Abstract
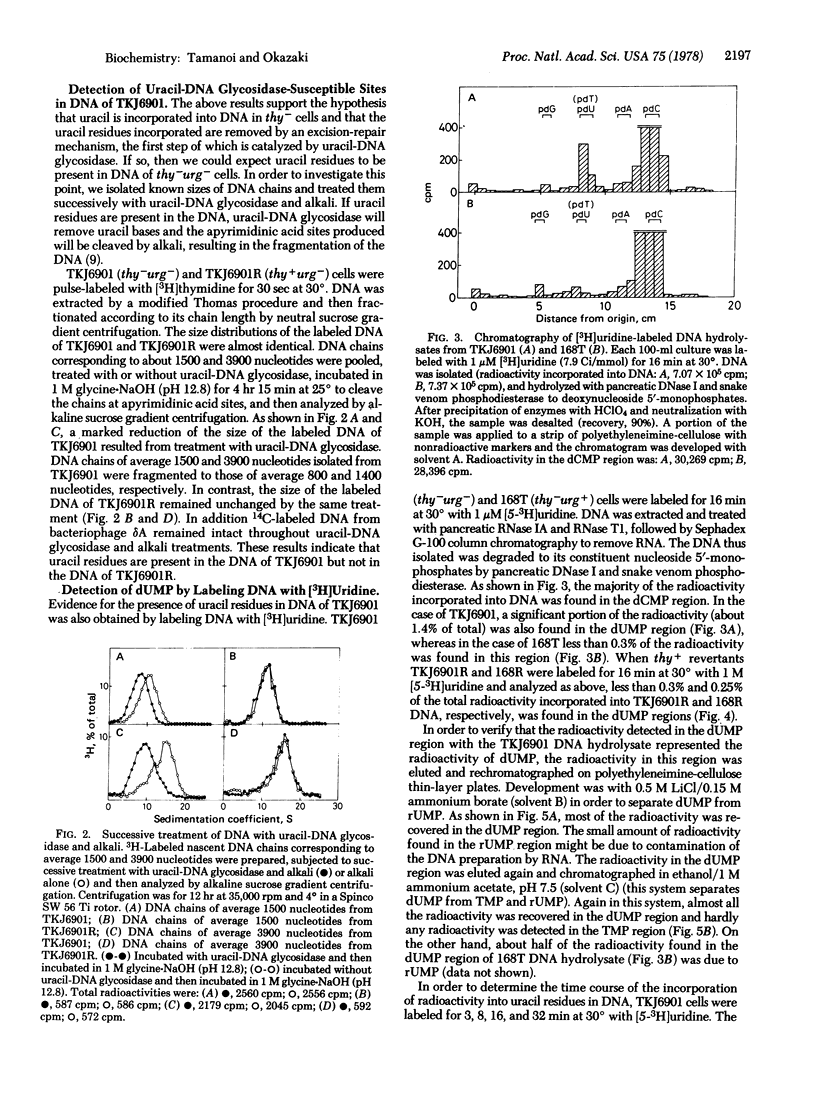
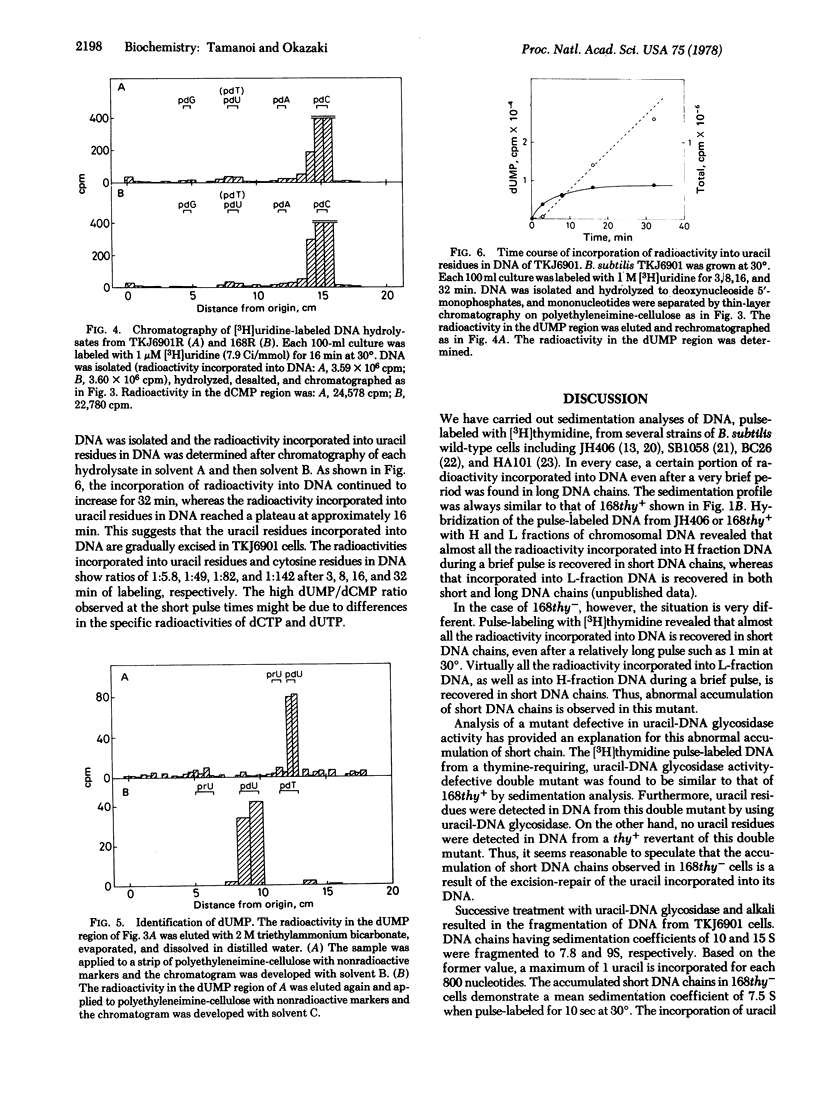
A thymine-requiring mutant of Bacillus subtilis strain 168 accumulates short DNA chains after brief pulses with [3H]thymidine. Reversion of the thy mutation to thy+ abolishes the accumulation of short DNA chains, suggesting that the accumulation is related to the thy mutation. The reason for this accumulation has been further investigated by analysis of a mutant with a defective uracil-DNA glycosidase activity (urg). The accumulation of short DNA chains in thy- cells is abolished by the deficiency of uracil-DNA glycosidase activity. In thy+ cells, the deficiency of the glycosidase activity does not change the sedimentation profile of pulse-labeled DNA. DNA isolated from thy-urg- cells is fragmented by successive treatment with purified uracil-DNA glycosidase and alkali, indicating that uracil residues are present in this DNA. DNA isolated from thy+urg- cells is not fragmented by the same treatment. Significant radioactivity is detected in the dUMP region, when [3H]uridine-labeled DNA from thy-urg- cells is hydrolyzed and analyzed by thin-layer chromatography. Only a trace amount of radioactivity, which is not influenced by the deficiency of uracil-DNA glycosidase activity, is found in the dUMP region in DNA hydrolysates from thy+ cells. These results suggest that, in thy- cells, uracil is incorporated into DNA and the accumulation of short DNA chains results from the excision-repair of this uracil whereas in thy+ cells, uracil is seldom, if ever, incorporated into DNA.
