Abstract
Purpose
An optimal prostate biopsy in clinical practice is based on a balance between adequate detection of clinically significant prostate cancers (sensitivity), assuredness regarding the accuracy of negative sampling (negative predictive value [NPV]), limited detection of clinically insignificant cancers, and good concordance with whole-gland surgical pathology results to allow accurate risk stratification and disease localization for treatment selection. Inherent within this optimization is variation of the core number, location, labeling, and processing for pathologic evaluation. To date, there is no consensus in this regard. The purpose of this review is 3-fold: 1. To define the optimal number and location of biopsy cores during primary prostate biopsy among men with suspected prostate cancer, 2. To define the optimal method of labeling prostate biopsy cores for pathologic processing that will provide relevant and necessary clinical information for all potential clinical scenarios, and 3. To determine the maximal number of prostate biopsy cores allowable within a specimen jar that would not preclude accurate histologic evaluation of the tissue.
Materials and Methods
A bibliographic search covering the period up to July, 2012 was conducted using PubMed®. This search yielded approximately 550 articles. Articles were reviewed and categorized based on which of the three objectives of this review was addressed. Data was extracted, analyzed, and summarized. Recommendations based on this literature review and our clinical experience is provided.
Results
The use of 10–12-core extended-sampling protocols increases cancer detection rates (CDRs) compared to traditional sextant sampling methods and reduces the likelihood that patients will require a repeat biopsy by increasing NPV, ultimately allowing more accurate risk stratification without increasing the likelihood of detecting insignificant cancers. As the number of cores increases above 12 cores, the increase in diagnostic yield becomes marginal. Only limited evidence supports the use of initial biopsy schemes involving more than 12 cores or saturation. Apical and laterally directed sampling of the peripheral zone increases CDR, reduces the need for repeat biopsies, and predicts pathological features on prostatectomy while transition-zone biopsies do not. There is little data to suggest that knowing the exact site of an individual positive biopsy core provides meaningful clinical information. However, determining laterality of cancer on biopsy may be helpful for both predicting sites of extracapsular extension and therapeutic planning. Placement of multiple biopsy cores in a single container (>2) appears to compromise pathologic evaluation, which can reduce CDR and increase the likelihood of equivocal diagnoses.
Conclusions
A 12-core systematic biopsy that incorporates apical and far-lateral cores in the template distribution allows maximal cancer detection, avoidance of a repeat biopsy, and adequate information for both identifying men who need therapy and planning that therapy while minimizing the detection of occult, indolent prostate cancers. This literature review does not provide compelling evidence that individual site-specific labeling of cores benefits clinical decision-making regarding the management of prostate cancer. Based upon the available literature, we recommend packaging no more than two cores in each jar to avoid reduction of CDR through inadequate tissue sampling.
INTRODUCTION
An optimal prostate biopsy in clinical practice is based on a balance between adequate detection of clinically significant prostate cancers (sensitivity), assuredness regarding the accuracy of negative sampling (negative predictive value or NPV), limited detection of clinically insignificant cancers, and good concordance with whole-gland surgical pathology results to allow accurate risk stratification for treatment selection. A variety of biopsy techniques have emerged for optimizing these attributes, including computerized and image-guided techniques, but systematic sampling with variable core numbers remains the standard in practice.
Several biopsy strategies have been employed to increase cancer detection rate (CDR) including sampling more cores or sampling additional areas such as the peripheral, transitional, or anterior zones. These strategies, however, run the risk of increasing the detection of indolent or non-lethal prostate cancer, which may result in overtreatment. Furthermore, increasing the number of cores has led to increased costs for specimen processing, pathologic evaluation, and cancer therapy. As a result, an optimal biopsy strategy includes an adequate number of cores to provide confidence in a negative finding while limiting the number of cores and pathologic specimens sufficiently to avoid over-detection and cost escalation. Consequently, today’s biopsy protocols typically involve extracting 10–12 cores per biopsy,1 which has been endorsed by expert panels in the United States, Canada, and Italy.2–4 No consensus exists regarding the optimal labeling of these prostate biopsy cores for pathologic processing or the number of allowable cores per container without compromise of histologic evaluation. Given the controversy regarding the optimal strategy for prostate biopsy with regard to core number, location, labeling, and pathologic processing1, 3, we undertook a review of the literature to address the following primary objectives:
Define the optimal number and location of biopsy cores during initial prostate biopsy among men with suspected prostate cancer. In doing so, we address the CDR, NPV, detection of clinically insignificant cancer, and pathologic concordance with radical prostatectomy (RP) pathology results for each biopsy strategy.
Define the optimal method of labeling prostate biopsy cores for pathologic processing that will provide relevant and necessary clinical information for all potential clinical scenarios.
Determine the maximal number of prostate biopsy cores allowable within a specimen jar that would not preclude accurate histologic evaluation of the tissue.
Several indications for prostate biopsy exist, including primary biopsy at the time of suspicion of cancer; repeat biopsy for persistent suspicion or premalignant/atypical findings; surveillance biopsy for low-risk cancer; and staging biopsy for therapeutic planning or, most recently, focal ablation. Because the desired outcome of each biopsy indication is distinct, we focus our efforts on the initial biopsy. The analysis for this review did not evaluate the additional value of nodule-directed or image-directed biopsies nor did it not evaluate the imaging method of biopsy guidance (transperineal vs transrectal). Other important considerations exist regarding prostate biopsies, such as adverse events, but were beyond the scope of this review.
