Abstract
Context
A newly-recognized pathogenic mechanism underlying light chain amyloidosis (AL) involves endothelial dysfunction and cell injury caused by misfolded light chain proteins (LC). Nanoliposomes (NL) are artificial phospholipid vesicles that could attach to misfolded proteins and reduce tissue injury.
Objective
To test whether co-treatment with NL reduce LC-induced endothelial dysfunction and cell death.
Methods
Abdominal subcutaneous adipose arterioles from 14 non-AL subjects were cannulated; dilator response to acetylcholine and papaverine were measured at baseline and following 1-hour exposure to LC (20 μg/mL, 2 purified from AL subjects’ urine, 1 from human recombinant LC [AL-09]) ± NL (phosphatidylcholine/cholesterol/phosphatidic acid 70/25/5 molar ratio) or NL alone. Human aortic artery endothelial cells (HAEC) were exposed to Oregon Green-labeled LC±NL for 24 hours and intracellular LC and apoptosis (Hoechst stain) were measured. Circular dichroism spectroscopy was performed on AL-09 LC±NL to follow changes in secondary structure and protein thermal stability.
Results
LC caused impaired dilation to acetylcholine that was restored by NL (control-94.0±1.8%, LC-65.0±7.1%, LC+NL-95.3±1.8%, p≤0.001 LC vs. control or LC+NL). NL protection was inhibited by L-NG-nitroarginine methyl ester. NL increased the beta sheet structure of LC, reduced endothelial cell internalization of LC and protected against LC-induced endothelial cell death.
Conclusions
LC induced human adipose arteriole endothelial dysfunction and endothelial cell death, which were reversed by co-treatment with NL. This protection may partly be due to enhancing LC protein structure and reducing LC internalization. Nanoliposomes represent a promising new class of agents to ameliorate tissue injury from protein misfolding diseases such as AL.
Keywords: nanoliposomes, lipid nanoparticles, amyloid, heart failure, endothelial function
Introduction
Light chain amyloidosis (AL) is a protein-misfolding disease associated with high morbidity and mortality that involves plasma cell overproduction of amyloidogenic light chain proteins (LC) leading to multiorgan injury, particularly heart failure (Falk, 2005, Migrino et al., 2009). We showed that soluble prefibrillar LC induce microvascular dysfunction in ex-vivo human adipose and coronary arterioles (Franco et al., 2012, Migrino et al., 2010, Migrino et al., 2011), consistent with clinical observations of endothelial dysfunction in early (Berghoff et al., 2003) and established disease (Modesto et al., 2007). Chemotherapy±autologous stem cell transplantation to eradicate the plasma cells is the only treatment available but it is associated with high treatment related mortality and cannot be given in many patients with advanced disease (Dispenzieri et al., 2004). A novel approach to directly attack the amyloidogenic light chains using monoclonal antibodies has had initial preliminary success in an animal model (Solomon et al., 2003) but remains to be tested in humans. Another potential approach is to use nanoliposomes (NL) which are artificial phospholipid vesicles that may have an advantage over immunotherapy of not eliciting an immune response. Nanoliposomes were found to bind amyloidogenic Aβ1–42 proteins, proteins that are relevant in Alzheimer’s disease (Gobbi et al., 2010, Re et al., 2011) as well as interact with amyloid light chain protein (SMA) (Meng et al., 2008). This points to the potential of nanoliposomes to modify injury by misfolded proteins. We aim to test the hypothesis that NL attenuate LC-induced human adipose arteriole endothelial dysfunction and protect against LC-induced human endothelial cell injury.
Methods
AL light chain proteins
The methods for LC isolation have been previously described (Migrino et al., 2011). In brief, urine was collected from 2 AL subjects with cardiac involvement (both males, 58±15 years old, both lambda type). LC were purified by dialysis, size exclusion filtration, Affigel blue filtration and lyophilization. Purified proteins were verified to be light chains using anti-human lambda ELISA quantitation kit (Bethyl Labs, Montgomery TX) and Western blot probed with anti-human lambda light chain antibody (Sigma-Aldritch, St Louis MO). All subjects provided informed consent and the study was approved and supervised by the Institutional Review Boards of the Phoenix VA and Medical College of Wisconsin. Human recombinant LC protein AL-09 was also produced as per previous methods (McLaughlin et al., 2006). AL-09 is derived from a κ1 light chain variable domain from a cardiac AL patient who died 1 year after diagnosis and the protein sequence was deposited in GenBank (accession AF490909). In this study, we used AL-09 full length. The purification protocol has been described previously (Levinson et al., 2013).
