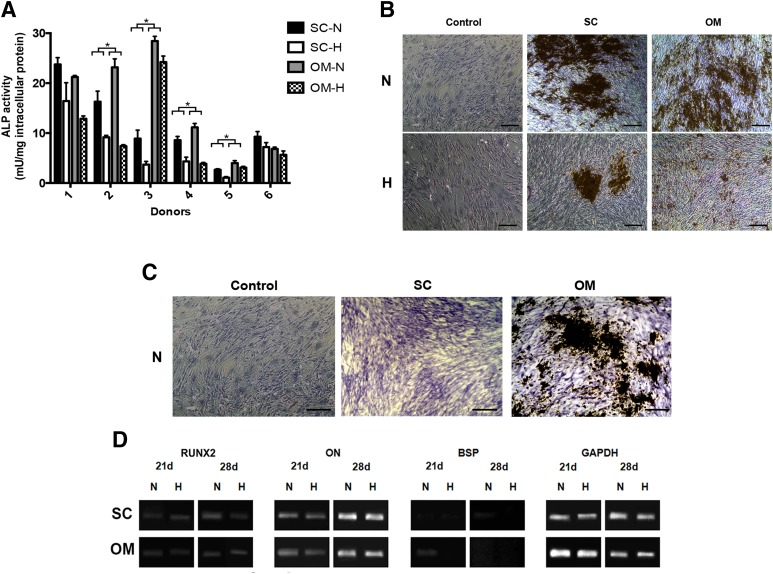
Osteogenic Differentiation Is Enhanced in ASCs Isolated From the Omentum and Under Normoxic Culture Conditions
For the subcutaneous and omentum ASCs, ALP enzyme activity (Fig. 6A) was significantly enhanced in all donor sets as compared with the non-induced controls. Under normoxic conditions, there was a trend toward higher average ALP enzyme activity in the omental ASCs (Fig. 2F). In the donor-matched analysis, the normoxic ALP activity was consistently higher for the omentum samples, and this difference was statistically significant for donors 2–5 (Fig. 6A). For both depots, the ALP enzyme activity was significantly lower in the cells cultured under hypoxic culture conditions (Figs. 2F, 6A). The von Kossa staining results were more variable. In donors 2 and 5, matrix mineralization was only observed in the omental ASCs (Fig. 6C). For the other donors, the effects of oxygen tension on matrix mineralization were consistent with the trends in ALP activity, with larger deposits observed under normoxic conditions for both depots (Fig. 7B). In terms of osteogenic gene expression (Fig. 7C), RUNX2 and ON were expressed at similar levels in the ASCs from both depots at 21 and 28 days, with slightly enhanced RUNX2 expression under normoxic conditions for the subcutaneous samples at 28 days. BSP expression was only detected at low levels under normoxic conditions in the subcutaneous ASCs at 28 days, as well as at qualitatively higher levels in the omentum samples at 21 days.
Figure 6.
Osteogenic differentiation of ASCs isolated from subcutaneous fat and the omentum. (A): ALP enzyme activity of induced donor-matched P2 ASCs at 28 days. Data are expressed as mean ± SD (n = 3, N = 6). With the exception of donor 1 (SC), donor 5 (OM), and donor 6 (SC + OM), a significant difference was observed in ALP activity in both depots under normoxic versus hypoxic conditions. ∗, Significant difference between donor-matched subcutaneous and omentum ASCs (p < .05). (B): Representative von Kossa staining at 28 days post-induction. Matrix mineralization was observed in all induced samples, but was dramatically reduced under hypoxic conditions for both depots. Scale bars represent 100 μm. (C): In donors 2 and 5, matrix mineralization was observed in ASCs isolated from the omentum but not subcutaneous fat. Scale bars represent 100 μm. (D): Representative endpoint RT-PCR analysis of osteogenic gene expression at 21 and 28 days (n = 3, N = 2) with GAPDH as the housekeeping gene. Abbreviations: ALP, alkaline phosphatase; H, hypoxic (5% O2/90% N2/5% CO2); N, normoxic (95% air/5% CO2); OM, omentum; SC, subcutaneous.