MATERIALS AND METHODS
By invitation from the American Urological Association, the authors were selected as an expert committee on prostate biopsies. Over the following year, the committee met in person and by phone conference to define the objectives of the study.
Search Strategy
Based on the study objectives, we searched PubMed® for English-language articles published up to July of 2012 using combinations of the following key terms: prostatic neoplasms, biopsy methods, cores, saturation, sextant, cancer detection rate, repeat biopsy, negative predictive value, pathology concordance, insignificant cancer, apex, transition zone, lateral, extracapsular extension, surgical margin, labeling, clinical trial, meta-analysis, practice guideline, comparative study, consensus development conference, evaluation study, and multicenter study.
Study Selection
This search yielded approximately 550 articles. We reviewed each of these articles to determine whether they met our inclusion criteria of describing a prostate biopsy protocol, availability of data on patients who had not had a previous prostate biopsy, indication of biopsy entry site, and availability of data on patients who underwent TRUS only if the study included TRUS and another biopsy entry site.
Data Extraction
We categorized articles that met inclusion based on which of the three objectives of this review the articles addressed. After data extraction, the study committee was again reconvened to assess and analyzed relevant data from these studies to determine the influence of core number, core location, and saturation technique on CDR, NPV, surgical pathology concordance, and detection of insignificant cancers. We evaluated data regarding specimen processing to determine the influence of location-based labeling of cores on risk stratification, therapeutic planning, and assessment of biopsy adequacy.
RESULTS AND DISCUSSION
Cancer Detection Rates
The Committee has focused on the workflow of the biopsy process. However, we note that workflow of histologic processing also influences the cancer detection rate as well.5 The number of levels examined varies amongst laboratories.6 The CDR is the result of both the biopsy process and histology processing, and thus includes workflows from both Urology and Pathology.
Comparisons of CDRs between standard sextant biopsy protocols and extended-core biopsy protocols (involving 10–12 cores) have demonstrated an overall trend of increasing CDRs with greater numbers of cores. Several studies comparing sextant biopsies to an 11–12 core approach have demonstrated an increased in CDR of approximately 31%.7 These findings have been corroborated in a large review of 87 studies involving 20,698 patients.8 In contrast, numerous researchers have evaluated the utility of an 18-core or saturation biopsy (21-core) as an initial biopsy strategy and found no statistically significant gain in CDR.9 La Taille et al. (n=303) found that the CDRs using sextant, extended 12-core, 18-core, and 21-core biopsy schemes were 22.7%, 28.3%, 30.7%, and 31.3%, respectively.10 Diagnostic yield improved by 24.7% when the number of cores increased from 6 to 12, but only by 10.6% when the number of cores increased from 12 to 21. When Ploussard and colleagues compared CDRs in 2,753 consecutive patients, CDRs using sextant, 12-core, and 21-core schemes were 32.5%, 40.4%, and 43.3%, respectively.11 The 12-core procedure improved the CDR by 19.4% (p=0.004) compared to the sextant approach, and the 21-biopsy scheme improved the CDR by 6.7% overall (p<0.001). In their review of the diagnostic value of systematic prostate biopsies, Eichler et al noted taking more than 12 cores did not significantly improve cancer yield.8
In terms of biopsy core location, both apical and far-lateral sampling appears to increase CDR while transition-zone biopsies do not improve prostate CDR at initial extended biopsy. Babaian et al. evaluated an 11-core biopsy strategy in 362 patients, including 85 (23%) who were undergoing a first biopsy.7 The CDR for patients undergoing an initial biopsy was 34%, and 9 cancers were uniquely identified by non-sextant sites. Of the cancers identified uniquely by cores from non-sextant sites, 7 were identified by anterior-horn biopsies and 2 by transition-zone biopsies. Because the entire apex is composed of peripheral zone, biopsies performed at the apex or lateral apex might not sample the anterior apex. Additional studies have demonstrated that biopsy cores directed at the anterior apex exclusively contribute to cancer detection in 4–6% of men.12 In a prospective study evaluating men undergoing a 12-core biopsy with 2 additional cores from the extreme anterior apex, Moussa et al. (n=181) reported apical cores achieved the highest CDR (73.6% of all cancers). The additional extreme anterior apical cores (one on each side) achieved the highest rate of unique cancer detection (p=0.011).13 Initial diagnostic transition-zone biopsies have demonstrated a low percentage of patients (2.9%) have cancer exclusively in the transition zone on first biopsy.14 However, in a prospective study of 1,000 men, Guichard et al. did find a significant improvement in CDR (by 7.2%, p=0.023) with the addition of transition-zone biopsies to a 12-core scheme, for an overall CDR of 41.5%.9
Negative Predictive Value: Avoidance of Repeat Biopsy
In addition to detecting cancer, the goal of a biopsy scheme should be to increase the NPV and reduce the number of false-negative results from the initial biopsy. Sextant biopsies have false-negative rates of 15–34% based on repeated biopsies and computer simulations.15, 16 Simply by performing a second sextant biopsy during the same office visit, Levine et al. increased the number of cancers detected by 30%.16 Other researchers have demonstrated that prostate CDRs on repeat biopsy vary as a function of the extent of the initial biopsy.17 If a prior negative biopsy involved a sextant scheme, the CDR was 39% with a repeat extended biopsy, whereas if a prior negative biopsy involved an extended scheme, the CDR of the repeat biopsy decreased to 21–28%. Use of repeat saturation (20 to 24 cores) biopsy after initial saturation biopsy has been shown to have a CDR of 24%, similar to the CDR of 29% for biopsies following an initial sextant biopsy (p=0.08).17 The authors from this study concluded that the false-negative rate for repeat prostate biopsies after an initial saturation biopsy is equivalent to that following traditional biopsy and they recommended against saturation prostate biopsies as an initial strategy.