Nanoliposomes
Nanoliposomes (phosphatidylcholine/cholesterol/phosphatidic acid 70/25/5 molar ratio; 20 mg lipid/ml) were prepared by probe sonication as described (Lasch et al., 2003). All lipids were purchased from Avanti Polar Lipids (Alabaster AL). Briefly, a mixture of all lipids was dissolved in chloroform followed by removal of the organic solvent using a rotary evaporator. After adding 5 mM HEPES (pH 7.4) to the dry lipid film, the sample was probe sonicated with a Sonic Dismembrator (Model100, Fischer Scientific) at a power output of approximately 10 Watts for 30 minutes. To remove any titanium particles, the sample was centrifuged for 10 minutes at 30000× g. Liposome size and zeta potential (determined using a Coulter N4 Submicron Particle Size Analyzer) were 29±6 nm and −11.1 mV, respectively.
Ex-vivo human adipose arteriole vasoreactivity
The methods for arteriole preparation and testing have previously been described (Franco et al., 2012, Migrino et al., 2011). In brief, 14 subjects (all males, 64±3 years old) without AL/diabetes/vascular disease undergoing routine abdominal surgery agreed to donate subcutaneous adipose tissue obtained by their surgeons. Arterioles (~100–200 μM diameter) were isolated, cannulated and pressurized to 60 mmHg. Baseline control vasoreactivity was performed following preconstriction with endothelin-1 to 60% baseline diameter and successive administration of acetylcholine (10−9-10−4M) to measure endothelium-dependent dilation and papaverine (10−4M) to measure smooth-muscle dependent dilation using videomicrometer. After washing, the vessels were exposed to LC (20 μg/mL) with or without NL (1:10 LC:NL mass ratio) for 1 hour and a second vasoreactivity response to acetylcholine and papaverine was measured. In three additional arterioles, LC and NL were co-treated with L-NG-nitroarginine methyl ester (L-NAME, 5 mmol, Sigma Aldrich, St. Louis MO).
Circular dichroism (CD) spectroscopy
The secondary structure of AL-09 LC protein was characterized by following the Far-UV-CD spectrum from 260 to 200 nm as per previous methods (McLaughlin et al., 2006) except we followed it at 14°C. AL-09 LC at 10 μM was mixed 1:1 with NL and incubated for 30 min at 14°C before the Far-UV-CD spectrum was obtained.. The buffer and NL baselines were subtracted from the AL-09+NL spectrum. Thermal denaturation curves of AL-09±NL were monitored at the maximum β-sheet signal (217 nm) and ellipticity was measured from 14–80°C. Three replicates were performed.
Oregon Green (OG) labeling
OG labeling of LC was achieved using OG 488 protein labeling kit (Molecular Probes, Eugene OR). 50 μL of 1M bicarbonate was added to LC (1 mg) and added to 1 vial of OG reactive dye, stirring the mixture for 1 hour at room temperature. Labeled protein was purified by passing the mixture through a column with purification resin while adding elution buffer until the labeled protein has been eluted. Using handheld UV lamp, the first band representing labeled protein was collected while slower moving band consisting of unincorporated dye was discarded. The degree of OG protein labeling was determined by measuring the absorbance of the conjugate solution at 280 nm and 496 nm. The concentration of the protein in the sample was calculated as:
Where 203,000 cm−1M−1 is the molar extinction coefficient of a typical IgG and 0.12 is the correction factor to account for absorption of the dye at 280 nm. The degree of labeling was calculated as:
The degree of labeling was 3.6 M dye/M protein.
Endothelial cell LC entry and cell death
Human aortic endothelial cells (HAEC, Lonza, Portsmouth NH) were passaged 24–48 hours prior to exposure to OG labeled LC (20 μg/mL) ± NL (LC:NL 1:10 mass ratio) for 24 hours (cells were not serum-starved). Cells were fixed using 4% formaldehyde and stained using 1 μM Hoechst 33258 stain (Sigma-Aldritch). Confocal microscopy was performed using Zeiss 710 laser scanning confocal microscope. Oregon green was visualized with 488nm laser excitation and 515–535 emission while Hoechst was identified by 405nm laser excitation and 415–460nm emission. OG signal was compared versus control (HAEC exposed to OG labeled LC for 10 minutes). ImageJ (National Institutes of Health, Bethesda MD) was used to measure OG signal. Apoptotic cell death was determined using Hoechst staining by measuring the percentage of cells with dense concentrated granular nuclear fluorescence; cells were considered viable if they had diffuse fluorescence in the nuclei. Measurement was performed by a reader blind to treatment allocation.
