Abstract
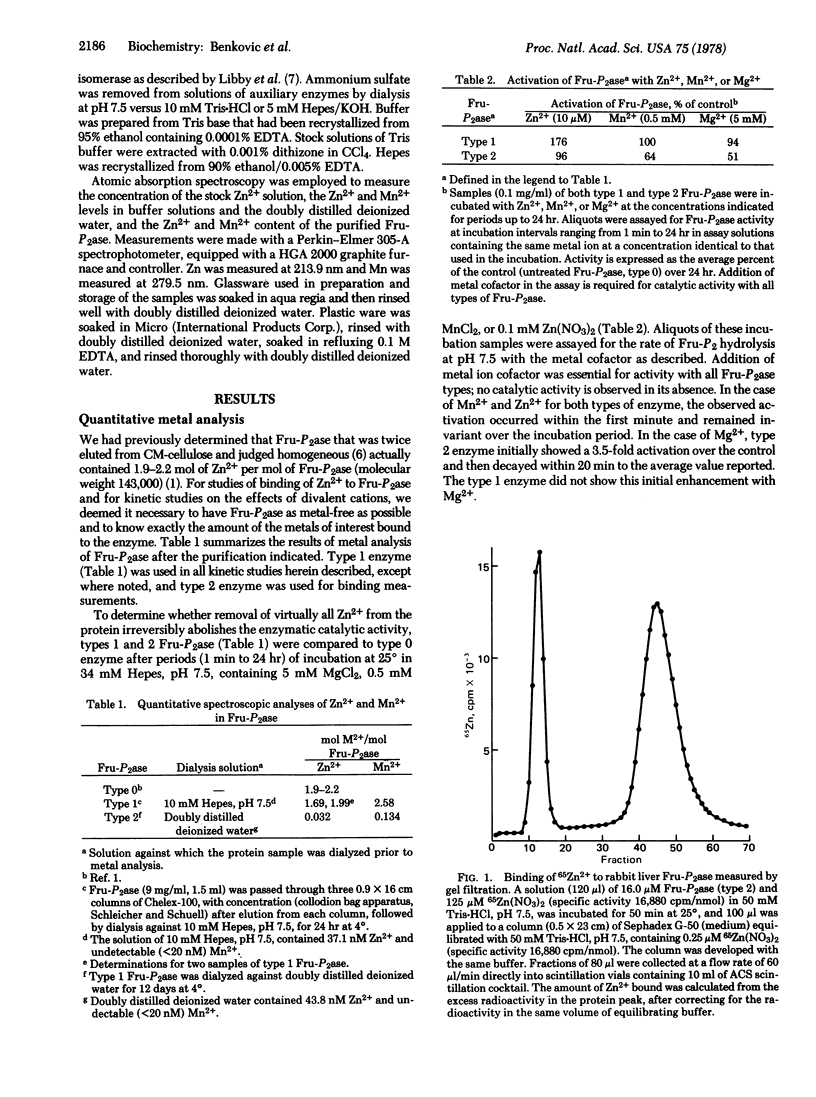
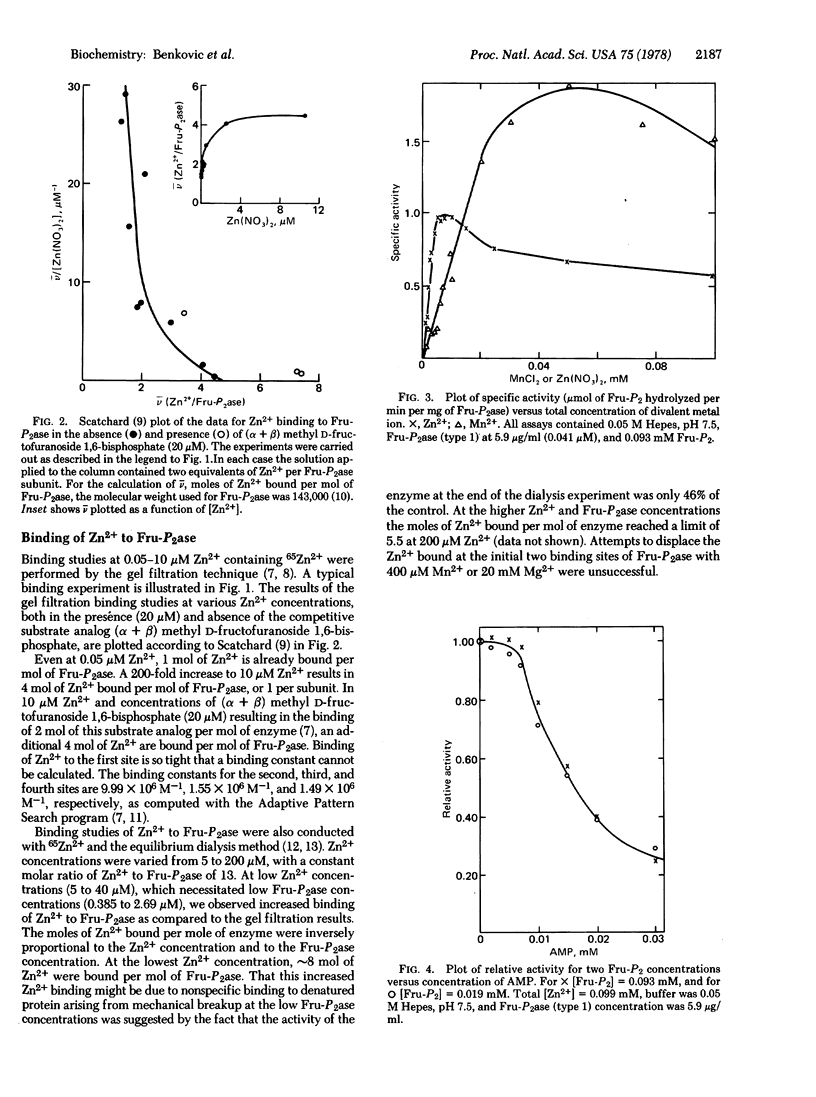
Atomic absorption determinations of zinc content were employed to demonstrate the technique to obtain zinc-free rabbit liver fructose-1,6-bisphosphatase (D-fructose-1,6-bisphosphate 1-phosphohydrolase, EC 3.1.3.11). Reactivation of the apoenzyme by Zn2+ is rapid (within 1 min) and restores up to 96% of the initial specific activity. Gel filtration measurements showed that the enzyme contains four binding sites for zinc per molecule, one per subunit. The dissociation constants for the initial two binding sites are less than 0.1 μM. In the presence of a substrate analog, (α + β) methyl D-fructofuranoside 1,6-bisphosphate, at a level where two analog molecules are bound per phosphatase molecule, a total of eight Zn2+ ions bind at 8 μM Zn2+, revealing the presence of additional binding sites, including the catalytic one. The activity in the presence of Zn2+ is maximal at ca. 8 μM Zn2+, which corresponds to saturation of the four subunit sites plus the catalytic sites in the presence of substrate. At metal ion concentrations less than 10 μM, the order of activation is Zn2+ > Mn2+ > Mg2+. In kinetic assays with two metal cofactors the effect of Zn2+ at concentrations less than 10 μM on either the Mg2+ or the Mn2+ assays is inhibitory owing to the apparent formation of mixed (two different elements) metal ion-enzyme complexes possessing a catalytic activity that is measureable but lower than anticipated if the catalysis by the various metal ions is simply additive. Hence the activation by EDTA of the Mg2+ and Mn2+ assays is explicable in terms of Zn2+ removal, thus eliminating mixed metal species. Collectively these observations suggest that fructose-1,6-bisphosphatase may function in vivo as a Zn2+ metalloprotein.
Keywords: Mg2+, Mn2+, EDTA, apoenzyme, mixed metal ion-enzyme complexes
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benkovic S. J., Frey W. A., Libby C. B., Villafranca J. J. The role of tryptophan in neutral fructose diphosphatase. Biochem Biophys Res Commun. 1974 Mar 15;57(1):196–203. doi: 10.1016/s0006-291x(74)80376-1. [DOI] [PubMed] [Google Scholar]