Similar to CDRs, apical and far-lateral directed cores reduce the need for repeat biopsies and increases NPV while transitional zone biopsies do not. Sampling the anterior apical peripheral zone on repeat biopsy identified 36.0% with cancer exclusively in the anterior apical peripheral zone cores. The CDR from the anterior apical peripheral zone sites was significantly higher in the repeat biopsies than in the initial biopsies (p<0.01), suggesting a predominance of missed cancers in this location.18 Apical cores and extreme anterior apical cores have been shown to increase unique cancer detection and minimize the potential for misdiagnosis and need for repeat biopsy.13 Because relatively few cancers are found uniquely in the transition zone, it is unlikely that repeat biopsies would be avoided by routine transition-zone sampling. No difference was seen in the number of men requiring a repeat biopsy when evaluating the role of transition-zone sampling on initial and repeat biopsy.19 Few studies have evaluated the NPV of far-lateral sampling of the prostate. However, lateral sampling appears to improve clinical NPV because several cancers are identified only in the lateral sample.
Pathology Concordance
Several studies have demonstrated that extended biopsy schemes improve biopsy concordance with prostatectomy specimens. Concordance rates of prostate cancer grade, when an extended biopsy scheme is used, are as high as 85%, compared to 50% with a sextant biopsy.20–22 Upgrading of the Gleason score has been shown to be significantly less likely with the extended scheme (17% vs. 41% for the sextant scheme, p<0.001).21 Similarly, 14% of the prostate cancers detected using extended biopsy schemes have been shown to be under-graded compared to 25% of cancers detected using sextant schemes (p=0.01).22 The results of biopsy schemes involving saturation biopsies (more than 12 cores) appear to have a higher concordance rate with results from prostatectomy (59%) than a scheme involving fewer than 12 cores (47%, p=0.05).23
Apical and laterally directed sampling improves the ability to predict pathological features on prostatectomy, while the concordance of transition-zone biopsies with radical resection is poor. In a study evaluating individually labeled, preoperative apical core biopsies and corresponding prostatectomy specimens, Rogatsch et al. determined the positive predictive value (PPV) for identifying the tumor location correctly was 71.1%, while the lack of cancer in the apical biopsy had an NPV of 75.5%.24 Cancer concordance of transition-zone biopsies and prostatectomy specimens range from approximately 20–40%.25 The role of lateral sampling of the prostate was evaluated by Singh et al. who showed that laterally directed cores were independent predictors of pathological features at prostatectomy.26
Insignificant Cancer Detection
A potential drawback of the extended-core biopsy scheme and the resulting increased CDR is the increased likelihood of detecting insignificant prostate cancers. Although few studies exist which observed a higher detection rate of clinically insignificant prostate cancer with sextant biopsy schemes, the majority of reports found no significant differences in the detection rate of insignificant cancers between sextant and extended biopsy schemes.27 In a large database study (n=4,072), Meng and colleagues found that increasing the number of biopsy cores did not result in the identification of a disproportionate number of lower-risk tumors.27 However, increasing the number of cores beyond the extended biopsy strategy appears to increase insignificant cancer detection. Haas et al. showed that an extended-biopsy 18-core strategy increased the detection rate of insignificant prostate cancers by 22%.28 In one study, a 21-core protocol increased the rate of prostate cancers eligible for active surveillance (62.5% vs. 48.4%, p=0.036) compared to the rate detected by a 12-core (and sextant) scheme without significantly increasing the rate of insignificant prostate cancers detected (p=0.503) since cancers detected by the six-core protocol were significantly more aggressive.11
Specimen Processing for Analysis
In processing prostate biopsies for pathologic analysis, urologists must choose the number of cores to place in each specimen container and the optimal method of labeling these cores to indicate the prostate site from which the cores were extracted. Several factors can have an impact on urologists’ decisions, including the influence of cancer location on therapeutic planning and surveillance, quality of pathology analysis when multiple cores are placed in a single container, and assurance of biopsy quality through identification of core location. The first two factors can be critically assessed from the existing urologic and pathologic literature.
For the latter factor, the importance of individually labeling core location might only be meaningful if individual cores are deemed non-informative or substandard for processing. If cores are grouped together, urologists cannot determine whether any region of the prostate was under-sampled. Because the importance of apical and far-lateral sampling is well demonstrated in the existing literature, assessment of biopsy adequacy would seem to require, at least, demonstration of adequate sampling of these regions.