Data and statistical analyses
Data are expressed as means±standard error of means; significant value is set at p<0.05 (two-sided). Baseline control and post-treatment dilator response at each acetylcholine/papaverine dose was compared using paired Student’s t-test. Overall dilator response to acetylcholine was analyzed by deriving the log effective concentration 50% (logEC50) using nonlinear regression and variable slope (4 parameters) and least squares fit (Migrino et al., 2011). For multiple group analyses, one-way or two way analysis of variance with Bonferroni post-test were utilized. Statistical calculations were done using GraphPad Prism 5.0 (San Diego CA).
Results
LC reduced dilation to acetylcholine and, to a lesser extent, papaverine in adipose arterioles (Figure 1A–B). Co-treatment with NL fully restored dilator responses. The protective effect of NL was reversed by co-treatment of the nitric oxide synthase inhibitor L-NAME. In separate vessels, dilator responses when arterioles were treated with NL alone were not significantly different from baseline control, either to acetylcholine (Figure 1B), or papaverine (10−4M: 89.5±6.0 versus 91.4±6.2% control, p=0.4, n=5).
Figure 1.
Arteriole vasoreactivity and CD spectroscopy. A and B. Dilator response to acetylcholine and papaverine was reduced with LC and restored with by NL. L-NAME abolished NL protective effect. In separate arterioles, NL alone did not change dilator response to acetylcholine versus baseline control. C. Far-UV-CD spectroscopy shows increased ellipticity (more negative value) at 211–220 nm wavelengths when AL-09 LC is mixed with NL signifying increased beta-structure. D. Thermal denaturation profile of AL-09 was not changed by NL.
NL increased the ellipticity of AL-09 LC at 211–220 wavelength (n=3, 2-way ANOVA p<0.001) suggesting increased AL-09 beta-sheet structure (Figure 1C). AL-09 thermal denaturation profile was not affected by NL (Figure 1D).
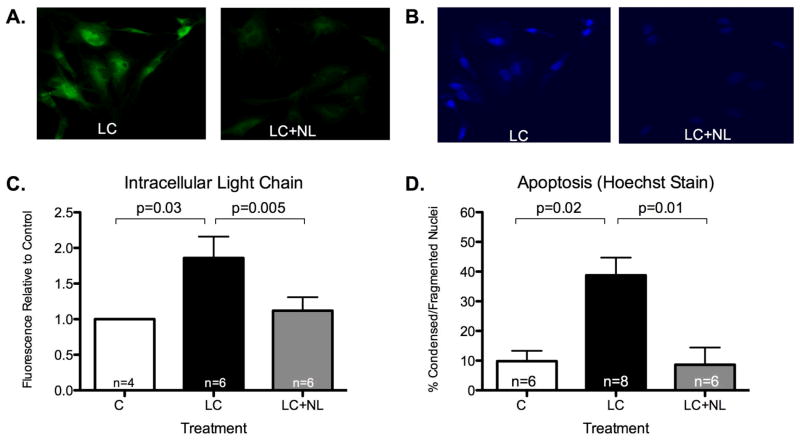
NL decreased HAEC LC internalization (Figure 2A/C) and reduced apoptotic death (Figure 2B/D).
Figure 2.
Endothelial cell light chain internalization and apoptotic injury. A and C. Intracellular Oregon green-stained LC was significantly reduced by co-treatment with NL. B and D. Hoecsht staining demonstrates reduced apoptotic injury with NL co-treatment.
Discussion
We present the novel observation that nanoliposomes composed of phosphatidylcholine/cholesterol/phosphatidic acid reverse LC-induced human arteriole endothelial dysfunction, smooth muscle dysfunction and endothelial cell death, possibly through NL effects on increasing the amount of folded LC and reducing LC cellular internalization.