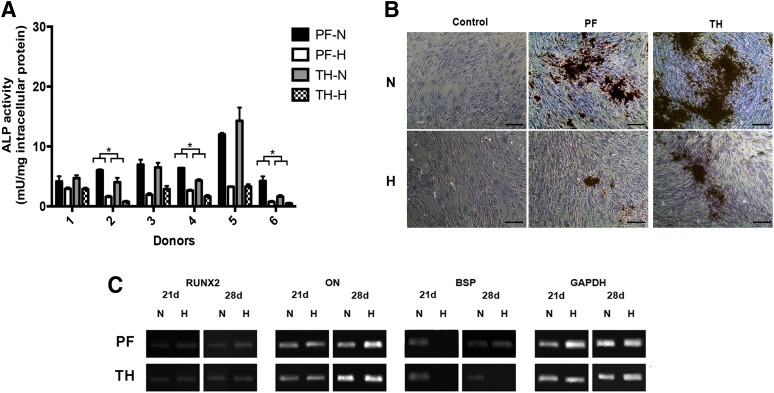
Figure 7.
Osteogenic differentiation of ASCs isolated from pericardial adipose tissue and the thymic remnant. (A): ALP enzyme activity of induced donor-matched P2 ASCs at 28 days. Data are expressed as mean ± SD (n = 3, N = 6). For all donors, a significant difference was observed for both depots under normoxic versus hypoxic conditions. ∗, Significant difference between donor-matched pericardial and thymic remnant ASCs (p < .05). (B): Representative von Kossa staining at 28 days post-induction. Matrix mineralization was observed in all induced samples, but was reduced under hypoxic conditions for both depots. Scale bars represent 100 μm. (C): Representative endpoint RT-PCR analysis of osteogenic gene expression at 21 and 28 days (n = 3, N = 2) with GAPDH as the housekeeping gene. Abbreviations: ALP, alkaline phosphatase; H, hypoxic (5% O2/90% N2/5% CO2); N, normoxic (95% air/5% CO2); PF, pericardial fat; TH, thymic remnant.
For the intrathoracic samples, the average ALP enzyme activity levels were similar for both depots (Fig. 2F). However, in the donor-matched analysis, significantly higher ALP activity was noted under normoxic conditions in the pericardial ASCs from donors 2, 4, and 6 (Fig. 7A). All induced cultures stained positively for matrix mineralization (Fig. 7B), with the exception of the donor 6 samples. Similar to the subcutaneous and omental ASCs, osteogenic differentiation was reduced under hypoxic conditions (Fig. 7A, 7B). In the gene expression studies (Fig. 7C), RUNX2 and ON were expressed in both sample groups, at qualitatively higher levels at 28 days. BSP was expressed under normoxic conditions for both depots at 21 and 28 days, as well as in the pericardial ASCs cultured under hypoxic conditions at 28 days.
In comparing all four depots, the omentum had the highest average ALP activity levels of all four depots, although the differences were not statistically significant (Fig. 2F). Furthermore, it was noted that there were differences in the sensitivity of the cell populations to the hypoxic culture conditions between depots (supplemental online Fig. 1B). More specifically, the percentage decrease in average ALP activity under hypoxic conditions versus normoxic conditions was more pronounced for the ASCs from the intrathoracic depots.
Intrathoracic ASCs Undergo Adipogenic Differentiation in Osteogenic Culture Conditions
Interestingly, extensive intracellular lipid accumulation was observed in the osteogenic studies with the pericardial and thymic remnant ASCs from donors 2, 4, and 5 under normoxic conditions, suggesting that these cells were predisposed toward the adipogenic lineage (supplemental online Fig. 2). No evidence of adipogenesis was observed in any of the osteogenic cultures for the ASCs from subcutaneous adipose tissue or the omentum.
Discussion
In obesity and metabolism research, depot- and donor-dependent differences in adipose tissue structure and function, along with variations in the mature adipocyte and adipose progenitor populations, have long been recognized [18, 39–41]. In terms of the clinical translation of cellular therapies involving ASCs, these studies highlight that “all fat is not equal” and emphasize the importance of consideration of adipose depot selection. A better understanding of the characteristics of ASCs from different depots and donors will help to identify whether a specific fat source is more promising for certain regenerative applications. If an autologous approach is to be applied, there are many other influencing factors, including donor health status, tissue availability and accessibility, and timing, as well as cost of treatment.
The majority of comparative studies to date have focused on assessing differences in proliferation and adipogenic differentiation in cells isolated from subcutaneous adipose tissue and the omentum [14, 18, 39–41]. A smaller number of studies have looked at the effects of adipose source on osteogenic potential [16, 42, 43]. Interestingly, there is no clear consensus in terms of the specific trends for proliferation or differentiation, and the results from different groups can appear contradictory. However, there is a lack of standardization in methods, including cell extraction protocols, culture conditions, and passage number, as well as inherent variability in the donor populations, which can make it difficult to compare the data. Furthermore, in reviewing past work, it becomes clear that it is advantageous to use a range of characterization assays to more fully understand the observed cellular responses.