Importance of Clinical Information Derived from the Labeling of Cores
Prediction of Extracapsular Extension (ECE)
Taneja et al. retrospectively compared the results of the diagnostic biopsies of 243 men undergoing RP with their final surgical pathology results.29 In this study, 103 men had individually labeled cores for specimen processing and only the right and left cores from the remaining 141 were labeled. The presence of cancer in an individually labeled core was associated with an 8.9±2.2% PPV and 96.9±1.4% NPV for the ECE location compared to a PPV of 12.9 ±3.0% and NPV of 95.8±1.8% when cores were packaged in two containers. The authors concluded that packaging cores in individual containers is substantially more expensive than packaging samples in just two containers without providing much clinical benefit.
Few other studies have evaluated the relationship between biopsy location and ECE location, but several studies have integrated site-specific core data into predictive models. Naya et al. showed that maximum tumor length of at least 7 mm and positive basal core location were the strongest independent predictors of ECE on a side (p<0.0001 and 0.002, respectively).30 In a review of 2,660 cases, Tsuzuki et al. demonstrated that the percentage of side-specific cores with tumor (greater than 33.3% vs. 33.3% or less) and average percent involvement of each positive core (greater than 20% vs. 20% or less) were independent predictors of neurovascular bundle penetration in multivariate analysis.31 Ohori et al. demonstrated that a nomogram constructed with biopsy core laterality only could accurately predict the laterality of ECE.32 In a study of 124 patients who underwent RP for clinically localized cancer diagnosed using individually labeled sextant cores, Tombal et al. concluded that the topography of the positive biopsies was predictive of ECE, specifically, a greater likelihood of organ-confined disease was observed if the cores were from adjacent locations (p<0.01).33 However, the number and topography of positive sextants and the percentage of positive cores correlated almost linearly, suggesting on first analysis that identifying the exact position of the biopsy has no benefit. In a separate study of 223 men undergoing RP, the best predictors of the risk of ECE on a side were an average percentage of biopsy cores positive for cancer overall of 15 or greater (odds ratio 8.4, p<0.0001) and an average from 3 ipsilateral biopsies of 15 or greater (odds ratio 7.4, p<0.0001).34 The sextant-specific percentage of biopsy cores positive for cancer predicted risk of ECE in a sextant (odds ratio 2.5, p<0.020).
Other researchers have evaluated the importance of base and apical positive core sampling. Badalament et al. demonstrated that, in decreasing order, quantitative nuclear grade, preoperative PSA, total percent tumor involvement, number of positive sextant cores, preoperative Gleason score, and involvement of more than 5% of a base and/or apex biopsy were significant (p ≤0.006) for predicting disease organ confinement status.35 Kamat et al. showed that a core tumor length of 7 mm and a positive basal biopsy core of any tumor length and tumor grade predict ipsilateral extraprostatic extension (EPE).36 In a study of 371 men, a positive biopsy at the apex was not predictive of a positive apical SM or EPE, but a positive biopsy at the base was predictive of a positive basal SM and EPE.37 A positive SM, in turn, correlated with EPE on final pathology. A positive basal SM correlated with EPE in 75% of cases, whereas a positive apical SM showed EPE in only 33% of cases (P<0.02).
Prediction of Surgical Margin Status
The location of cancer can influence the likelihood of a positive SM at the time of resection. The ability of a positive biopsy location to predict the risk of SM violation probably depends on the predictive accuracy of the biopsy with regard to location. Rogatsch et al. correlated apical biopsies with the apical prostate cancer in the final surgical specimens and found the PPV of a single positive apical core for identifying tumor location correctly in the prostatectomy specimen was 71.1%, whereas the absence of cancer in the apical biopsy had an NPV of 75.5%.24 Sensitivity was 44.5% for a positive biopsy core. In this context, the predictive value of an individual positive apical core biopsy was only 28.8% for SM positivity at the apex.
Influence of Core Number on Pathologic Analysis
A substantial literature suggests that placing more cores in a specimen container reduces the likelihood of cancer detection and the accuracy of cancer assessment, possibly because of tissue tangling, fragmentation, and inability to align tissue fragments at the time of sectioning (Figure 1). In a retrospective analysis of data on 1,448 men who underwent a 6–12-core prostate needle biopsy, Gupta et al. compared 515 biopsy specimens submitted in 1 or 2 containers to 933 biopsy specimens submitted in 6–12 containers.38 Monthly equivocal diagnoses were less frequent in the 6–12-container group than in the 1–2-container group (2.8% vs. 6.0%, respectively, p=0.003). The use of 6–12 containers also significantly reduced rates of atypical glands suspicious for adenocarcinoma (p=0.042) and high-grade prostatic intraepithelial neoplasia with adjacent atypical gland suspicious for adenocarcinoma (p=0.038) compared to the 1–2-container group. Reis et al. demonstrated that pathologists often receive more cores than the number sampled by the urologist and suggested that these changes are due to core fragmentation.39 In their study, biopsies resulted in 21.54 (±3.56) cores, whereas pathologists examined 24.08 (±4.77, p<0.01) cores. Core numbers by all prostate gland areas (such as right and left base, mid-gland, and apex) were statistically different between biopsy and pathological examination reports (p<0.01). Fajardo et al. also evaluated factors that can lead to core fragmentation.40 They examined 463 biopsies that contained prostatic adenocarcinoma in fragmented cores, as well as 200 control sets lacking fragmented cores. The mean number of cores per specimen container was significantly higher in the fragmented group than in the unfragmented group (2.6 vs. 2.1, respectively, p=0.004). The mean number of containers with cancer in the fragmented group was significantly higher, at 2.8 (1–13), than in the unfragmented group, at 1.6 (1–13, p<0.001). Mean Gleason score was 6.6 (6–10) in the fragmented group and 6.2 (6–10) in the unfragmented group, p<0.001. The authors concluded that the number of cores per container, presence of cancer, and increased Gleason score all contribute to the likelihood of tissue fragmentation.
