Abstract
Delivery of therapeutic compounds to the inner ear via absorption through the round window membrane (RWM) has advantages over direct intracochlear infusions; specifically, minimizing impact upon functional hearing measures. However, previous reports show that significant basal-to-apical concentration gradients occur, with the potential to impact treatment efficacy. Here we present a new approach to inner ear drug delivery with induced advection aiding distribution of compounds throughout the inner ear in the murine cochlea. Polyimide microtubing was placed near the RWM niche through a bullaostomy into the middle ear cavity allowing directed delivery of compounds to the RWM. We hypothesized that a posterior semicircular canalostomy would induce apical flow from the patent cochlear aqueduct to the canalostomy due to influx of cerebral spinal fluid. To test this hypothesis, young adult CBA/CaJ mice were divided into two groups: bullaostomy approach only (BA) and bullaostomy + canalostomy (B+C). Cochlear function was evaluated by distortion product otoacoustic emission (DPOAE) and auditory brainstem response (ABR) thresholds during and after middle ear infusion of salicylate in artificial perilymph (AP), applied near the RWM. The mice recovered for 1 week, and were re-tested. The results demonstrate there was no significant impact on auditory function utilizing the RWM surgical procedure with or without the canalostomy, and DPOAE thresholds were elevated reversibly during the salicylate infusion. Comparing the threshold shifts for both methods, the B+C approach had more of a physiological effect than the BA approach, including at lower frequencies representing more apical cochlear locations. Unlike mouse cochleostomies, there was no deleterious auditory functional impact after 1 week recovery from surgery. The B+C approach had more drug efficacy at lower frequencies, underscoring potential benefits for more precise control of delivery of inner ear therapeutic compounds.
Keywords: mouse, drug delivery, infusion, concentration gradient, cochlea, inner ear
Introduction
Auditory dysfunction and permanent hearing loss impact more than 10% of the population, over 30 million people in the United States. Currently there are no medical treatments to address the biological basis of permanent hearing loss, with prosthetics such as hearing aids and cochlear implants being the primary treatment options for impacted individuals. Extensive efforts are underway to develop biomedical interventions, including new medications or drug regimens to prevent or reverse permanent hearing loss. One approach is to understand the molecular pathways involved in normal organ of Corti development, including differential cell signaling and lineage fates, and then mimic or re-capitulate these developmental sequences to regenerate the organ of Corti in deaf patients, to restore hearing [1–3]. However, when these therapeutic interventions become available, a challenge still remains of how to deliver these treatments to the inner ear locally, which will avoid systemic side effects of the new combinations of drugs, gene therapy and/or stem cells, and open up many more therapeutic options for regenerating hair cells and the organ of Corti. However, drug delivery to the inner ear is challenging due to its small size and anatomical location in the temporal bone.
Intra-cochlear delivery of drugs or genes has been successfully accomplished in animal models by injection through the round window membrane [4], injection into the endolymphatic space via scala media [5–6] and endolymphatic sac [7], and injection or infusion into the perilymphatic space via the semicircular canals [8], scala vestibuli [9–10], and most commonly the scala tympani [11–13]. Drug delivery is generally accomplished via a syringe pump [14] or osmotic pump [15] for continuous infusion, or a reciprocating pump for zero net volume delivery [16]. While approaches that directly enter the perilymphatic space can preserve auditory function acutely, reports of successful recovery and chronic retention of hearing are rare, particularly in small rodents. This impacts both the utility of the approaches for hearing loss and deafness therapy research, and the potential for clinical translation.
One approach that has been used clinically is transtympanic delivery with absorption through the round window membrane (RWM). Gentamicin has been successfully delivered with this technique for the management of Meniere’s disease with intractable vertigo [17–18]. For cases of idiopathic sudden sensorineural hearing loss where use of systemic steroids is contraindicated due to concomitant medical conditions or ineffectiveness, transtympanic delivery of glucocorticoids has proven an effective treatment [19]. Tinnitus is also treated clinically via this technique with gentamicin, dexamethasone, and lidocaine [20]. This approach has also been utilized in animal model systems, with significant apical-basal concentration gradients measured in scala tympani (e.g., [21]) due to diffusion-limited transport, since the fluids of the inner ear are relatively stagnant without intrinsic flow or movement [22]. Frequency dependent physiological responses to agents directly infused into the basal turn of scala tympani suggest similar gradients exist for direct infusion approaches (e.g., [23]). This gradient will impact treatment efficacy for pharmaceuticals with a narrow therapeutic window, or molecular therapies involving protection or regeneration in apical cochlear structures. Advanced therapies which address the biological basis of auditory and vestibular dysfunction will require understanding and control of concentration gradients associated with different inner ear drug delivery protocols. Therapy developments will involve utilization of mice as animal models, due to the extensive research already performed on mouse sensory and nervous systems, including the peripheral and central auditory systems (e.g., [24–25]), and the extensive options for mouse genetics.