With this guiding principle, we applied consistent methods to more deeply probe the influence of adipose tissue depot source on the human ASC populations. Subcutaneous fat is the most widely investigated ASC source, due to its abundance and ease in acquisition. However, the omentum has long been recognized for its unique angiogenic properties, and omental flaps have been used in many clinical applications to stimulate blood vessel formation and healing, which may suggest the presence of distinct regenerative cell populations [44–47]. In the context of heart surgery through a sternotomy, pericardial adipose tissue and the thymic remnant become accessible, expendable, and often abundant adipose sources that could be used in cell therapies for cardiovascular regeneration. Previous studies have identified multipotent cell populations in epicardial fat, which is closely interconnected with the myocardium [19], and in the thymus of pediatric [48] and adult [49] donors. However, pericardial adipose tissue, as well as the thymic remnant from elderly donors, have not yet been explored as ASC sources. Furthermore, to the best of our knowledge, this is the first comparative adipose depot study to assess the effects of oxygen tension by comparing the ASC responses under normoxic and hypoxic culture conditions.
In terms of yield, the number of viable cells in the SVF from the omentum was significantly higher than from abdominal subcutaneous adipose tissue, which is consistent with the findings of van Harmelen et al. [41] and may be attributable to the presence of more endothelial cells in the richly vascularized omentum. For the intrathoracic depots, there was substantial donor variability in the thymic remnant samples. Combined with the histological results, it is likely that this difference in the SVF yield is at least partly associated with the presence of other cell types, such as lymphocytes, that were not eliminated during the extraction process.
Flow cytometric analysis of P2 ASCs showed that, consistent with the literature, all cultured ASCs were positive for CD44, CD73, CD90, and CD29, with very low expression of CD31 and CD4 [50–52]. CD166 expression was more variable, but the patterns are in line with previous reports of low expression of this adhesion molecule in the SVF of subcutaneous ASCs, with enhanced expression through successive passaging [51, 53]. Similarly, several studies have demonstrated that SVF from subcutaneous adipose tissue and the omentum contains CD34+ populations, but expression decreases substantially during culturing [15, 51, 54]. However, we found that a high percentage of P2 ASCs from the intrathoracic depots was CD34+ after expansion. This enhanced CD34 expression may be favorable for cardiovascular cell therapy. More specifically, testing in animal models of chronically ischemic myocardium has provided evidence of the safety and efficacy of intramyocardial transplantation of CD34+-enriched cell suspensions for promoting neovascularization [55–57]. Based on these findings, recent clinical trials have focused on strategies with autologous CD34+ bone marrow-derived MSCs in patients with refractory angina [58] and myocardial infarction [59], with significant improvements noted in angina frequency and exercise tolerance in the first study and a dose response observed in terms of the number of injected CD34+ cells and increased left ventricular ejection fraction in the second.
In terms of in vitro clonogenic potential, it was interesting that the P2 ASCs derived from subcutaneous adipose tissue and the omentum formed more colonies on average in the CFU-F assay, despite having lower levels of CD34 expression than the ASCs derived from pericardial adipose tissue and thymic remnant. Previous work with subcutaneous ASCs and bone marrow-derived MSCs has indicated that there is a positive correlation between CD34 expression and colony formation [60–62]. However, our results are consistent with our proliferation data, which showed that the intrathoracic ASCs had longer average doubling times. Although the proliferation rates were not altered under the level of hypoxia used in our study, there was enhanced colony formation observed for all depots when the cells were cultured at 5% O2. These results are similar to past studies demonstrating the positive effects of hypoxia on colony formation for bone marrow-derived MSCs [63, 64].
In terms of adipogenesis, our donor-matched subcutaneous and omentum results were consistent with published studies indicating that subcutaneous ASCs are more adipogenic than those derived from the omentum [15, 16, 18, 41]. Interestingly, unlike ASCs from epicardial adipose tissue that reportedly do not differentiate into adipocytes [19], the cells derived from both pericardial adipose tissue and thymic remnant could be stimulated to undergo adipogenic differentiation, although the thymic remnant results were more inconsistent. In general, there was donor variability in the GPDH activity levels observed for all depots, which may be attributable to a multitude of factors, including gender, age, and BMI, as well as insulin sensitivity [65]. In terms of the effect of oxygen tension on adipogenic differentiation, GPDH activity and intracellular lipid accumulation were not majorly influenced by culturing at 5% O2 for most of the donors. However, there was a trend toward reduced enzyme activity under hypoxic conditions, as well as some differences in the gene expression patterns for the subcutaneous and omentum ASCs, which may suggest that hypoxia had a slight negative impact on adipogenesis. Based on the results of Lin et al. and Lee and Kemp [27, 66], it is possible that further reductions in the oxygen levels to 1% or 2% could more profoundly influence the adipogenic response.