Figure 1.
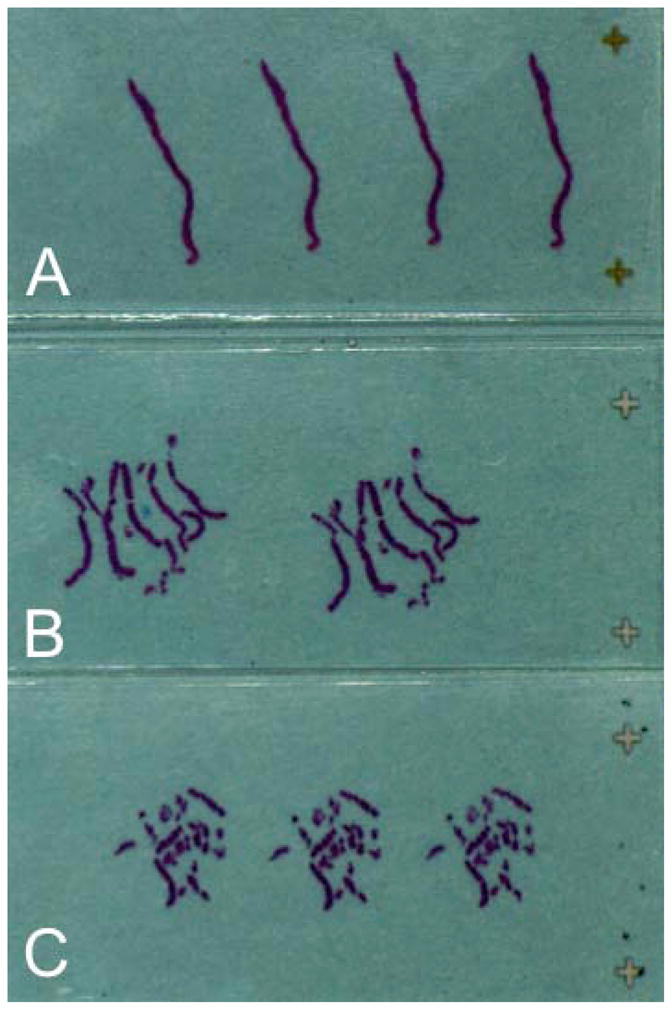
One prostate biopsy core submitted in one specimen container and embedded in one cassette (A). Multiple prostate biopsy cores submitted in the same specimen container and embedded in one cassette (B, C). (Image courtesy of Ming Zhou MD, PhD, Department of Pathology, New York University).
Despite the observation that the placement of multiple cores in a single container compromises pathologic evaluation, no consensus opinion exists on how many cores can be safely placed in a container to allow adequate pathologic analysis. Researchers have demonstrated that simultaneously including 3 biopsy cores in the same cassette can lead to the loss of a mean length of 1.15 cm of assessable tissue, which corresponds to the average length of one prostate biopsy.41 In addition, computer simulation of a biopsy demonstrated that packaging multiple ipsilateral biopsies in a single container often entangles the specimens and can result in loss of 40% of the tissue surface area with only a 5-degree shift in the angle of the needle biopsy within the tissue block. This probably increases the rate of equivocal biopsies, resulting in the need for repeat biopsies.42
Some authors have proposed alternative methods to overcome tissue entangling and fragmentation. Pre-embedding cores that were stretched and oriented between two nylon meshes led to a higher frequency of cancer diagnosis, reduction in the number of cases with atypical foci, and significantly lower number of cancers diagnosed in only one core.43 Firoozi et al. bundled two adjacent cores in a single container and marked the lateral core in each container with India ink.44 Thirteen of 64 (20%) men undergoing RP had ECE and 10 (15%) had a positive SM. The location of ECE and positive SM on whole-mount specimens correlated with a positive biopsy site in 70% and 60% of men, respectively. The tissue-labeling protocol used did not increase procedure time or introduce any tissue artifacts.