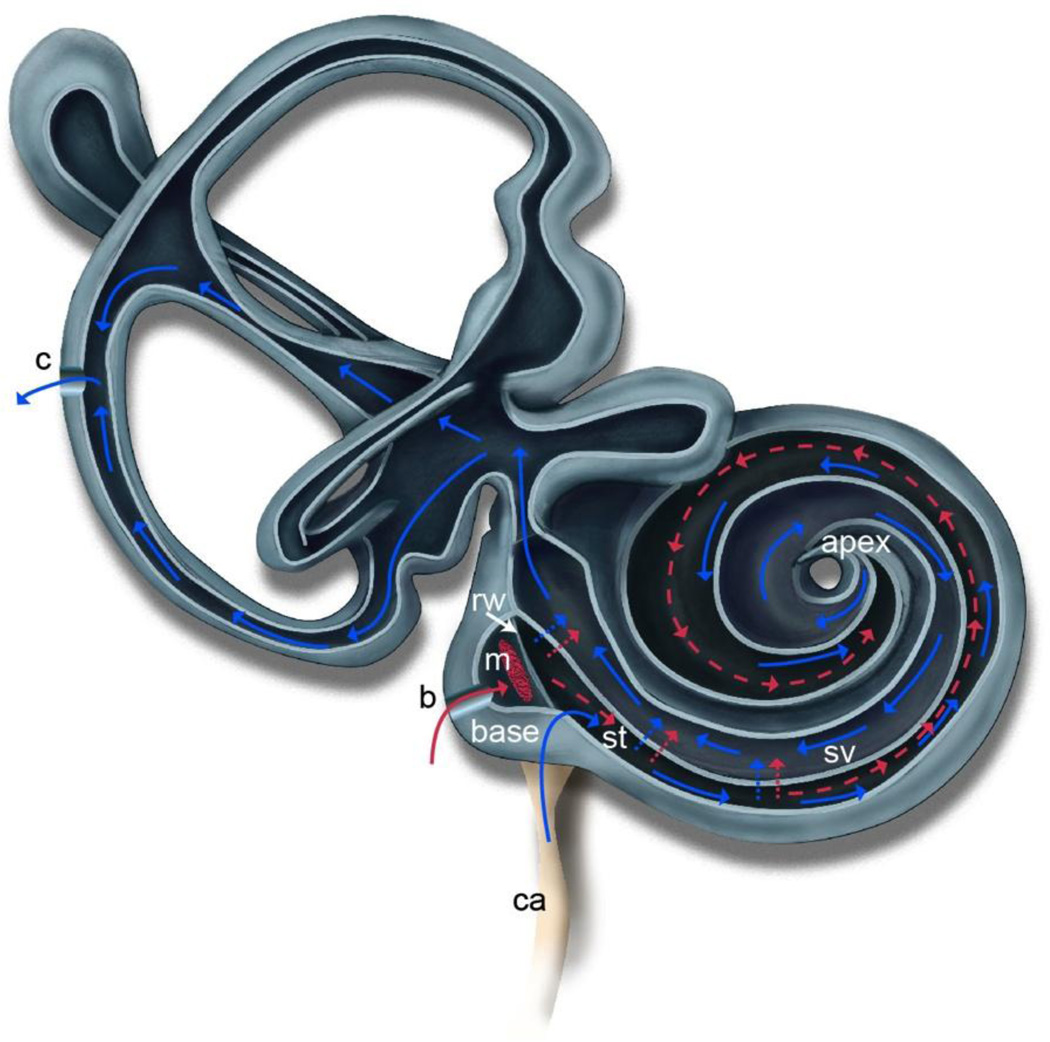
The present report provides a potential path for control of intracochlear concentration gradients in the mouse model system. We previously discovered that perfusion from a basal turn, scala tympani cochleostomy to a fluidic exit created by a posterior semicircular canal canalostomy reduced concentration gradients in the mouse cochlea [14]. While this approach had no impact on acute hearing function, recovery surgeries evidenced loss of hearing as measured by both distortion product otoacoustic emissions (DPOAEs) and auditory brainstem responses (ABRs) one week post-surgery. Here the clinically successful RWM delivery approach is coupled with the concept of directed perfusion to explore opportunities for control of concentration gradients within the cochlea without long-term impact to cochlear function. The conceptual approach and hypothetical flow paths for distribution of compounds presented at the RWM niche is depicted in Figure 1. Creation of a fluidic exit in the posterior semicircular canal induces advective flow via influx of cerebral spinal fluid (CSF) from the cochlear aqueduct, driven by intracranial pressure. Ionic tracer monitoring experiments where the otic capsule was perforated at the apex in guinea pigs verify flow of perilymph in scala tympani due to influx of CSF from the patent cochlear aqueduct [22]. It is this induced advective flow that is leveraged in the present work, with the outlet shifted to the vestibular system where detrimental impact to cochlear function is mitigated.
Figure 1.
Illustration of the mouse inner ear depicting the hypothesized advection flow induced by creation of a fluidic exit via canalostomy (c) in the posterior semicircular canal. Drug is delivered to the round window (rw) via polyimide microtubing inserted through a bullaostomy (b) into the middle ear (m) and positioned at the RWM niche. The drug is absorbed through the RWM into scala tympani (st). Diffusion is shown in red as dotted lines. Induced advection flow due to CSF influx from the cochlear aqueduct (ca) is shown as solid lines in blue. This flow carries the drug throughout the cochlea via a flow path through st, to the helicotrema at the apex, through scala vestibuli (sv) and into the vestibular system to the canalostomy (c). Adapted from [14].
Salicylate has been shown to act as a competitive antagonist at the anion-binding site of prestin, resulting in an inhibition of outer hair cell electromotility [26]. This inhibition decreases the magnitude of otoacoustic emissions [27], and can be measured as a reversible elevation of DPOAE thresholds. A physiological place-frequency map of the mouse [28], coupled with DPOAE threshold shifts enables estimation of the spatio-temporal concentration of salicylate delivered to the inner ear. In the present investigation, infusion of 50 mM sodium salicylate in artificial perilymph (AP) into the RWM niche of the middle ear was used to evaluate a new concept for distributing drugs throughout the cochlea without directly penetrating the cochlear scalae. A ventral surgical approach facilitated consistent placement of infusion tubing at the RWM niche. The approach leveraged induced advection due to influx of CSF from the patent cochlear aqueduct to a canalostomy in the posterior semicircular canal. Cochlear function was evaluated via DPOAE and ABR thresholds, allowing an indirect physiological estimation of drug distribution within the cochlea, and confirmation of intact cochlear function one week post-surgery.
Material and Methods
A preliminary dose-response study was conducted prior to initiation of the current study to identify an appropriate concentration and flow rate for this RWM niche delivery approach. Eight salicylate concentrations from 1.6 mg/ml to 100 mg/ml were examined at flow rates from 16 nl/min to 100 nl/min with DPOAE responses measured during delivery. A concentration of 8 mg/ml (50 mM) at 50 nl/min flow rate with 1000 nl total delivery yielded measurable responses within the acute timeframe of the planned experiments. This concentration is similar to that employed with dexamethasone delivery to the RWM in guinea pigs (10 mg/ml) [21], with both concentration and flow rate higher than those used in our past studies with direct intracochlear infusion into scala tympani (1.6 mg/ml (10mM), 16nl/min) [14] as expected with diffusion through the RWM.