In our study, which is the first to compare ASCs extracted from matched subcutaneous/omentum fat and matched intrathoracic adipose tissue under varying oxygen levels, osteogenic differentiation was enhanced in the omental ASCs relative to all other depots. The lack of mineralization observed in some subcutaneous samples, despite elevated ALP activity, emphasizes the importance of analyzing multiple differentiation markers. The enhanced osteogenic activity combined with the reduced adipogenic capacity of the omental ASCs may be indicative that the heterogeneous population includes progenitors that have more restricted potential in culture and are predisposed toward the osteogenic lineage, as has been observed with MSCs derived from human bone marrow [67–70]. Consistent with the results of He et al. and Merceron et al. [26, 30], ALP activity and mineral deposition were inhibited when the cells were cultured at 5% O2 for all depots. These results are concordant with the fact that under physiological conditions bone is highly vascularized and that vascularization is key for normal bone development [71].
For three donors under normoxic conditions, the intrathoracic ASCs accumulated substantial intracellular lipid under osteogenic differentiation conditions, which may be related to the dexamethasone in the osteogenic formulation, as this potent corticosteroid can have inductive effects on both osteogenic and adipogenic pathways [72, 73]. Furthermore, whereas vitamin D3 has been used to stimulate osteogenic differentiation of ASCs [74], it can have diverse effects on different cell populations and has been shown in previous studies with fetal rat calvaria cells to promote adipogenesis synergistically with dexamethasone and inhibit osteogenesis [75, 76]. This phenomenon was only observed in the intrathoracic ASCs, and the differential response may indicate that these populations are more committed toward the adipogenic lineage.
Conclusion
The results of our study emphasize the relevance of depot selection in the development of autologous or allogenic cell-based regenerative strategies with ASCs. Although there was donor variability, particularly in the adipogenic and osteogenic differentiation levels, notable differences were observed between the populations isolated from the subcutaneous, omentum, and intrathoracic depots. Moreover, we have shown for the first time that ASCs can be extracted from pericardial adipose tissue and the thymic remnant and that these cells have unique characteristics, including enhanced CD34 expression at P2 and increased sensitivity to hypoxic culture conditions. Of the intrathoracic depots studied, the pericardial ASC population was more homogenous and may have potential clinical utility in cardiovascular applications. The enhanced osteogenic capacity of the omentum, combined with its reduced adipogenic differentiation potential, suggests that omental ASCs may hold particular promise for bone regeneration strategies.
Supplementary Material
Acknowledgments
We thank Dr. S.D. Waldman for the use of the microplate reader and Oliver Jones for technical assistance with sectioning of the tissue samples. Furthermore, we thank Laurie Dodd, Shelley Simpson, Dr. J.F. Watkins, and Karen Martin for support with clinical collaborations. Funding for this study was provided by the Kingston General Hospital/Hotel Dieu Hospital Cardiac Program Research Award and the Canadian Institutes of Health Research. Scholarship support for Valerio Russo was provided through the Human Mobility Research Centre NSERC (Natural Sciences and Engineering Research Council)-CREATE Training Program in Bone and Joint Health Technologies. Infrastructure was purchased with funding from the Canadian Foundation for Innovation Leader's Opportunity Fund, the Ministry of Research and Innovation Ontario Research Fund - Research Infrastructure, and the NSERC Research Tools and Instruments programs.
