Abstract
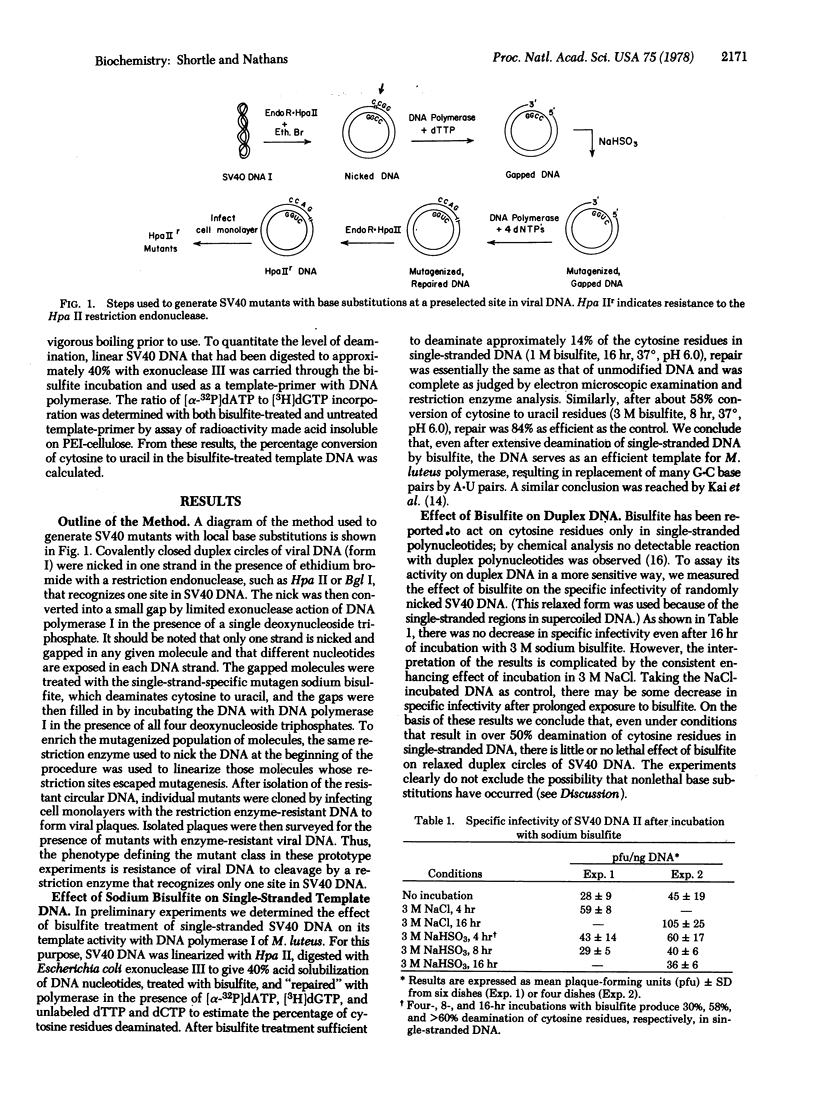
DNA from simian virus 40 (SV40) was prepared for local mutagenesis by nicking the molecule at a specific site with a restriction endonuclease that recognizes one site in SV40 DNA and then extending the nick enzymatically to expose a short, single-stranded segment of DNA. The "gapped" DNA was treated with a single-strand-specific mutagen, sodium bisulfite, which converts cytosine to uracil. After mutagenesis, the gap was repaired with DNA polymerase, generating molecules resistant to the restriction enzyme used to make the initial nick. From cells infected with DNA thus modified, SV40 mutants were isolated that had enzyme-resistant genomes. In some cases, precise positions of G.C to A.T transitions could be inferred from the patterns of susceptibility of mutant DNA to other restriction endonucleases whose recognition sequences were altered by the mutagenesis procedure. One of the restriction endonuclease sites mutagenized (Bgl I) maps at the origin of SV40 DNA replication and near sequences corresponding to the 5' ends of viral mRNAs. Many of the resulting Bgl I-resistant mutants yielded small plaques, suggesting partial defectiveness in DNA replication or transcription.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borrias W. E., Wilschut I. J., Vereijken J. M., Weisbeek P. J., van Arkel G. A. Induction and isolation of mutants in a specific region of gene A of bacteriophage phi chi 174. Virology. 1976 Mar;70(1):195–197. doi: 10.1016/0042-6822(76)90251-8. [DOI] [PubMed] [Google Scholar]
- Brockman W. W., Nathans D. The isolation of simian virus 40 variants with specifically altered genomes. Proc Natl Acad Sci U S A. 1974 Mar;71(3):942–946. doi: 10.1073/pnas.71.3.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon J., Shenk T. E., Berg P. Biochemical procedure for production of small deletions in simian virus 40 DNA. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1392–1396. doi: 10.1073/pnas.72.4.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole C. N., Landers T., Goff S. P., Manteuil-Brutlag S., Berg P. Physical and genetic characterization of deletion mutants of simian virus 40 constructed in vitro. J Virol. 1977 Oct;24(1):277–294. doi: 10.1128/jvi.24.1.277-294.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danna K., Nathans D. Specific cleavage of simian virus 40 DNA by restriction endonuclease of Hemophilus influenzae. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2913–2917. doi: 10.1073/pnas.68.12.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell R. A., Sabo D. L., Bandle E. F., Weissmann C. Site-directed mutagenesis: effect of an extracistronic mutation on the in vitro propagation of bacteriophage Qbeta RNA. Proc Natl Acad Sci U S A. 1975 Jan;72(1):367–371. doi: 10.1073/pnas.72.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayatsu H. Bisulfite modification of nucleic acids and their constituents. Prog Nucleic Acid Res Mol Biol. 1976;16:75–124. doi: 10.1016/s0079-6603(08)60756-4. [DOI] [PubMed] [Google Scholar]
- Kai K., Tsuruo T., Hayatsu H. The effect of bisulfite modification on the template activity of DNA for DNA polymerase I. Nucleic Acids Res. 1974 Jul;1(7):889–899. doi: 10.1093/nar/1.7.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly T. J., Jr, Nathans D. The genome of simian virus 40. Adv Virus Res. 1977;21:85–173. doi: 10.1016/s0065-3527(08)60762-9. [DOI] [PubMed] [Google Scholar]