AL, like other protein misfolding diseases such as Alzheimer’s disease, confers significant morbidity/mortality. We showed previously that LC induce endothelial dysfunction in human adipose/coronary arterioles and apoptosis through reduced nitric oxide bioavailability and oxidative/nitrosative stress (Migrino et al., 2011). Nanoliposomes may be ideal for human treatment and reducing misfolded protein toxicity. Unlike other nanoparticles, they are considered non-toxic, non-immunogenic, fully biodegradable and structurally versatile (Re et al.). NL with anionic phospholipids (phosphatidic acid/cardiolipin-containing) were shown to bind Aβ1–42 peptides with high affinity (Gobbi et al., 2010, Re et al., 2011) reducing cell and tissue exposure to amyloid proteins. Our findings also show interaction between NL with phosphatidic acid and LC, although it is not known whether the interaction is similar to the interaction between NL and Aβ. Nanoliposomes reduced LC internalization while causing an increase in protein beta-sheet structure (although thermal denaturation profile was not altered). These results suggest that the presence of NL shifts the equilibrium towards the folded state for AL-09, reducing the amount of partially folded states sampled by the protein. These partially folded states were implicated as the initiators of amyloid formation and cytotoxicity for amyloid diseases (Chiti et al., 1999). Our observations suggest that part of NL protection may be through enhancement of LC protein structure, potentially decreasing its toxicity, while impairing LC internalization through a yet undetermined means. Previous work by a coinvestigator show that AL-09 internalized more rapidly into cardiomyocytes versus germline protein devoid of somatic mutations (κIO18/08) and that the rate of fibril formation correlates with the degree of intracellular internalization (Levinson et al., 2013), pointing to the important role of the ensemble of conformations adopted by the proteins in LC internalization and possibly pathologic effects. It remains unknown whether NL have additional direct cell protective effects, a focus of future investigations.
Our results of phosphatidic acid-containing NL interacting with LC are consistent with the findings of Meng and colleagues (Meng et al., 2008). The authors investigated the affinity of a variety of liposomal membranes, made of binary mixtures of different types of neutral phospholipids as well as a variety of negatively charged phospholipids such as phosphatidic acid and phosphatidyl glycerol, to interact with a different amyloid LC (SMA, kappa type, variable region). They found that liposomes containing phosphatidic acid, i.e. phospholipids with the smallest head group, displayed a strong affinity towards SMA. Their results strongly suggest that a negatively charged liposomal membrane alone is not sufficient to interact with amyloid proteins; the nature of the phospholipid head group seems to be at least equally important. In the study of Meng and colleagues, it is salient to point out that when cholesterol is incorporated to phosphatidic acid-containing liposomal membranes in a proportion similar to that used in our study (25%), the liposomal membranes were found not to promote amyloid protein fibrillation.
Limitations and future directions
Our ex-vivo human arteriole model does not fully recapitulate in-vivo microvascular conditions since the model does not replicate blood flow and other in-vivo conditions; therefore, this initial proof-of-concept finding of the protective effect of NL against LC-induced microvascular dysfunction needs to be validated and optimized in an in-vivo animal setting in the future. In addition, although our data show interaction between nanoliposomes and LC, the exact mechanism of the interaction remains unknown and we also do not know whether the affinity of nanoliposomes is specific to LC. Although the composition of NL tested in our study showed protective effect, the ideal phospholipid composition and concentration to optimize NL protection should be tested in the future.
Conclusions
Amyloidogenic light chain-induced human adipose arteriole endothelial dysfunction and endothelial cell deaths were reversed by co-treatment with NL. This protection may partly be due to enhancing LC protein structure and reducing LC internalization. Nanoliposomes represent a promising new class of agents to ameliorate tissue injury from protein misfolding diseases such as AL.
Acknowledgments
We thank our research volunteers, the Phoenix Veterans Affairs Surgery Service, including Howard Bourdages, Lillian Dawes, William Dolan, Maher Huttam, John Pyeatt III, John Hatfield and Paulina Iacoban. The contents do not represent the views of the Veterans Affairs or the United States government.
Footnotes
Declarations of interest
Funding and support were provided by the Amyloidosis Foundation, VA Merit BLRD I01BX007080 (VA ORD), RO1 GM071514 (MRA/WO) and Carl T. Hayden Medical Research Foundation.