Author Contributions
V.R.: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing; C.Y.: collection and assembly of data; P.B.: provision of study material, final approval of manuscript; A.H.: conception and design, financial support, provision of study material, final approval of manuscript; L.E.F.: conception and design, financial support, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Bourin P, Bunnell BA, Casteilla L, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT) Cytotherapy. 2013;15:641–648. doi: 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mizuno H, Tobita M, Uysal AC. Concise review: Adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells. 2012;30:804–810. doi: 10.1002/stem.1076. [DOI] [PubMed] [Google Scholar]
- 3.Gimble JM, Bunnell BA, Guilak F. Human adipose-derived cells: An update on the transition to clinical translation. Regen Med. 2012;7:225–235. doi: 10.2217/rme.11.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rehman J, Traktuev D, Li J, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 5.Suga H, Glotzbach JP, Sorkin M, et al. Paracrine mechanism of angiogenesis in adipose-derived stem cell transplantation. Ann Plast Surg. 2013 doi: 10.1097/SAP.0b013e318264fd6a. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McIntosh K, Zvonic S, Garrett S, et al. The immunogenicity of human adipose-derived cells: Temporal changes in vitro. Stem Cells. 2006;24:1246–1253. doi: 10.1634/stemcells.2005-0235. [DOI] [PubMed] [Google Scholar]
- 7.Puissant B, Barreau C, Bourin P, et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: Comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005;129:118–129. doi: 10.1111/j.1365-2141.2005.05409.x. [DOI] [PubMed] [Google Scholar]
- 8.Melief SM, Zwaginga JJ, Fibbe WE, et al. Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts. Stem Cells Transl. Med. 2013;2:455–463. doi: 10.5966/sctm.2012-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao Y, Sun Z, Liao L, et al. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun. 2005;332:370–379. doi: 10.1016/j.bbrc.2005.04.135. [DOI] [PubMed] [Google Scholar]
- 10.Baer PC, Brzoska M, Geiger H. Epithelial differentiation of human adipose-derived stem cells. Methods Mol Biol. 2011;702:289–298. doi: 10.1007/978-1-61737-960-4_21. [DOI] [PubMed] [Google Scholar]
- 11.Banas A, Teratani T, Yamamoto Y, et al. Adipose tissue-derived mesenchymal stem cells as a source of human hepatocytes. Hepatology. 2007;46:219–228. doi: 10.1002/hep.21704. [DOI] [PubMed] [Google Scholar]
- 12.Ashjian PH, Elbarbary AS, Edmonds B, et al. In vitro differentiation of human processed lipoaspirate cells into early neural progenitors. Plast Reconstr Surg. 2003;111:1922–1931. doi: 10.1097/01.PRS.0000055043.62589.05. [DOI] [PubMed] [Google Scholar]
- 13.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 14.Tchkonia T, Giorgadze N, Pirtskhalava T, et al. Fat depot origin affects adipogenesis in primary cultured and cloned human preadipocytes. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1286–R1296. doi: 10.1152/ajpregu.00653.2001. [DOI] [PubMed] [Google Scholar]
- 15.Park HT, Lee ES, Cheon Y-P, et al. The relationship between fat depot-specific preadipocyte differentiation and metabolic syndrome in obese women. Clin Endocrinol. 2012;76:59–66. doi: 10.1111/j.1365-2265.2011.04141.x. [DOI] [PubMed] [Google Scholar]
- 16.Toyoda M, Matsubara Y, Lin K, et al. Characterization and comparison of adipose tissue-derived cells from human subcutaneous and omental adipose tissues. Cell Biochem Funct. 2009;27:440–447. doi: 10.1002/cbf.1591. [DOI] [PubMed] [Google Scholar]
- 17.Schipper BM, Marra KG, Zhang W, et al. Regional anatomic and age effects on cell function of human adipose-derived stem cells. Ann Plast Surg. 2008;60:538–544. doi: 10.1097/SAP.0b013e3181723bbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baglioni S, Cantini G, Poli G, et al. Functional differences in visceral and subcutaneous fat pads originate from differences in the adipose stem cell. PLoS One. 2012;7:e36569. doi: 10.1371/journal.pone.0036569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bayes-Genis A, Soler-Botija C, Farré J, et al. Human progenitor cells derived from cardiac adipose tissue ameliorate myocardial infarction in rodents. J Mol Cell Cardiol. 2010;49:771–780. doi: 10.1016/j.yjmcc.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 20.Iacobellis G. Epicardial and pericardial fat: Close, but very different. Obesity. 2009;17:625. doi: 10.1038/oby.2008.575. author reply 626–327. [DOI] [PubMed] [Google Scholar]