Drug infusion system and solutions
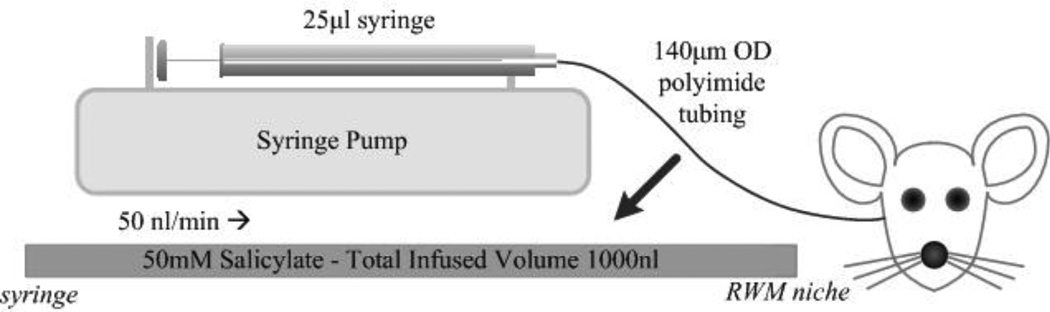
Sodium salicylate solution was delivered to the RWM niche via a 30–40 cm length of US Pharmacopoeia Class VI polyimide tubing (044-I; ID 110 µm; OD, 139 µm; Microlumen, Tampa, FL). The infusion tubing was connected to a 25 µl Hamilton syringe (1702 RNR 22S/2”) using a PEEK nanotight fitting (Upchurch Scientific, Oak Harbor, WA). The syringe was mounted in a syringe pump (UMP2, World Precision Instruments, Sarasota, FL) allowing precise control of infusion rates. A schematic illustration of the infusion setup is shown in Figure 2. The syringe was carefully filled with the salicylate solution, with all trapped air removed via repeated rapid aspiration and ejection. The infusion tubing was then connected and filled via syringe ejection.
Figure 2.
Illustration depicting the experimental infusion setup. Polyimide microtubing was preloaded with 50 mM salicylate solution. The setup was attached to a syringe pump to precisely control infusion rate. The infusion tubing was inserted through the bullaostomy and positioned at the RWM niche. Adapted from [14].
The fluidic system elements were cleaned following each use by forced flow of sterile double-distilled water through the syringe, tubing, and fittings. The system was disassembled and components placed in autoclave bags for sterilization at 121 °C for 1hr (Tuttnauer 2540 MLV autoclave) prior to use in surgery.
The salicylate solution had a composition (in mM) of: NaCl, 120; KCl, 3.5; CaCl2, 1.5; glucose, 5.5; and HEPES buffer, 20; sodium salicylate, 50. The pH was adjusted to 7.5 with NaOH. All solutions were mixed on the day of the experiments using sterile double-distilled water.
Animals and surgical procedures
A total of 13 young adult (age 2–4 months) CBA/CaJ mice, bred and raised in-house, were used for this study, with one animal expiring after anesthesia delivery. The remaining 12 animals were divided into two groups: bullaostomy (BA) (N= 6) and bullaostomy+canalostomy (B+C) (N = 6). All animals were infused with 50 mM salicylate, at a flow rate of 50 nl/min, for a total volume of 1000 nl (20 min). The mice were allowed to recover for 1 week to retest their hearing. All animal experiments were approved by the University of Rochester Committee on Animal Resources, and were performed using accepted NIH and veterinary standards.
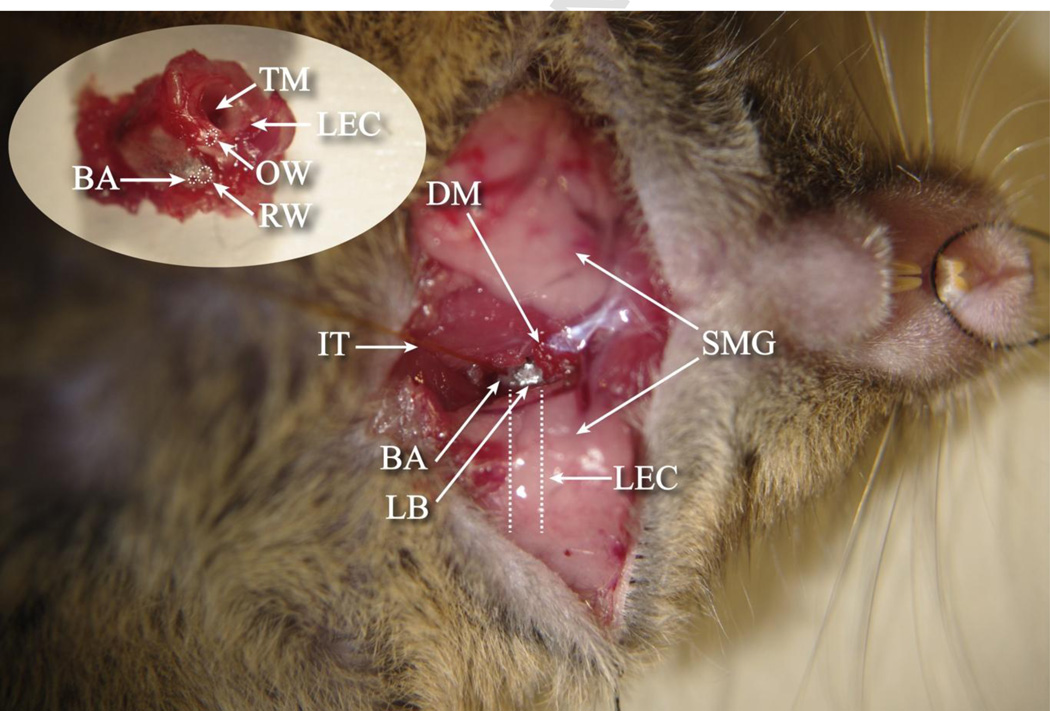
For the bullaostomy only (BA approach), animals were deeply anesthetized with a mixture of ketamine/xylazine (120 and 10 mg/kg body weight, respectively, intraperitoneal injection [IP]), and the left ventral surface of the neck was shaved and cleaned. For animals also receiving the canalostomy (B+C approach), the left post-auricular region was shaved and cleaned. The animal was positioned on a heated operative plane on its back under aseptic conditions. Surgery was performed on the left (ipsilateral) ear, following procedures outlined by Borkholder and colleagues [14]. The tympanic bulla was exposed by a ventral approach as shown in Figure 3. Under an operating microscope, an incision was made longitudinally along the ventral surface of the neck, extending from the angle of the mandible to the level of the clavicle. The submandibular gland was retracted laterally to expose the digastric muscle which was cut with an electrocautery to expose the bony tympanic bulla and the stapedial artery. The surrounding tissue was removed to expose the inferior-medial aspect of the bulla which was carefully cleaned and dried. A bullaostomy was drilled by hand at a location approximately 1.5 mm laterally below the stapedial artery using a 175 µm diameter carbide micro drill bit modified to include insertion stops [14]. Sequentially longer insertion-depth 175 µm bits were used (300 and 350 µm), with bulla entry determined by a subtle change in mechanical resistance and visualization of the middle ear cavity.
Figure 3.