- Kirtley M. E., Dix J. C. Activation of fructose diphosphatase by manganese, magnesium and cobalt. Arch Biochem Biophys. 1971 Dec;147(2):647–652. doi: 10.1016/0003-9861(71)90423-1. [DOI] [PubMed] [Google Scholar]
- Libby C. B., Frey W. A., Villafranca J. J., Benkovic S. J. Kinetic and binding studies of Mn (II) and fructose 1,6-bisphosphate with rabbit liver hexosebisphosphatase. J Biol Chem. 1975 Oct 10;250(19):7564–7573. [PubMed] [Google Scholar]
- Nimmo H. G., Tipton K. F. The purification of fructose 1,6-diphosphatase from ox liver and its activation by ethylenediaminetetra-acetate. Biochem J. 1975 Feb;145(2):323–334. doi: 10.1042/bj1450323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrosa F. O., Pontremoli S., Horecker B. L. Binding of Zn2+ to rat liver fructose-1,6-bisphosphatase and its effect on the catalytic properties. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2742–2745. doi: 10.1073/pnas.74.7.2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THIERS R. E., VALLEE B. L. Distribution of metals in subcellular fractions of rat liver. J Biol Chem. 1957 Jun;226(2):911–920. [PubMed] [Google Scholar]
- Tejwani G. A., Pedrosa F. O., Pontremoli S., Horecker B. L. Dual role of Zn2+ as inhibitor and activator of fructose 1,6-bisphosphatase of rat liver. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2692–2695. doi: 10.1073/pnas.73.8.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traniello S., Pontremoli S., Tashima Y., Horecker B. L. Fructose 1, 6-diphosphatase from liver: isolation of the native form with optimal activity at neutral pH. Arch Biochem Biophys. 1971 Sep;146(1):161–166. doi: 10.1016/s0003-9861(71)80052-8. [DOI] [PubMed] [Google Scholar]
- Ulm E. H., Pogell B. M., De Maine M. M., Libby C. B., Benkovic S. J. Fructose-1, 6-diphosphatase from rabbit liver. Methods Enzymol. 1975;42:369–374. doi: 10.1016/0076-6879(75)42143-7. [DOI] [PubMed] [Google Scholar]