- 21.Lynch HE, Goldberg GL, Chidgey A, et al. Thymic involution and immune reconstitution. Trends Immunol. 2009;30:366–373. doi: 10.1016/j.it.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haynes BF, Sempowski GD, Wells AF, et al. The human thymus during aging. Immunol Res. 2000;22:253–261. doi: 10.1385/IR:22:2-3:253. [DOI] [PubMed] [Google Scholar]
- 23.Grayson WL, Zhao F, Bunnell B, et al. Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochem Biophys Res Commun. 2007;358:948–953. doi: 10.1016/j.bbrc.2007.05.054. [DOI] [PubMed] [Google Scholar]
- 24.Dos Santos F, Andrade PZ, Boura JS, et al. Ex vivo expansion of human mesenchymal stem cells: a more effective cell proliferation kinetics and metabolism under hypoxia. J Cell Physiol. 2010;223:27–35. doi: 10.1002/jcp.21987. [DOI] [PubMed] [Google Scholar]
- 25.Zhu W, Chen J, Cong X, et al. Hypoxia and serum deprivation-induced apoptosis in mesenchymal stem cells. Stem Cells. 2006;24:416–425. doi: 10.1634/stemcells.2005-0121. [DOI] [PubMed] [Google Scholar]
- 26.Merceron C, Vinatier C, Portron S, et al. Differential effects of hypoxia on osteochondrogenic potential of human adipose-derived stem cells. Am J Physiol Cell Physiol. 2010;298:C355–C364. doi: 10.1152/ajpcell.00398.2009. [DOI] [PubMed] [Google Scholar]
- 27.Lin Q, Lee Y-J, Yun Z. Differentiation arrest by hypoxia. J Biol Chem. 2006;281:30678–30683. doi: 10.1074/jbc.C600120200. [DOI] [PubMed] [Google Scholar]
- 28.Thangarajah H, Vial IN, Chang E, et al. IFATS collection: adipose stromal cells adopt a proangiogenic phenotype under the influence of hypoxia. Stem Cells. 2009;27:266–274. doi: 10.1634/stemcells.2008-0276. [DOI] [PubMed] [Google Scholar]
- 29.Hu X, Yu SP, Fraser JL, et al. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg. 2008;135:799–808. doi: 10.1016/j.jtcvs.2007.07.071. [DOI] [PubMed] [Google Scholar]
- 30.He J, Genetos DC, Yellowley CE, et al. Oxygen tension differentially influences osteogenic differentiation of human adipose stem cells in 2D and 3D cultures. J Cell Biochem. 2010;110:87–96. doi: 10.1002/jcb.22514. [DOI] [PubMed] [Google Scholar]
- 31.Chung H-M, Won C-H, Sung J-H. Responses of adipose-derived stem cells during hypoxia: Enhanced skin-regenerative potential. Expert Opin Biol Ther. 2009;9:1499–1508. doi: 10.1517/14712590903307362. [DOI] [PubMed] [Google Scholar]
- 32.Flynn L, Semple JL, Woodhouse KA. Decellularized placental matrices for adipose tissue engineering. J Biomed Mater Res A. 2006;79:359–369. doi: 10.1002/jbm.a.30762. [DOI] [PubMed] [Google Scholar]
- 33.Pochampally R. Colony forming unit assays for MSCs. Methods Mol Biol. 2008;449:83–91. doi: 10.1007/978-1-60327-169-1_6. [DOI] [PubMed] [Google Scholar]
- 34.Hauner H, Skurk T, Wabitsch M. Cultures of human adipose precursor cells. Methods Mol Biol. 2001;155:239–247. doi: 10.1385/1-59259-231-7:239. [DOI] [PubMed] [Google Scholar]
- 35.Turner AEB, Yu C, Bianco J, et al. The performance of decellularized adipose tissue microcarriers as an inductive substrate for human adipose-derived stem cells. Biomaterials. 2012;33:4490–4499. doi: 10.1016/j.biomaterials.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 36.Flynn L, Prestwich GD, Semple JL, et al. Adipose tissue engineering with naturally derived scaffolds and adipose-derived stem cells. Biomaterials. 2007;28:3834–3842. doi: 10.1016/j.biomaterials.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 37.Wang V, Misra G, Amsden B. Immobilization of a bone and cartilage stimulating peptide to a synthetic bone graft. J Mater Sci Mater Med. 2008;19:2145–2155. doi: 10.1007/s10856-007-3306-0. [DOI] [PubMed] [Google Scholar]
- 38.Zhao Y, Waldman SD, Flynn LE. The effect of serial passaging on the proliferation and differentiation of bovine adipose-derived stem cells. Cells Tissues Organs. 2012;195:414–427. doi: 10.1159/000329254. [DOI] [PubMed] [Google Scholar]
- 39.Shahparaki A, Grunder L, Sorisky A. Comparison of human abdominal subcutaneous versus omental preadipocyte differentiation in primary culture. Metabolism. 2002;51:1211–1215. doi: 10.1053/meta.2002.34037. [DOI] [PubMed] [Google Scholar]