Ventral surgical approach exposing the tympanic bulla with middle ear access for blind alignment to the RWM niche. (SMG) submandibular gland, (LEC) left ear canal, (DM) digastric muscle, (IT) infusion tubing, (BA) bullaostomy, (LB) left bulla. Inset: Explanted left cochlea highlighting the location of the bullaostomy relative to the round window. (TM) tympanic membrane, (OW) oval window, (RW) round window.
Using a micromanipulator (MM3–3, World Precision Instruments, Sarasota, FL), a fine metal probe, and the polyimide infusion tubing attached with adhesive (3M Repositionable 75 spray adhesive, St. Paul, MN) to the probe, the infusion tubing assembly was inserted into the bullaostomy to a depth of approximately 300–500 µm, near the opening of the round window. Medical grade adhesive (Loctite 4206, Rocky Hill, CT) was used to temporarily secure the infusion tubing to the bulla opening, with subsequent application of dental cement (3M ESPE Duralon), providing a more permanent and robust bond, sealing the cannula to the bullaostomy site. The surgery site was loosely sutured closed to provide strain relief for the infusion tubing.
For animals also receiving the canalostomy (B+C approach), following the bullaostomy procedure described above, the animal was positioned on a heated operative plane on their stomach under aseptic conditions. Under an operating microscope, an incision was made behind the left pinna and the muscles separated to expose the posterior semicircular canal. A hole was drilled in the posterior semicircular canal with a 100 µm diameter drill bit modified with an insertion stop. Sequentially longer bits were used (102 and 127 µm), with canal entry determined by a subtle change in mechanical resistance. The canalostomy was covered with muscle for the duration of the experiment.
During infusions, mice remained immobilized by anesthesia as described above, with supplementary doses (1/3 of the initial dose) administered as needed to maintain the proper levels of general anesthesia. Parameters such as foot or tail pinch, palpebral reflex and respiratory rate were monitored to indicate the need for supplemental doses.
After 20 min of infusion, the pump was stopped with anesthesia maintained for 40 more min to capture additional auditory assessment data. After that, the micro-infusion tubing was gently removed, and the bullaostomy was sealed with bone wax and the area of the incision was closed with sutures. A 4% lidocaine solution was applied to the wound margins, and a 1 mg/kg dose of banamine nonsteroidal anti-inflammatory was injected intramuscularly for each animal. Mice were allowed to recover from anesthesia on a heating pad under the observation of the surgeon or surgical assistant. The recovering mice were checked frequently to make sure they were behaving, eating and drinking normally, and not scratching or rubbing the wound margins, or exhibiting other signs of mild to moderate pain or distress, including partially closed eyelids, increased vibrissal movement, biting or hunched posture. If signs of infection were observed, animals were closely monitored and, if necessary, treated with an Polyotic antibiotic in the drinking water at a concentration of 1.8 gm per 8 oz water until the experiments were complete and the animal was euthanized. At post-surgery one week, the hearing of the mice was measured again.
Auditory Function Assessment
Mice were anesthetized as described above with supplementary doses administered as needed. Prior to recordings, the ear canals and eardrums were inspected for signs of obstruction or infection, and only those animals with clear outer and middle ears were used. While under anesthesia, body temperature was maintained at 38°C with a heating pad. Recording sessions were completed in a soundproof acoustic chamber (IAC) with measurements performed on the left ear. Auditory function was assessed via automated DPOAE threshold measurements at F2 frequencies 8.9, 13.5, 17.9, 24.6, 35.8, and 51.4 kHz. Measurements were performed prior to surgery to use as a baseline for subsequent DPOAE threshold shifts. Additional measurements were made immediately following surgery, at the initiation of infusion, at 10 and 20 minutes during infusion, and at 20 and 40 minutes following termination of infusion.
Tucker Davis hardware (TDT; Alachua, FL) was controlled via ActiveX from a custom Matlab r13 (Mathworks; Natick, MA) graphical user interface. Sound stimuli were generated and signals acquired using Tucker Davis RP2.1 processors running at a sample rate of 200 kHz. All signals were played through two electrostatic speakers (TDT EC1) connected by 4 cm tubes to a probe containing an ER10B+ microphone (Etymotic; Elk Grove Village, IL); the entire speaker and probe assembly were mounted in an adjustable vibration-isolating frame on a micromanipulator arm. All recorded signals were loaded into Matlab r13 for analysis. Waveforms from each individual presentation were windowed using a Hamming window and high-resolution 390625-point fast Fourier transforms (FFTs) (2× sample rate) were calculated. The resulting FFTs had a bin size of 0.5 Hz allowing for accurate measurement of signal level as a function of frequency. Frequency-domain averaging was used to minimize artifacts; FFTs for multiple repetitions of the same stimulus were averaged together before subsequent analysis. The probe microphone was calibrated relative to a ¼” B&K microphone (Type 4938, Bruel & Kjaer; Naerum, Denmark) using a 0.1 cc coupler (simulating the mouse ear canal).
DPOAE amplitudes were measured in the following manner: two primaries (F1 and F2) were generated at 65 and 50 dB SPL, respectively. The ratio of the two frequencies was 1.25. Waveforms of the output of the ER10B+ probe microphone were captured on a TDT RP2.1. FFTs for each presentation were averaged together and the signal level at five frequencies was sampled: F1, F2, DP (2F1-F2), and two noise bins above and below the distortion product (DP) frequency. Following FFT sampling, dBV was converted to SPL based on the ER10B+ microphone calibration.
DPOAE Thresholds were defined as the F1 level required to produce a DP of 0 dB SPL (+/− 1 dB). We developed an automatic threshold search algorithm implemented in Matlab r13 using TDT hardware and the Etymotic ER10B+ probe microphone. The measurement of DP threshold was interleaved with DP amplitude measures described above and began with two primaries at 65 and 50 dB SPL. Based on the distance of the DP from its target level of 0 dB SPL (distance henceforth: DP error), F1 and F2 level on the subsequent trial was incremented (or decremented) by 0.6 of this distance; e.g., if the DP was at 10 dB SPL, F1 and F2 were decremented by 6 dB. F2 level was always F1–15 dB. The 0.6 “approach factor” was determined empirically to be an optimal rate of approach combining rapid acquisition of threshold and minimal oscillation around the target. Due to extremely steep DP I/O functions around 0 dB and the resulting overshoot of DP amplitude, occasionally on successive trials the DP amplitude oscillated around 0 dB. In each case of oscillation, defined as three trials in which the sign of the DP error changes each trial, the approach factor was automatically made smaller by a factor of 1.5. This iterative procedure allowed rapid convergence on DP threshold while preventing overshoot. Once the DP was measured to be within 1 dB of 0 dB SPL, the identical F1 level was presented again for confirmation. Identification of thresholds requires two successive trials of F1 F2 levels that evoked a 0 dB SPL DP amplitude.