If cores are not individually labeled, specimen numbers per jar can be reduced using a strategy for grouping cores when submitting specimens. One potential labeling strategy when packaging up to two cores in each jar is to separate cores from the right and left lobe and label those from the base (one core), mid-gland (one core), apex (two cores, from the medial and lateral locations), and far-lateral zone (two cores, from the mid-gland and base) (Figure 2A). When this strategy is used, eight specimen jars containing no more than two cores per jar are submitted. An alternative methodology is to use six specimen containers, each containing two cores, for the medial and lateral locations in the base, mid-gland, and apex on each side. Inking the lateral core in each container can provide additional information (Figure 2B). One core in the jar for orientation could, in fact, be inked when using any grouping method. Additional containers with image-directed samples or nodule-directed samples might be indicated in some cases.
Figure 2.
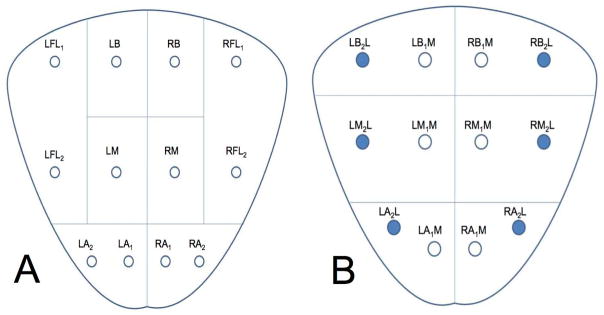
Potential labeling strategies when packaging up to two prostate biopsy cores in a single specimen container using a total of either 8 (A) or 6 (B) containers. Inking the lateral core in each container can provide additional information regarding orientation (B). LFL; left far lateral, RFL; right far lateral, LB; left base, RB; right base, LM; left mid, RM; right mid, LA; left apex, RA; right apex, LBL; left base lateral, RBL; right base lateral, LML; left mid lateral, RML right mid lateral, LMM; left mid medial, RMM; right mid medial, LAL; left apex lateral, RAL; right apex lateral, LAM; left apex medial, RAM; right apex medial.
CONCLUSIONS
An optimized diagnostic prostate biopsy allows maximal cancer detection, avoidance of a repeat biopsy, and adequate information for both identifying men who need therapy and planning that therapy. Ideally, such a biopsy minimizes the detection of occult, indolent prostate cancers that are unlikely to reduce the patient’s longevity. In performing a biopsy, these goals appear to be best achieved through a 12-core systematic sampling methodology that incorporates apical and far-lateral cores in the template distribution. The results of our literature review suggest that collecting more than 12 cores or sampling the transition zone offer no benefit for initial diagnostic biopsies. However, such approaches might be useful for resampling following a negative biopsy, when indicated, and for planning the use of novel therapeutic approaches, such as focal ablation. In some cases, at the discretion of the individual urologist, less rigorous sampling might be indicated. Simple sextant biopsies might be sufficient to obtain tissue confirmation for a diagnosis in obvious locally advanced or metastatic disease.
Most of the literature reviewed for this paper does not suggest that knowing the exact site of an individual positive biopsy core provides meaningful clinical information for determining the location of ECE or a potentially positive SM. The literature does strongly support the necessity of determining the laterality of cancer on biopsy for both predicting sites of ECE and therapeutic planning.
The pathology literature suggests that increasing the number of cores in a specimen jar leads to increased tissue fragmentation, tangling of cores, and reduced tissue sampling, which can reduce CDRs and increase the likelihood of equivocal diagnoses (such as atypical small acinar proliferation). Although the literature does not identify the maximum number of cores that should be packaged in a single container, including fewer cores in each container appears to improve detection outcomes. We recommend packaging no more than two cores in each jar based on our assessment of the literature. Site-specific knowledge of disease location can be obtained by inking one of the two cores in the specimen without affecting the quality of the tissue assessment.
Acknowledgments
The authors would like to thanks Ming Zhou, MD, PhD for providing images of the prostate biopsy slides.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chrouser KL, Lieber MM. Extended and saturation needle biopsy for the diagnosis of prostate cancer. Curr Urol Rep. 2004;5:226. doi: 10.1007/s11934-004-0041-7. [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network. [Accessed February 10, 2013];NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Prostate Cancer Early Detection. 2012 Version 1.2012. Available at http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 3.Bertaccini A, Fandella A, Prayer-Galetti T, et al. Systematic development of clinical practice guidelines for prostate biopsies: a 3-year Italian project. Anticancer Res. 2007;27:659. [PubMed] [Google Scholar]
