Abstract
Purpose
Increasing step rate has been shown to elicit changes in joint kinematics and kinetics during running, and has been suggested as a possible rehabilitation strategy for runners with patellofemoral pain. The purpose of this study was to determine how altering step rate affects internal muscle forces and patellofemoral joint loads, and then to determine what kinematic and kinetic factors best predict changes in joint loading.
Methods
We recorded whole body kinematics of 30 healthy adults running on an instrumented treadmill at three step rate conditions (90%, 100%, and 110% of preferred step rate). We then used a 3D lower extremity musculoskeletal model to estimate muscle, patellar tendon, and patellofemoral joint forces throughout the running gait cycles. Additionally, linear regression analysis allowed us to ascertain the relative influence of limb posture and external loads on patellofemoral joint force.
Results
Increasing step rate to 110% of preferred reduced peak patellofemoral joint force by 14%. Peak muscle forces were also altered as a result of the increased step rate with hip, knee and ankle extensor forces, and hip abductor forces all reduced in mid-stance. Compared to the 90% step rate condition, there was a concomitant increase in peak rectus femoris and hamstring loads during early and late swing, respectively, at higher step rates. Peak stance phase knee flexion decreased with increasing step rate, and was found to be the most important predictor of the reduction in patellofemoral joint loading.
Conclusion
Increasing step rate is an effective strategy to reduce patellofemoral joint forces and could be effective in modulating biomechanical factors that can contribute to patellofemoral pain.
Keywords: Patellofemoral Pain, Cadence, Knee, Stride Length, Rehabilitation
Introduction
Running is a very popular mode of exercise around the world, with over 13.9 million people participating in the US alone (28). Despite its health benefits, injury due to long distance running is frequent, with studies reporting injury incidence rates as high as 79% within a six month period (18, 34). The most common site of injury is the knee, with patellofemoral pain being the most frequent complaint (32). Prevention and treatment of this type of pain are essential for keeping runners active.
The cause of patellofemoral pain has been described as multifactorial, and many biomechanical risk factors have been identified as possible contributors. These factors include kinematic abnormalities, patellar maltracking, overuse, and excessive compressive stresses on the patellofemoral joint cartilage (8, 11, 15, 19–20). Net biomechanical loading at the patellofermoral joint, a major determinant of cartilage stress, is estimated to reach 4.5–7.6 times body weight during running (2, 10, 27, 29), which is higher than most other everyday activities (2, 25). Hence, finding a method to reduce the magnitude of the patellofemoral joint force during running may be effective in mitigating patellofemoral pain for runners.
Prior work has shown that increasing running step rate, while keeping a constant forward velocity, significantly alters lower extremity joint kinetics and kinematics. For example, increasing step rate by 5–10% above preferred will reduce stance phase knee flexion angle and knee extension torque (14). Conversely, reducing step rate, i.e. over-striding, tends to increase these variables. In addition to the biomechanical changes, this strategy has been found beneficial in reducing pain and increasing training ability in runners with patellofemoral pain (3, 37). As both knee flexion and quadriceps muscle forces are recognized as primary factors affecting patellofemoral compression (39), an increase in step rate may be a simple means of modulating internal joint loading.
The objective of this study was to assess changes in muscle and patellofemoral loading with systematic variations in step rate in healthy runners. We hypothesized that increasing step rate would decrease patellofemoral joint force, with the net reduction arising from changes in external loading and limb posture. This hypothesis was tested by using dynamic musculoskeletal models to estimate internal muscle and joint contact loads from kinematic and kinetic measures collected during treadmill running at step rates of 90%, 100%, and 110% of preferred.
Methods
Participants
Thirty healthy subjects (15 males, mean ± SD, 33 ± 14 years, 68.6 ± 10.9 kg, 1.75 ± 0.11 m) agreed to participate in the study. All subjects were recreational runners (running at least 24 km/week for 3 months, 44 ± 21 km/week) who were currently pain-free while running and had not previously undergone lower extremity surgery or sustained a leg injury in the past three months. The protocol was approved by the University of Wisconsin-Madison’s Health Sciences Institutional Review Board, and all volunteers gave written informed consent prior to participation.
Experimental Protocol
Each participant’s preferred step rate was first determined during the final minute of a five-minute treadmill run at his/her preferred speed. Participants were then asked to perform a series of randomly ordered running trials at their preferred speed at three specified step rates: preferred step rate (100%), 10% greater than preferred (110%) and 10% less than preferred (90%). All step rates were maintained using an audible metronome. Whole body kinematics were recorded for 15 seconds during each of the running trials using a passive eight-camera motion capture system (Motion Analysis Corporation, Santa Rosa, CA). A total of 40 markers were tracked at 200 Hz, including 21 markers on anatomical landmarks (8 upper extremity, 5 pelvis, 8 lower extremity), and 14 tracking markers adhered to rigid plates that were strapped to the thigh and shank segments. Marker data was subsequently low-pass filtered at 12 Hz using generalized cross-validation splines (38). Ground reaction forces were simultaneously recorded at 2000 Hz using an instrumented treadmill (Bertec Corporation, Columbus, OH), and then low-pass filtered at 50 Hz, also using generalized cross-validation splines (38).