ABR thresholds were measured before surgery and one week post-surgery following recovery as a secondary assessment of surgical approach impact to cochlear function, utilizing the same anesthetic as the DPOAE procedure. The ABR testing procedures were similar to our previous reports [29–32], and are summarized here. ABRs were recorded with subcutaneous platinum needle electrodes placed at the vertex (non-inverting input), right mastoid prominence (inverted input) and tail (indifferent site). Calibrated tone pips, 5 ms duration, 0.5 ms rise–fall time (phase alternating 90o) were utilized at 8, 12, 16, 22, 32, and 40kHz. Electroencephalographic (EEG) activity was differentially amplified (50 or 100 k; Grass [Quincy, MA] model P511 EEG amplifier), then input to an A/D converter (Tucker-Davis Technologies [TDT, Alachua, FL] AD1), and digitized at 50 kHz. Each averaged response was based on 300–500 stimulus repetitions recorded over 10-ms epochs. Contamination by muscle or cardiac activity was prevented by rejecting data epochs in which the single-trace EEG contained peak-to-peak amplitudes exceeding 50 µV. The threshold was defined as the first level that did not evoke a response to a measured frequency, i.e., no difference from the baseline. Normal body temperature was maintained at 38° C with a servo heating pad. The ABR was recorded in a small sound attenuating chamber (IAC).
Statistical Analyses
DPOAE and ABR threshold shift data were collected from each experiment. Two-way analysis of variance (ANOVA) with repeated measures was used to compare threshold shifts achieved with each surgical approach (Prism, GraphPad, La Jolla, CA). Responses are graphically shown as a mean ± SEM for all animals receiving each surgical approach. Unless otherwise noted, the p<0.05 level was used for statistical significance.
Results and Discussion
Intracochlear spread of agents delivered to the RWM is traditionally dominated by diffusion mechanisms. The present study aimed to significantly impact the longitudinal component of this spread through an induced longitudinal advective flow. The induced advection flow originates at the cochlear aqueduct which in the mouse is adjacent to the RWM [33]. With base to apex flow, agents absorbed through the RWM are carried to more apical regions of the cochlea.
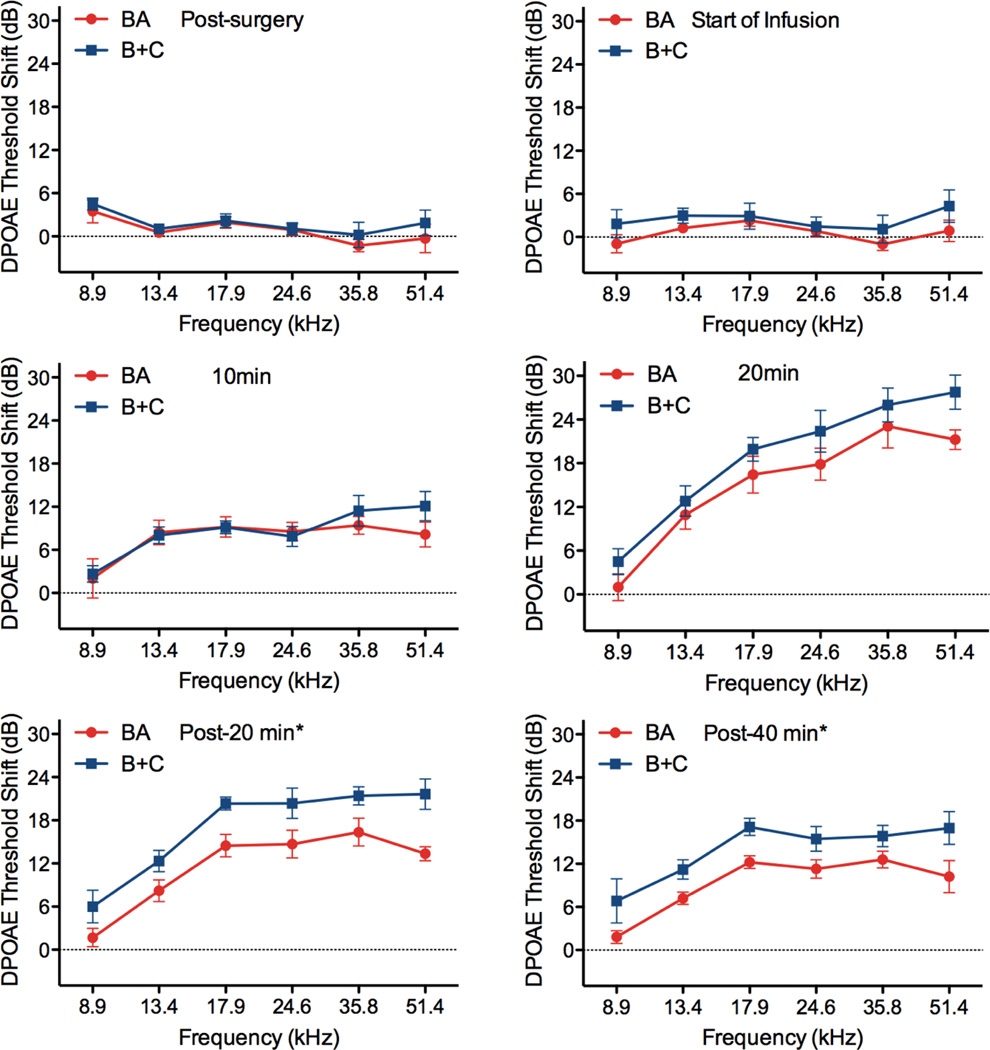
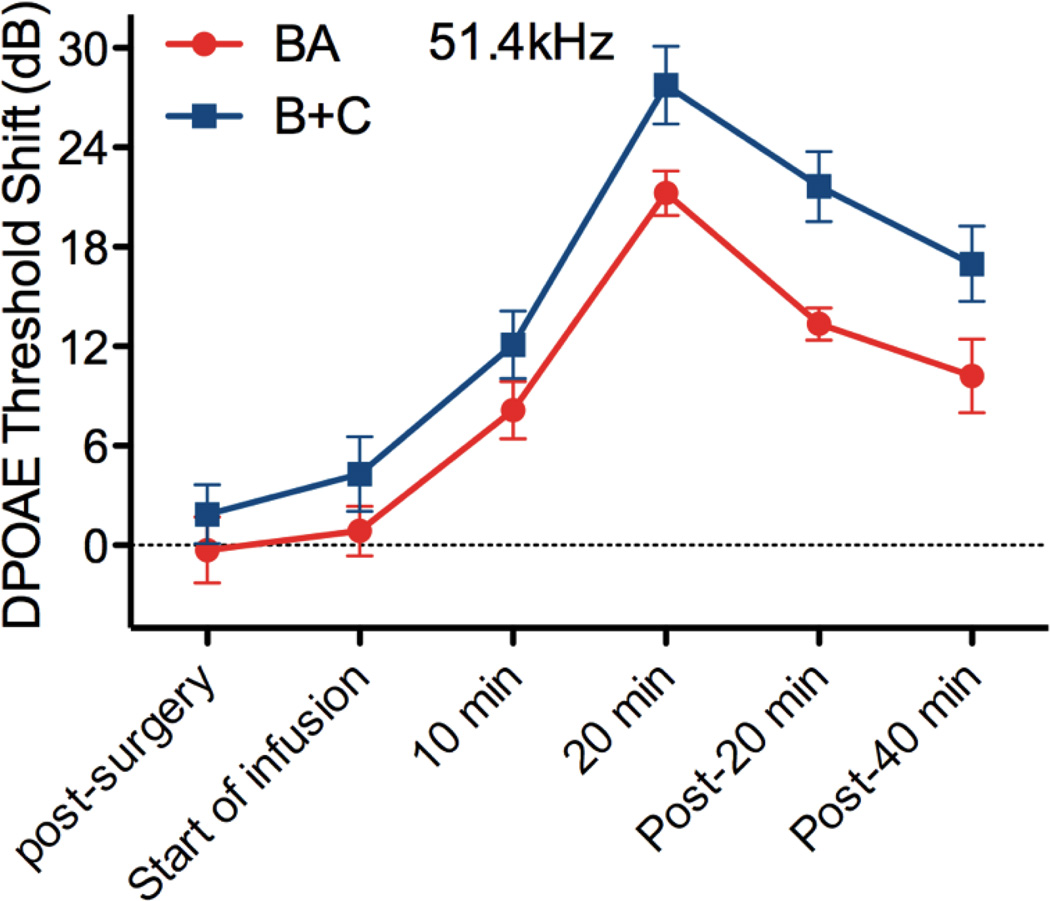
DPOAE threshold shifts for both surgical approaches are shown in Figure 4. The surgical approach and initiation of infusion had no impact on cochlear function as evidenced by stable DPOAE thresholds as compared to pre-surgery levels. As 50 mM salicylate was delivered to the RWM niche, the compound diffuses through the RWM and ultimately impacts outer hair cell function and the DPOAE thresholds. While both surgical approaches demonstrate significant shifts from baseline, with more pronounced shifts at higher, basal frequencies, the B+C approach provides greater threshold shifts at all frequencies. This is true even at the lowest, most apical frequencies where the induced advection flow of CSF from the cochlear aqueduct to the fluidic exit in the posterior semicircular canal presumably carries salicylate from base to apex in scala tympani, through the helicotrema, and from apex to base in scala vestibuli. In contrast the BA approach relies entirely on diffusion mechanisms for distribution of the salicylate.