References
- BERGHOFF M, KATHPAL M, KHAN F, SKINNER M, FALK R, FREEMAN R. Endothelial dysfunction precedes C-fiber abnormalities in primary (AL) amyloidosis. Ann Neurol. 2003;53:725–30. doi: 10.1002/ana.10552. [DOI] [PubMed] [Google Scholar]
- CHITI F, WEBSTER P, TADDEI N, CLARK A, STEFANI M, RAMPONI G, DOBSON CM. Designing conditions for in vitro formation of amyloid protofilaments and fibrils. Proc Natl Acad Sci U S A. 1999;96:3590–4. doi: 10.1073/pnas.96.7.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DISPENZIERI A, KYLE RA, LACY MQ, THERNEAU TM, LARSON DR, PLEVAK MF, RAJKUMAR SV, FONSECA R, GREIPP PR, WITZIG TE, LUST JA, ZELDENRUST SR, SNOW DS, HAYMAN SR, LITZOW MR, GASTINEAU DA, TEFFERI A, INWARDS DJ, MICALLEF IN, ANSELL SM, PORRATA LF, ELLIOTT MA, GERTZ MA. Superior survival in primary systemic amyloidosis patients undergoing peripheral blood stem cell transplantation: a case-control study. Blood. 2004;103:3960–3. doi: 10.1182/blood-2003-12-4192. [DOI] [PubMed] [Google Scholar]
- FALK RH. Diagnosis and management of the cardiac amyloidoses. Circulation. 2005;112:2047–60. doi: 10.1161/CIRCULATIONAHA.104.489187. [DOI] [PubMed] [Google Scholar]
- FRANCO DA, TRURAN S, BURCIU C, GUTTERMAN DD, MALTAGLIATI A, WEISSIG V, HARI P, MIGRINO RQ. Protective role of clusterin in preserving endothelial function in AL amyloidosis. Atherosclerosis. 2012;225:220–3. doi: 10.1016/j.atherosclerosis.2012.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOBBI M, RE F, CANOVI M, BEEG M, GREGORI M, SESANA S, SONNINO S, BROGIOLI D, MUSICANTI C, GASCO P, SALMONA M, MASSERINI ME. Lipid-based nanoparticles with high binding affinity for amyloid-beta1–42 peptide. Biomaterials. 2010;31:6519–29. doi: 10.1016/j.biomaterials.2010.04.044. [DOI] [PubMed] [Google Scholar]
- LASCH J, WEISSIG V, BRANDL M. Preparation of liposomes. In: TORCHILIN VP, WEISSIG V, editors. Liposomes. Oxford University Press; 2003. [Google Scholar]
- LEVINSON RT, OLATOYE OO, RANDLES EG, HOWELL KG, DICOSTANZO AC, RAMIREZ-ALVARADO M. Role of mutations in the cellular internalization of amyloidogenic light chains into cardiomyocytes. Sci Rep. 2013;3:1278. doi: 10.1038/srep01278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCLAUGHLIN RW, DE STIGTER JK, SIKKINK LA, BADEN EM, RAMIREZ-ALVARADO M. The effects of sodium sulfate, glycosaminoglycans, and Congo red on the structure, stability, and amyloid formation of an immunoglobulin light-chain protein. Protein Sci. 2006;15:1710–22. doi: 10.1110/ps.051997606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MENG X, FINK AL, UVERSKY VN. The effect of membranes on the in vitro fibrillation of an amyloidogenic light-chain variable-domain SMA. J Mol Biol. 2008;381:989–99. doi: 10.1016/j.jmb.2008.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIGRINO RQ, HARI P, GUTTERMAN DD, BRIGHT M, TRURAN S, SCHLUNDT B, PHILLIPS SA. Systemic and microvascular oxidative stress induced by light chain amyloidosis. Int J Cardiol. 2010;145:67–8. doi: 10.1016/j.ijcard.2009.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIGRINO RQ, MAREEDU RK, EASTWOOD D, BOWERS M, HARMANN L, HARI P. Left ventricular ejection time on echocardiography predicts long-term mortality in light chain amyloidosis. J Am Soc Echocardiogr. 2009;22:1396–402. doi: 10.1016/j.echo.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIGRINO RQ, TRURAN S, GUTTERMAN DD, FRANCO DA, BRIGHT M, SCHLUNDT B, TIMMONS M, MOTTA A, PHILLIPS SA, HARI P. Human microvascular dysfunction and apoptotic injury induced by AL amyloidosis light chain proteins. Am J Physiol Heart Circ Physiol. 2011;301:H2305–12. doi: 10.1152/ajpheart.00503.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MODESTO KM, DISPENZIERI A, GERTZ M, CAUDURO SA, KHANDHERIA BK, SEWARD JB, KYLE R, WOOD CM, BAILEY KR, TAJIK AJ, MILLER FA, PELLIKKA PA, ABRAHAM TP. Vascular abnormalities in primary amyloidosis. Eur Heart J. 2007;28:1019–24. doi: 10.1093/eurheartj/ehm066. [DOI] [PubMed] [Google Scholar]
- RE F, CAMBIANICA I, SESANA S, SALVATI E, CAGNOTTO A, SALMONA M, COURAUD PO, MOGHIMI SM, MASSERINI M, SANCINI G. Functionalization with ApoE-derived peptides enhances the interaction with brain capillary endothelial cells of nanoliposomes binding amyloid-beta peptide. J Biotechnol. 2011;156:341–6. doi: 10.1016/j.jbiotec.2011.06.037. [DOI] [PubMed] [Google Scholar]
- SOLOMON A, WEISS DT, WALL JS. Therapeutic potential of chimeric amyloid-reactive monoclonal antibody 11-1F4. Clin Cancer Res. 2003;9:3831S–8S. [PubMed] [Google Scholar]