- 40.Tchkonia T, Tchoukalova YD, Giorgadze N, et al. Abundance of two human preadipocyte subtypes with distinct capacities for replication, adipogenesis, and apoptosis varies among fat depots. Am J Physiol Endocrinol Metab. 2005;288:E267–E277. doi: 10.1152/ajpendo.00265.2004. [DOI] [PubMed] [Google Scholar]
- 41.Van Harmelen V, Röhrig K, Hauner H. Comparison of proliferation and differentiation capacity of human adipocyte precursor cells from the omental and subcutaneous adipose tissue depot of obese subjects. Metabolism. 2004;53:632–637. doi: 10.1016/j.metabol.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 42.Aksu AE, Rubin JP, Dudas JR, et al. Role of gender and anatomical region on induction of osteogenic differentiation of human adipose-derived stem cells. Ann Plast Surg. 2008;60:306–322. doi: 10.1097/SAP.0b013e3180621ff0. [DOI] [PubMed] [Google Scholar]
- 43.Levi B, James AW, Glotzbach JP, et al. Depot-specific variation in the osteogenic and adipogenic potential of human adipose-derived stromal cells. Plast Reconstr Surg. 2010;126:822–834. doi: 10.1097/PRS.0b013e3181e5f892. [DOI] [PubMed] [Google Scholar]
- 44.Ma CH, Kim MG. Laparoscopic primary repair with omentopexy for duodenal ulcer perforation: a single institution experience of 21 cases. J Gastric Cancer. 2012;12:237–242. doi: 10.5230/jgc.2012.12.4.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cartier R, Brunette I, Hashimoto K, et al. Angiogenic factor: A possible mechanism for neovascularization produced by omental pedicles. J Thorac Cardiovasc Surg. 1990;99:264–268. [PubMed] [Google Scholar]
- 46.Kanamori T, Watanabe G, Yasuda T, et al. Hybrid surgical angiogenesis: Omentopexy can enhance myocardial angiogenesis induced by cell therapy. Ann Thorac Surg. 2006;81:160–167. doi: 10.1016/j.athoracsur.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 47.Zhang C, Hou J, Zheng S, et al. Vascularized atrial tissue patch cardiomyoplasty with omentopexy improves cardiac performance after myocardial infarction. Ann Thorac Surg. 2011;92:1435–1442. doi: 10.1016/j.athoracsur.2011.05.054. [DOI] [PubMed] [Google Scholar]
- 48.Mouiseddine M, Mathieu N, Stefani J, et al. Characterization and histological localization of multipotent mesenchymal stromal cells in the human postnatal thymus. Stem Cells Dev. 2008;17:1165–1174. doi: 10.1089/scd.2007.0252. [DOI] [PubMed] [Google Scholar]
- 49.Krampera M, Sartoris S, Liotta F, et al. Immune regulation by mesenchymal stem cells derived from adult spleen and thymus. Stem Cells Dev. 2007;16:797–810. doi: 10.1089/scd.2007.0024. [DOI] [PubMed] [Google Scholar]
- 50.Gronthos S, Franklin DM, Leddy HA, et al. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001;189:54–63. doi: 10.1002/jcp.1138. [DOI] [PubMed] [Google Scholar]
- 51.Mitchell JB, McIntosh K, Zvonic S, et al. Immunophenotype of human adipose-derived cells: Temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006;24:376–385. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- 52.Tucker HA, Bunnell BA. Characterization of human adipose-derived stem cells using flow cytometry. Methods Mol Biol. 2011;702:121–131. doi: 10.1007/978-1-61737-960-4_10. [DOI] [PubMed] [Google Scholar]
- 53.Varma MJO, Breuls RGM, Schouten TE, et al. Phenotypical and functional characterization of freshly isolated adipose tissue-derived stem cells. Stem Cells Dev. 2007;16:91–104. doi: 10.1089/scd.2006.0026. [DOI] [PubMed] [Google Scholar]
- 54.Suga H, Shigeura T, Matsumoto D, et al. Rapid expansion of human adipose-derived stromal cells preserving multipotency. Cytotherapy. 2007;9:738–745. doi: 10.1080/14653240701679873. [DOI] [PubMed] [Google Scholar]
- 55.Iwasaki H, Kawamoto A, Ishikawa M, et al. Dose-dependent contribution of CD34-positive cell transplantation to concurrent vasculogenesis and cardiomyogenesis for functional regenerative recovery after myocardial infarction. Circulation. 2006;113:1311–1325. doi: 10.1161/CIRCULATIONAHA.105.541268. [DOI] [PubMed] [Google Scholar]
- 56.Kocher AA, Schuster MD, Szabolcs MJ, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 57.Kawamoto A, Iwasaki H, Kusano K, et al. CD34-positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation. 2006;114:2163–2169. doi: 10.1161/CIRCULATIONAHA.106.644518. [DOI] [PubMed] [Google Scholar]