Figure 4.
DPOAE threshold shifts versus F2 frequency for the BA (red traces) and B+C (blue traces) approaches during delivery of 50 mM salicylate to the RWM niche. Surgery had an insignificant impact on cochlear function. Clear threshold shifts are observed during delivery for both approaches, demonstrating transfer of the salicylate across the intact RWM. Asterisks indicate statistically significant main effects between methods. Data are plotted as mean ± SEM (n=6 animals for each approach).
At the last two time points of measurement, 20 minutes and 40 minutes following termination of flow of salicylate to the RWM niche, a statistically significant difference between methods was observed (p<0.05). This suggests that a longer infusion time would likely result in not only greater threshold shifts, but also a greater difference between methods. One limitation of the current technique is the requirement to maintain the animals under anesthesia during the duration of the experiment due to the syringe pump and infusion tubing. Advancements in implantable pump technologies suitable for use in mice [34–36] would enable longer-term experiments to fully explore the potential of this induced advection approach to enhance distribution of agents throughout the murine cochlea. They would enable determination of the limits of distribution uniformity achievable, and optimization of delivery paradigms to achieve uniformity. Additionally this chronic delivery capability would eliminate any potential impact of anesthesia on auditory measures as has been reported for ABR [37] and DPOAE [38] thresholds. Ultimately, these technologies will enable the development of advanced therapies addressing the biological basis of auditory function which involve repeated treatments over extended periods of time; therapies that will require chronic delivery of curative agents.
Analysis of the 51.4 kHz DPOAE threshold shifts after infusion at the RWM niche is terminated (Figure 5) reveals a decline in DPOAE threshold shifts of approximately 0.3 dB/min, correlating to a rapid decline in salicylate concentration in the basal turn. This suggests that modulation of the RWM niche source, in combination with the induced advective flow, could potentially enable a lower, but more stable concentration from base to apex in the cochlea. This has significant implications for hearing or deafness therapies where dose-response window is narrow, or toxicity is a concern.
Figure 5.
DPOAE threshold shifts for the most basal F2 frequency over time. After infusion is stopped, there is a reduction of approximately 0.3dB per minute for the B+C approach suggesting a decline in basal salicylate concentration. This rapid decline may enable concentration control via flow control at the RWM niche. Data are plotted as mean ± SEM (n=6 animals for each approach).