- Lai C. J., Nathans D. A map of temperature-sensitive mutants of simian virus 40. Virology. 1975 Jul;66(1):70–81. doi: 10.1016/0042-6822(75)90179-8. [DOI] [PubMed] [Google Scholar]
- Lai C. J., Nathans D. Deletion mutants of simian virus 40 generated by enzymatic excision of DNA segments from the viral genome. J Mol Biol. 1974 Oct 15;89(1):179–193. doi: 10.1016/0022-2836(74)90169-7. [DOI] [PubMed] [Google Scholar]
- Lai C. J., Nathans D. The B/C gene of simian virus 40. Virology. 1976 Dec;75(2):335–345. doi: 10.1016/0042-6822(76)90032-5. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Ljungquist S., Siegert W., Nyberg B., Sperens B. DNA N-glycosidases: properties of uracil-DNA glycosidase from Escherichia coli. J Biol Chem. 1977 May 25;252(10):3286–3294. [PubMed] [Google Scholar]
- Lindahl T. New class of enzymes acting on damaged DNA. Nature. 1976 Jan 1;259(5538):64–66. doi: 10.1038/259064a0. [DOI] [PubMed] [Google Scholar]
- McCutchan J. H., Pagano J. S. Enchancement of the infectivity of simian virus 40 deoxyribonucleic acid with diethylaminoethyl-dextran. J Natl Cancer Inst. 1968 Aug;41(2):351–357. [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. K., Wells R. D. Deoxyribonucleoside triphosphate stimulation of exonucleolytic activity of the Micrococcus luteus deoxyribonucleic acid polymerase. J Biol Chem. 1972 May 10;247(9):2675–2681. [PubMed] [Google Scholar]
- Miller L. K., Wells R. D. Properties of the exonucleolytic activities of the Micrococcus luteus deoxyribonucleic acid polymerase. J Biol Chem. 1972 May 10;247(9):2667–2674. [PubMed] [Google Scholar]
- Murray N. E., Murray K. Manipulation of restriction targets in phage lambda to form receptor chromosomes for DNA fragments. Nature. 1974 Oct 11;251(5475):476–481. doi: 10.1038/251476a0. [DOI] [PubMed] [Google Scholar]
- Parker R. C., Watson R. M., Vinograd J. Mapping of closed circular DNAs by cleavage with restriction endonucleases and calibration by agarose gel electrophoresis. Proc Natl Acad Sci U S A. 1977 Mar;74(3):851–855. doi: 10.1073/pnas.74.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. J., Myers P. A., Morrison A., Murray K. A specific endonuclease from Arthrobacter luteus. J Mol Biol. 1976 Mar 25;102(1):157–165. doi: 10.1016/0022-2836(76)90079-6. [DOI] [PubMed] [Google Scholar]
- Roberts R. J. Restriction endonucleases. CRC Crit Rev Biochem. 1976 Nov;4(2):123–164. doi: 10.3109/10409237609105456. [DOI] [PubMed] [Google Scholar]
- Rundell K., Collins J. K., Tegtmeyer P., Ozer H. L., Lai C. J., Nathans D. Identification of simian virus 40 protein A. J Virol. 1977 Feb;21(2):636–646. doi: 10.1128/jvi.21.2.636-646.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro R., Braverman B., Louis J. B., Servis R. E. Nucleic acid reactivity and conformation. II. Reaction of cytosine and uracil with sodium bisulfite. J Biol Chem. 1973 Jun 10;248(11):4060–4064. [PubMed] [Google Scholar]
- Subramanian K. N., Dhar R., Weissman S. M. Nucleotide sequence of a fragment of SV40 DNA that contains the origin of DNA replication and specifies the 5' ends of "early" and "late" viral RNA. III. Construction of the total sequence of EcoRII-G fragment of SV40 DNA. J Biol Chem. 1977 Jan 10;252(1):355–367. [PubMed] [Google Scholar]