- 4.El-Hakim A, Moussa S. CUA guidelines on prostate biopsy methodology. Can Urol Assoc J. 2010;4:89. doi: 10.5489/cuaj.10021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Egevad L, Allsbrook WC, Jr, Epstein JI. Current practice of diagnosis and reporting of prostate cancer on needle biopsy among genitourinary pathologists. Hum Pathol. 2006;37:292. doi: 10.1016/j.humpath.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Lane RB, Jr, Lane CG, Mangold KA, et al. Needle biopsies of the prostate: what constitutes adequate histologic sampling? Arch Pathol Lab Med. 1998;122:833. [PubMed] [Google Scholar]
- 7.Babaian RJ, Toi A, Kamoi K, et al. A comparative analysis of sextant and an extended 11-core multisite directed biopsy strategy. J Urol. 2000;163:152. [PubMed] [Google Scholar]
- 8.Eichler K, Hempel S, Wilby J, et al. Diagnostic value of systematic biopsy methods in the investigation of prostate cancer: a systematic review. J Urol. 2006;175:1605. doi: 10.1016/S0022-5347(05)00957-2. [DOI] [PubMed] [Google Scholar]
- 9.Guichard G, Larré S, Gallina A, et al. Extended 21-sample needle biopsy protocol for diagnosis of prostate cancer in 1000 consecutive patients. Eur Urol. 2007;52:430. doi: 10.1016/j.eururo.2007.02.062. [DOI] [PubMed] [Google Scholar]
- 10.de la Taille A, Antiphon P, Salomon L, et al. Prospective evaluation of a 21-sample needle biopsy procedure designed to improve the prostate cancer detection rate. Urology. 2003;61:1181. doi: 10.1016/s0090-4295(03)00108-0. [DOI] [PubMed] [Google Scholar]
- 11.Ploussard G, Nicolaiew N, Marchand C, et al. Prospective evaluation of an extended 21-core biopsy scheme as initial prostate cancer diagnostic strategy. Eur Urol. doi: 10.1016/j.eururo.2012.05.049. Epub ahead of print June 9, 2012. [DOI] [PubMed] [Google Scholar]
- 12.Meng MV, Franks JH, Presti JC, Jr, et al. The utility of apical anterior horn biopsies in prostate cancer detection. Urologic oncology. 2003;21:361. doi: 10.1016/s1078-1439(03)00031-0. [DOI] [PubMed] [Google Scholar]
- 13.Moussa AS, Meshref A, Schoenfield L, et al. Importance of additional “extreme” anterior apical needle biopsies in the initial detection of prostate cancer. Urology. 2010;75:1034. doi: 10.1016/j.urology.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 14.Bazinet M, Karakiewicz PI, Aprikian AG, et al. Value of systematic transition zone biopsies in the early detection of prostate cancer. J Urol. 1996;155:605. [PubMed] [Google Scholar]
- 15.Chen ME, Troncoso P, Johnston DA, et al. Optimization of prostate biopsy strategy using computer based analysis. J Urol. 1997;158:2168. doi: 10.1016/s0022-5347(01)68188-6. [DOI] [PubMed] [Google Scholar]
- 16.Levine MA, Ittman M, Melamed J, et al. Two consecutive sets of transrectal ultrasound guided sextant biopsies of the prostate for the detection of prostate cancer. J Urol. 1998;159:471. doi: 10.1016/s0022-5347(01)63951-x. [DOI] [PubMed] [Google Scholar]
- 17.Lane BR, Zippe CD, Abouassaly R, et al. Saturation technique does not decrease cancer detection during followup after initial prostate biopsy. J Urol. 2008;179:1746. doi: 10.1016/j.juro.2008.01.049. [DOI] [PubMed] [Google Scholar]
- 18.Orikasa K, Ito A, Ishidoya S, et al. Anterior apical biopsy: is it useful for prostate cancer detection? Int J Urol. 2008;15:900. doi: 10.1111/j.1442-2042.2008.02106.x. [DOI] [PubMed] [Google Scholar]
- 19.Terris MK, Pham TQ, Issa MM, et al. Routine transition zone and seminal vesicle biopsies in all patients undergoing transrectal ultrasound guided prostate biopsies are not indicated. J Urol. 1997;157:204. [PubMed] [Google Scholar]
- 20.Elabbady AA, Khedr MM. Extended 12-core prostate biopsy increases both the detection of prostate cancer and the accuracy of Gleason score. Eur Urol. 2006;49:49. doi: 10.1016/j.eururo.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 21.Mian BM, Lehr DJ, Moore CK, et al. Role of prostate biopsy schemes in accurate prediction of Gleason scores. Urology. 2006;67:379. doi: 10.1016/j.urology.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 22.San Francisco IF, DeWolf WC, Rosen S, et al. Extended prostate needle biopsy improves concordance of Gleason grading between prostate needle biopsy and radical prostatectomy. J Urol. 2003;169:136. doi: 10.1016/S0022-5347(05)64053-0. [DOI] [PubMed] [Google Scholar]
- 23.Kahl P, Wolf S, Adam A, et al. Saturation biopsy improves preoperative Gleason scoring of prostate cancer. Pathol Res Pract. 2009;205:259. doi: 10.1016/j.prp.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 24.Rogatsch H, Horninger W, Volgger H, et al. Radical prostatectomy: the value of preoperative, individually labeled apical biopsies. J Urol. 2000;164:754. doi: 10.1097/00005392-200009010-00031. [DOI] [PubMed] [Google Scholar]