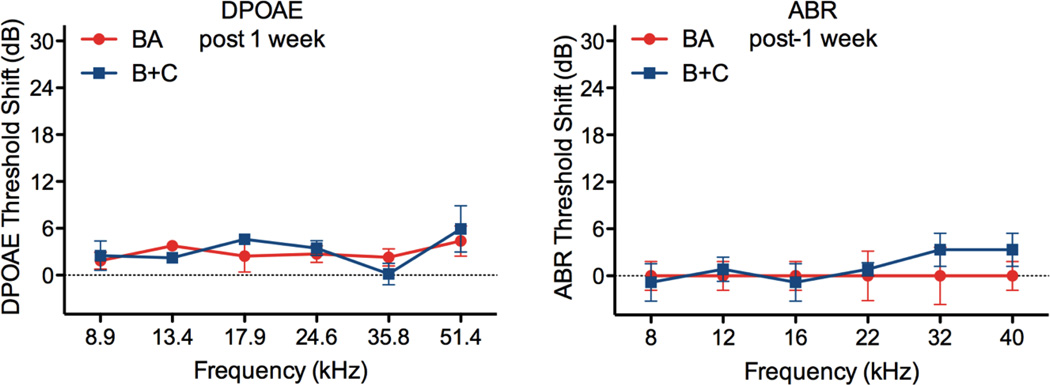
Figure 6 demonstrates the approaches used in the current report for delivering a pharmacological agent to the cochlea have no long-term impact to cochlear function. Threshold shifts for both DPOAEs and ABRs one week post-surgery are insignificant, compared to pre-surgery baselines. Other approaches with direct intracochlear access via a basal turn cochleostomy have been shown to have no acute impact to cochlear function [14, 23], but recovery surgeries result in hearing deficits. This limits their applicability to therapy development where the curative agent delivery approach should improve long-term hearing and not negatively impact cochlear function. Delivery of agents to the RWM niche in the middle ear eliminates the need to directly access the cochlea and avoids long term hearing deficits previously seen in some rodents, including mice. The creation of a canalostomy in the vestibular system retains the benefits of the RWM delivery, while facilitating transport of agents absorbed through the RWM throughout the cochlea. This induced advection approach could enable more rapid therapy development targeting all regions of the cochlea, providing improved solute distribution without chronic impact to functional auditory measures.
Figure 6.
In stark contrast to cochleostomy procedures in mice, DPOAE (left) and ABR (right) threshold shifts are insignificant as compared to pre-surgery baselines after one week of recovery. There is no difference between the BA (red) and B+C (blue) responses, and shifts from baseline are not significant, suggesting no impact to cochlear function with either approach. Data shown as mean ± SEM (n=6 animals for each approach).
Future work will couple micro-CT imaging of inner ear drug delivery [36] with quantitative modeling of this induced advection approach to provide predictive spatio-temporal intracochlear concentrations in each scala. This advanced capability will enable more rapid design of pharmacological delivery profiles for controlled target concentrations within the cochlea that will be essential for curative therapy development using mouse model systems.
Conclusions
A novel approach to delivering agents to the cochlear scalae in the mouse model system has been demonstrated providing evidence of enhanced distribution through the cochlea. The middle ear bullaostomy and vestibular system canalostomy had no deleterious impact to auditory functional measures one week post-surgery. The proposed hypothesis of induced advection from the cochlear aqueduct to the canalostomy is consistent with the measured DPOAE responses. The rapid shifts and recovery in response suggest potential for tuned control of spatio-temporal solute concentrations within the cochlea via modulation of the middle ear source, coupled with the induced advective currents. Advanced implantable microsystems to provide this level of control, and to enable longer term experiments without continuous anesthesia will be required to fully explore the potential of this new inner ear drug delivery paradigm.
Acknowledgements
This work supported by NIH Grants from the National Institute on Deafness and other Communication Disorders (K25-DC008291 and P30 DC05409) and the National Institute on Aging (P01 AG009524).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Xiaoxia Zhu, Email: xiaoxiazhu@usf.edu.
Robert D. Frisina, Email: rfrisina@usf.edu.
References
- 1.Driver EC, Kelley MW. Specification of cell fate in the mammalian cochlea. Birth Defects Res C Embryo Today. 2009;87(3):212–221. doi: 10.1002/bdrc.20154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Driver EC, Kelley MW. Transfection of mouse cochlear explants by electroporation. Curr Protoc Neurosci. 2010;Chapter 4(Unit 4.34):1–10. doi: 10.1002/0471142301.ns0434s51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Puligilla C, Dabdoub A, Brenowitz SD, Kelley MW. Sox2 induces neuronal formation in the developing mammalian cochlea. J Neurosci. 2010;30(2):714–722. doi: 10.1523/JNEUROSCI.3852-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Staecker H, Li D, O'Malley BW, et al. Gene expression in the mammalian cochlea: a study of multiple vector systems. Acta Otolaryngol. 2001;121:157–163. doi: 10.1080/000164801300043307. [DOI] [PubMed] [Google Scholar]
- 5.Ishimoto S-i, Kawamoto K, Kanzaki S, et al. Gene transfer into supporting cells of the organ of Corti. Hear Res. 2002;173:187–197. doi: 10.1016/s0378-5955(02)00579-8. [DOI] [PubMed] [Google Scholar]
- 6.Izumikawa M, Minoda R, Kawamoto K, et al. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. In: Nature Publishing Group, editor. Nature Medicine. 2005. pp. 271–276. [DOI] [PubMed] [Google Scholar]
- 7.Yamasoba T, Yagi M, Roessler BJ, et al. Inner ear transgene expression after adenoviral vector inoculation in the endolymphatic sac. Hum Gene Ther. 1999;10:769–774. doi: 10.1089/10430349950018526. [DOI] [PubMed] [Google Scholar]
- 8.Kawamoto K, Oh SH, Kanzaki S, et al. The functional and structural outcome of inner ear gene transfer via the vestibular and cochlear fluids in mice. Mol Ther. 2001;4:575–585. doi: 10.1006/mthe.2001.0490. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki M, Yagi M, Brown JN, et al. Effect of transgenic GDNF expression on gentamicin-induced cochlear and vestibular toxicity. Gene Ther. 2000;7:1046–1054. doi: 10.1038/sj.gt.3301180. [DOI] [PubMed] [Google Scholar]
- 10.Bowers WJ, Chen X, Guo H, et al. Neurotrophin-3 transduction attenuates cisplatin spiral ganglion neuron ototoxicity in the cochlea. Mol Ther. 2002;6:12–18. doi: 10.1006/mthe.2002.0627. [DOI] [PubMed] [Google Scholar]