- 58.Losordo DW, Henry TD, Davidson C, et al. Intramyocardial, autologous CD34+ cell therapy for refractory angina. Circ Res. 2011;109:428–436. doi: 10.1161/CIRCRESAHA.111.245993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Quyyumi AA, Waller EK, Murrow J, et al. CD34(+) cell infusion after ST elevation myocardial infarction is associated with improved perfusion and is dose dependent. Am Heart J. 2011;161:98–105. doi: 10.1016/j.ahj.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 60.Maumus M, Peyrafitte J-A, D’Angelo R, et al. Native human adipose stromal cells: localization, morphology and phenotype. Int J Obes. 2011;35:1141–1153. doi: 10.1038/ijo.2010.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaiser S, Hackanson B, Follo M, et al. BM cells giving rise to MSC in culture have a heterogeneous CD34 and CD45 phenotype. Cytotherapy. 2007;9:439–450. doi: 10.1080/14653240701358445. [DOI] [PubMed] [Google Scholar]
- 62.Suga H, Matsumoto D, Eto H, et al. Functional implications of CD34 expression in human adipose-derived stem/progenitor cells. Stem Cells Dev. 2009;18:1201–1210. doi: 10.1089/scd.2009.0003. [DOI] [PubMed] [Google Scholar]
- 63.Grayson WL, Zhao F, Izadpanah R, et al. Effects of hypoxia on human mesenchymal stem cell expansion and plasticity in 3D constructs. J Cell Physiol. 2006;207:331–339. doi: 10.1002/jcp.20571. [DOI] [PubMed] [Google Scholar]
- 64.Fehrer C, Brunauer R, Laschober G, et al. Reduced oxygen tension attenuates differentiation capacity of human mesenchymal stem cells and prolongs their lifespan. Aging Cell. 2007;6:745–757. doi: 10.1111/j.1474-9726.2007.00336.x. [DOI] [PubMed] [Google Scholar]
- 65.van Harmelen V, Skurk T, Röhrig K, et al. Effect of BMI and age on adipose tissue cellularity and differentiation capacity in women. Int J Obes Relat Metab Disord. 2003;27:889–895. doi: 10.1038/sj.ijo.0802314. [DOI] [PubMed] [Google Scholar]
- 66.Lee J-H, Kemp DM. Human adipose-derived stem cells display myogenic potential and perturbed function in hypoxic conditions. Biochem Biophys Res Commun. 2006;341:882–888. doi: 10.1016/j.bbrc.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 67.Muraglia A, Cancedda R, Quarto R. Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J Cell Sci. 2000;113:1161–1166. doi: 10.1242/jcs.113.7.1161. [DOI] [PubMed] [Google Scholar]
- 68.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 69.Russell KC, Lacey MR, Gilliam JK, et al. Clonal analysis of the proliferation potential of human bone marrow mesenchymal stem cells as a function of potency. Biotechnol Bioeng. 2011;108:2716–2726. doi: 10.1002/bit.23193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Russell KC, Phinney DG, Lacey MR, et al. In vitro high-capacity assay to quantify the clonal heterogeneity in trilineage potential of mesenchymal stem cells reveals a complex hierarchy of lineage commitment. Stem Cells. 2010;28:788–798. doi: 10.1002/stem.312. [DOI] [PubMed] [Google Scholar]
- 71.Schipani E, Maes C, Carmeliet G, et al. Regulation of osteogenesis-angiogenesis coupling by HIFs and VEGF. J Bone Miner Res. 2009;24:1347–1353. doi: 10.1359/jbmr.090602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arutyunyan IV, Rzhaninova AA, Volkov AV, et al. Effect of dexamethasone on differentiation of multipotent stromal cells from human adipose tissue. Bull Exp Biol Med. 2009;147:503–508. doi: 10.1007/s10517-009-0548-5. [DOI] [PubMed] [Google Scholar]
- 73.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kroeze RJ, Knippenberg M, Helder MN. Osteogenic differentiation strategies for adipose-derived mesenchymal stem cells. Methods Mol Biol. 2011;702:233–248. doi: 10.1007/978-1-61737-960-4_17. [DOI] [PubMed] [Google Scholar]
- 75.Zhang S, Chan M, Aubin JE. Pleiotropic effects of the steroid hormone 1,25-dihydroxyvitamin D3 on the recruitment of mesenchymal lineage progenitors in fetal rat calvaria cell populations. J Mol Endocrinol. 2006;36:425–433. doi: 10.1677/jme.1.01900. [DOI] [PubMed] [Google Scholar]
- 76.Bellows CG, Wang YH, Heersche JN, et al. 1,25-dihydroxyvitamin D3 stimulates adipocyte differentiation in cultures of fetal rat calvaria cells: Comparison with the effects of dexamethasone. Endocrinology. 1994;134:2221–2229. doi: 10.1210/endo.134.5.8156925. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.