Keywords: accumulation of short DNA chains, thy- and thy- cells, uracil-DNA glycosidase, deoxyuridylate
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cone R., Duncan J., Hamilton L., Friedberg E. C. Partial purification and characterization of a uracil DNA N-glycosidase from Bacillus subtilis. Biochemistry. 1977 Jul 12;16(14):3194–3201. doi: 10.1021/bi00633a024. [DOI] [PubMed] [Google Scholar]
- Gass K. B., Cozzarelli N. R. Further genetic and enzymological characterization of the three Bacillus subtilis deoxyribonucleic acid polymerases. J Biol Chem. 1973 Nov 25;248(22):7688–7700. [PubMed] [Google Scholar]
- Gass K. B., Hill T. C., Goulian M., Strauss B. S., Cozzarelli N. R. Altered deoxyribonucleic acid polymerase activity in a methyl methanesulfonate-sensitive mutant of Bacillus subtilis. J Bacteriol. 1971 Oct;108(1):364–374. doi: 10.1128/jb.108.1.364-374.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman M. M., Hicks M. L., Gellert M. Genetics and function of DNA ligase in Escherichia coli. J Mol Biol. 1973 Jul 15;77(4):531–547. doi: 10.1016/0022-2836(73)90221-0. [DOI] [PubMed] [Google Scholar]
- Isolation and characterization of a Bacillus subtilis mutant with a defective N-glycosidase activity for uracil-containing deoxyribonucleic acid. J Bacteriol. 1977 Aug;131(2):438–445. doi: 10.1128/jb.131.2.438-445.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad E. B., Lehman I. R. A conditional lethal mutant of Escherichia coli K12 defective in the 5' leads to 3' exonuclease associated with DNA polymerase I. Proc Natl Acad Sci U S A. 1974 May;71(5):2048–2051. doi: 10.1073/pnas.71.5.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad E. B., Modrich P., Lehman I. R. Genetic and enzymatic characterization of a conditional lethal mutant of Escherichia coli K12 with a temperature-sensitive DNA ligase. J Mol Biol. 1973 Jul 15;77(4):519–529. doi: 10.1016/0022-2836(73)90220-9. [DOI] [PubMed] [Google Scholar]
- Kuempel P. L., Veomett G. E. A possible function of DNA polymerase in chromosome replication. Biochem Biophys Res Commun. 1970 Nov 25;41(4):973–980. doi: 10.1016/0006-291x(70)90180-4. [DOI] [PubMed] [Google Scholar]
- Kurosawa Y., Ogawa T., Hirose S., Okazaki T., Okazaki R. Mechanism of DNA chain growth. XV. RNA-linked nascent DNA pieces in Escherichia coli strains assayed with spleen exonuclease. J Mol Biol. 1975 Aug 25;96(4):653–664. doi: 10.1016/0022-2836(75)90144-8. [DOI] [PubMed] [Google Scholar]
- Laipis P. J., Ganesan A. T. A deoxyribonucleic acid polymerase I-deficient mutant of Bacillus subtilis. J Biol Chem. 1972 Sep 25;247(18):5867–5871. [PubMed] [Google Scholar]
- Lindahl T. An N-glycosidase from Escherichia coli that releases free uracil from DNA containing deaminated cytosine residues. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3649–3653. doi: 10.1073/pnas.71.9.3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T., Andersson A. Rate of chain breakage at apurinic sites in double-stranded deoxyribonucleic acid. Biochemistry. 1972 Sep 12;11(19):3618–3623. doi: 10.1021/bi00769a019. [DOI] [PubMed] [Google Scholar]
- Mirzabekov A. D., Griffin B. E. 5 s RNA conformation. Studies of its partial T 1 ribonuclease digestion by gel electrophoresis and two-dimensional thin-layer chromatography. J Mol Biol. 1972 Dec 30;72(3):633–643. doi: 10.1016/0022-2836(72)90181-7. [DOI] [PubMed] [Google Scholar]
- Okazaki R., Arisawa M., Sugino A. Slow joining of newly replicated DNA chains in DNA polymerase I-deficient Escherichia coli mutants. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2954–2957. doi: 10.1073/pnas.68.12.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutberg B., Hoch J. A. Citric acid cycle: gene-enzyme relationships in Bacillus subtilis. J Bacteriol. 1970 Nov;104(2):826–833. doi: 10.1128/jb.104.2.826-833.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi M., Hayakawa H., Makino F., Tanaka K., Okada Y. A human enzyme that liberates uracil from DNA. Biochem Biophys Res Commun. 1976 Nov 22;73(2):293–299. doi: 10.1016/0006-291x(76)90706-3. [DOI] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamanoi F., Okazaki T., Okazaki R. Persistence of RNA attached to nascent short DNA pieces in Bacillus subtilis cells defective in DNA polymerase I. Biochem Biophys Res Commun. 1977 Jul 11;77(1):290–297. doi: 10.1016/s0006-291x(77)80195-2. [DOI] [PubMed] [Google Scholar]
- Tye B. K., Nyman P. O., Lehman I. R., Hochhauser S., Weiss B. Transient accumulation of Okazaki fragments as a result of uracil incorporation into nascent DNA. Proc Natl Acad Sci U S A. 1977 Jan;74(1):154–157. doi: 10.1073/pnas.74.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]