- 11.Kingma GG, Miller JM, Myers MW. Chronic drug infusion into the scala tympani of the guinea pig cochlea. Journal of Neuroscience Methods. 1992;45:127–134. doi: 10.1016/0165-0270(92)90050-n. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Van De Water TR, Bonny C, et al. A peptide inhibitor of c-Jun N-terminal kinase protects against both aminoglycoside and acoustic trauma-induced auditory hair cell death and hearing loss. J Neurosci. 2003;23:8596–8607. doi: 10.1523/JNEUROSCI.23-24-08596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Z, Mikulec AA, McKenna MJ, et al. A method for intracochlear drug delivery in the mouse. J Neurosci Methods 2006. 2006;150:67–73. doi: 10.1016/j.jneumeth.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 14.Borkholder DA, Zhu XX, Hyatt BT, Archilla AS, Livingston WJ, Frisina RD. Murine intracochlear drug delivery: reducing concentrations gradients within the cochlea. Hear Res. 2010;268:2–11. doi: 10.1016/j.heares.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eshraghi AA, Adil E, He J, et al. Local dexamethasone therapy conserves hearing in an animal model of electrode insertion trauma-induced hearing loss. Otol Neurotol. 2007;28:842–849. doi: 10.1097/mao.0b013e31805778fc. [DOI] [PubMed] [Google Scholar]
- 16.Chen Z, Kujawa SG, McKenna MJ, et al. Inner ear drug delivery via a reciprocating perfusion system in the guinea pig. J Control Release. 2005;110:1–19. doi: 10.1016/j.jconrel.2005.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffer ME, Balough BJ, Gottshall KR. Delivery of drugs to the inner ear. Curr Opin Otolaryngol Head Neck Surg. 2006;14:329–331. doi: 10.1097/01.moo.0000244190.51560.6b. [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann KK, Silverstein H. Inner ear perfusion: indications and applications. Curr Opin Otolaryngol Head Neck Surg. 2003;11:334–339. doi: 10.1097/00020840-200310000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Light JP, Silverstein H. Transtympanic perfusion: indications and limitations. Curr Opin Otolaryngol Head Neck Surg. 2004;12:378–383. doi: 10.1097/01.moo.0000134438.91734.38. [DOI] [PubMed] [Google Scholar]
- 20.Darlington CL, Smith PF. Drug treatments for tinnitus. Prog Brain Res. 2007;166:249–262. doi: 10.1016/S0079-6123(07)66023-3. [DOI] [PubMed] [Google Scholar]
- 21.Plontke SK, Biegner T, Kammerer B, et al. Dexamethasone concentration gradients along scala tympani after application to the round window membrane. Otol Neurotol. 2008;29:401–406. doi: 10.1097/MAO.0b013e318161aaae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salt AN, Ma Y. Quantification of solute entry into cochlear perilymph through the round window membrane. Hear Res. 2001;154:88–97. doi: 10.1016/s0378-5955(01)00223-4. [DOI] [PubMed] [Google Scholar]
- 23.Chen Z, Mikulec AA, McKenna MJ, et al. A method for intracochlear drug delivery in the mouse. J Neurosci Met. 2006;150:67–73. doi: 10.1016/j.jneumeth.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 24.Gordon-Salant S, Frisina RD, Popper A, Fay RR. The Aging Auditory System: Perceptual Characterization and Neural Bases of Presbycusis,”. New York: Springer-Verlag; 2010. p. 301. [Google Scholar]
- 25.Frisina RD, Zhu X, D’Souza M. Biological Bases of Age-Related Hearing Loss. Ch. 2; Phonak Proc. Int. Conf. Aging Auditory System; 2010. pp. 19–24. [Google Scholar]
- 26.Oliver D, He DZZ, Klöcker N, Ludwig J, Schulte U, Waldegger S, Ruppersberg JP, Dallos P, Fakler B. Intracellular anions as the voltage sensor of prestin, the outer hair cell motor protein. Science. 2001;292:2340–2343. doi: 10.1126/science.1060939. [DOI] [PubMed] [Google Scholar]
- 27.Brownell WE. Outer hair cell electromotility and otoacoustic emissions. Ear Hear. 1990;11:82–92. doi: 10.1097/00003446-199004000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Müller M, von Hünerbein K, Hoidis S, Smolders JWT. A physiological place-frequency map of the cochlea in the CBA/J mouse. Hear Res. 2005;202:63–73. doi: 10.1016/j.heares.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 29.Varghese GI, Zhu X, Frisina RD. Age-Related Declines in Contralateral Suppression of Distortion Product Otoacoustic Emissions Utilizing Pure Tones in CBA/CaJ Mice. Hear Res. 2005;209:60–67. doi: 10.1016/j.heares.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 30.Zhu X, Vasilyeva ON, Kim S-H, Jacobson M, Romney J, Waterman MS, Tuttle D, Frisina RD. Auditory efferent system declines precede age-related hearing loss: Contralateral suppression of otoacoustic emissions in mice. J Comp Neurol. 2007;503:593–604. doi: 10.1002/cne.21402. [DOI] [PubMed] [Google Scholar]
- 31.Frisina RD, Newman SR, Zhu X. Auditory efferent activation in CBA mice exceeds that of C57s for varying levels of noise. J Acoust Soc Am. 2007;121:EL-29–EL-34. doi: 10.1121/1.2401226. [DOI] [PubMed] [Google Scholar]
- 32.Frisina RD, Singh A, Bak M, Bozorg S, Seth R, Zhu X. F1 (CBA × C57) mice show superior hearing in old age relative to their parental strains: Hybrid vigor or a new animal model for "Golden Ears"? Neurobiol Aging. 2011;32:1716–1724. doi: 10.1016/j.neurobiolaging.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Santi PA, Rapson I, Voie AH. Development of the mouse cochlea database (MCD) Hear. Res. 2008;243:11–17. doi: 10.1016/j.heares.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson DG, Zhu X, Frisina RD, Borkholder DA. Micro-Molded Cannulae for Intracochlear Infusions in Small Rodents; 29th IEEE EMBS Annual International Conf. Proc.: Medi Biol; 2007. pp. 6616–6619. [DOI] [PubMed] [Google Scholar]
- 35.Johnson DG, Waldron MJ, Frisina RD, Borkholder DA. Implantable Micropump Technologies for Murine Intracochlear Infusions. IEEE Engineer Med Biol Soc. 2010:6441–6444. doi: 10.1109/IEMBS.2010.5627335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haghpanahi M, Gladstone MB, Zhu XX, Frisina RD, Borkholder DA. Noninvasive technique for monitoring drug transport through the murine cochlea using micro-computed tomography. Ann Biomed Engineer. 2013 doi: 10.1007/s10439-013-0816-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Looij MAJ, Liem SS, van der Burg H, van der Wees J, De Zeeuw CI, van Zanten BGA. Impact of conventional anesthesia on auditory brainstem responses in mice. Hearing Research. 2004;193:75–82. doi: 10.1016/j.heares.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 38.Cederholm JME, Froud KE, Wong ACY, Ko M, Ryan AF. Differential actions of isoflurane and ketamine-based anesthetics on cochlear function in the mouse. Hearing Research. 2012;292:71–79. doi: 10.1016/j.heares.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]