- 25.Haarer CF, Gopalan A, Tickoo SK, et al. Prostatic transition zone directed needle biopsies uncommonly sample clinically relevant transition zone tumors. J Urol. 2009;182:1337. doi: 10.1016/j.juro.2009.06.042. [DOI] [PubMed] [Google Scholar]
- 26.Singh H, Canto EI, Shariat SF, et al. Six additional systematic lateral cores enhance sextant biopsy prediction of pathological features at radical prostatectomy. J Urol. 2004;171:204. doi: 10.1097/01.ju.0000100220.46419.8b. [DOI] [PubMed] [Google Scholar]
- 27.Meng MV, Elkin EP, DuChane J, et al. Impact of increased number of biopsies on the nature of prostate cancer identified. J Urol. 2006;176:63. doi: 10.1016/S0022-5347(06)00493-9. [DOI] [PubMed] [Google Scholar]
- 28.Haas GP, Delongchamps NB, Jones RF, et al. Needle biopsies on autopsy prostates: sensitivity of cancer detection based on true prevalence. J Natl Cancer Inst. 2007;99:1484. doi: 10.1093/jnci/djm153. [DOI] [PubMed] [Google Scholar]
- 29.Taneja SS, Penson DF, Epelbaum A, et al. Does site specific labeling of sextant biopsy cores predict the site of extracapsular extension in radical prostatectomy surgical specimen? J Urol. 1999;162:1352. [PubMed] [Google Scholar]
- 30.Naya Y, Ochiai A, Troncoso P, et al. A comparison of extended biopsy and sextant biopsy schemes for predicting the pathological stage of prostate cancer. J Urol. 2004;171:2203. doi: 10.1097/01.ju.0000127729.71350.7f. [DOI] [PubMed] [Google Scholar]
- 31.Tsuzuki T, Hernandez DJ, Aydin H, et al. Prediction of extraprostatic extension in the neurovascular bundle based on prostate needle biopsy pathology, serum prostate specific antigen and digital rectal examination. J Urol. 2005;173:450. doi: 10.1097/01.ju.0000151370.82099.1a. [DOI] [PubMed] [Google Scholar]
- 32.Ohori M, Kattan MW, Koh H, et al. Predicting the presence and side of extracapsular extension: a nomogram for staging prostate cancer. J Urol. 2004;171:1844. doi: 10.1097/01.ju.0000121693.05077.3d. [DOI] [PubMed] [Google Scholar]
- 33.Tombal B, Tajeddine N, Cosyns JP, et al. Does site-specific labelling and individual processing of sextant biopsies improve the accuracy of prostate biopsy in predicting pathological stage in patients with T1c prostate cancer? BJU Int. 2002;89:543. doi: 10.1046/j.1464-410x.2002.02672.x. [DOI] [PubMed] [Google Scholar]
- 34.Elliott SP, Shinohara K, Logan SL, et al. Sextant prostate biopsies predict side and sextant site of extracapsular extension of prostate cancer. J Urol. 2002;168:105. [PubMed] [Google Scholar]
- 35.Badalament RA, Miller MC, Peller PA, et al. An algorithm for predicting nonorgan confined prostate cancer using the results obtained from sextant core biopsies with prostate specific antigen level. J Urol. 1996;156:1375. [PubMed] [Google Scholar]
- 36.Kamat AM, Jacobsohn KM, Troncoso P, et al. Validation of criteria used to predict extraprostatic cancer extension: a tool for use in selecting patients for nerve sparing radical prostatectomy. J Urol. 2005;174:1262. doi: 10.1097/01.ju.0000173914.26476.7c. [DOI] [PubMed] [Google Scholar]
- 37.Touma NJ, Chin JL, Bella T, et al. Location of a positive biopsy as a predictor of surgical margin status and extraprostatic disease in radical prostatectomy. BJU Int. 2006;97:259. doi: 10.1111/j.1464-410X.2006.05968.x. [DOI] [PubMed] [Google Scholar]
- 38.Gupta C, Ren JZ, Wojno KJ. Individual submission and embedding of prostate biopsies decreases rates of equivocal pathology reports. Urology. 2004;63:83. doi: 10.1016/j.urology.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 39.Reis LO, Reinato JA, Silva DC, et al. The impact of core biopsy fragmentation in prostate cancer. Int Urol Nephrol. 2010;42:965. doi: 10.1007/s11255-010-9720-0. [DOI] [PubMed] [Google Scholar]
- 40.Fajardo DA, Epstein JI. Fragmentation of prostatic needle biopsy cores containing adenocarcinoma: the role of specimen submission. BJU Int. 2010;105:172. doi: 10.1111/j.1464-410X.2009.08737.x. [DOI] [PubMed] [Google Scholar]
- 41.Yfantis HG, Loffe OB, Silverberg SG. Prostate core biopsies processing: evaluating current practice. United States and Canadian Academy of Pathology Annual Meeting; Chicago, Illinois. 2002. pp. 347–1447. [Google Scholar]
- 42.Kao J, Upton M, Zhang P, et al. Individual prostate biopsy core embedding facilitates maximal tissue representation. J Urol. 2002;168:496. [PubMed] [Google Scholar]
- 43.Rogatsch H, Moser P, Volgger H, et al. Diagnostic effect of an improved preembedding method of prostate needle biopsy specimens. Hum Pathol. 2000;31:1102. doi: 10.1053/hupa.2000.9837. [DOI] [PubMed] [Google Scholar]
- 44.Firoozi F, Nazeer T, Fisher HA, et al. Tissue-marking scheme for a cost-effective extended prostate biopsy protocol. Urologic oncology. 2009;27:21. doi: 10.1016/j.urolonc.2007.09.002. [DOI] [PubMed] [Google Scholar]