Computational modeling
A 3D 29 degree-of-freedom (DOF) whole body model was used to analyze joint kinematics and kinetics during running. The pelvis was the base segment with six DOF. Each lower limb included a three DOF ball-and-socket representation of the hip, a one DOF ankle, a one DOF tibiofemoral joint and a one DOF patellofemoral joint. Rolling and gliding at the tibiofemoral joint were accounted for by specifying the tibiofemoral translations and non-sagittal tibiofemoral rotations as constrained functions of the knee flexion angle (1). The patella was assumed to translate within a fixed path relative to the femur with the patella position determined assuming a constant patella tendon length (1). The hip joint center in the pelvic reference frame was then calibrated using a hip circumduction task and a functional joint center identification routine (16). All other segment dimensions in the model were scaled to each subject using anatomical marker positions measured in a standing upright trial. These calibration trials included use of 10 additional markers, with 8 of 10 in the lower extremity. We analyzed lower extremity muscle and joint loading using a musculoskeletal model that included geometric descriptions of the patellar tendon and 92 additional musculotendon units acting about the low back, hip, knee and ankle joints (1). Musculotendon attachment points were scaled in proportion to the factors used to scale the segments to which they were attached.
The generalized coordinates of the model were first calculated at each frame of a running trial using a global optimization inverse kinematics routine, which minimized the weighted sum of squared differences between measured and model marker positions (17). Patella translation was computed assuming that the patella tendon length was constant (5.5 cm in the nominal model) (5). Generalized coordinates were then fit with 5th order generalized cross-validation splines (38), and then differentiated to ascertain the generalized speeds and accelerations. The equations of motion describing whole body linked segment dynamics were derived using SIMM/Pipeline (Musculographics Inc, Santa Rosa, CA) and SDFast (Parametric Technology Corporation, Needham, MA). Muscle forces were assumed to vary linearly with muscle activation from zero to the maximum isometric force for that muscle, i.e. F=aFo, where a is a muscle’s activation level and Fo is the maximum isometric force (1). At each frame of motion, numerical optimization was used to estimate lower extremity muscle and patellar tendon forces by determining the activations that generated the measured accelerations at the hip, tibiofemoral, patellofemoral, and ankle joints while minimizing a weighted sum of squared muscle activations (13). The weighting factor for each muscle was taken as that muscle’s volume, which was the product of the muscle’s optimal fiber length and physiological cross-sectional area. Accelerations at the lower back and upper extremity joints were prescribed to measured values. To assess the veracity of the model estimates, the average muscle force patterns over a gait cycle were cross-correlated with ensemble EMG data collected from the vastus lateralis, rectus femoris, tibialis anterior, medical gastrocnemius, biceps femoris, semimembranosus, gluteus medius and gluteus maximus. These EMG data were previously presented in Chumanov et al (4), and included the subjects analyzed in this study. Cross-correlations were computed at varying lags between EMG and force to account for electromechanical delays, and the lag resulting in the peak correlation was used.
Upon computation of the muscle forces, the multi-body dynamics model was queried for the net reaction force vector acting across the patellofemoral joint. The magnitude of the patellofemoral loading per unit body weight (BW) was computed at each time frame. The patellofemoral loading rate was then determined by numerically differentiating (central difference) the force magnitude-time curve. The patellofemoral impulse was determined by numerically integrating (trapezoidal integration) the force magnitude-time curve. The specific metrics extracted from these curves were: peak patellofemoral force, peak patellofemoral loading rate, and the patellofemoral impulse during stance. Peak muscle forces during stance and swing were also extracted from each stride, and subsequently normalized to body weight. Five right footed strides were analyzed for each subject at each step rate condition.
Repeated measures ANOVA, with both step rate and stride number being repeated factors, was used to compare of peak muscle forces and patellofemoral loading metrics between the different step rate conditions. Post-hoc analyses were performed using Tukey’s Honest Significance Tests. The criterion significance level was set to p=0.05. Finally, univariate regression analysis determined whether patellofemoral force was more closely associated with posture or load characteristics. Lines of best fit and coefficients of determination were computed with peak patellofemoral force magnitude as the dependent variable. Maximum knee flexion angle during stance and peak vertical ground reaction force were used as independent variables in separate analyses. All statistical analyses were performed using STATISTICA 6.1 (Statsoft, Inc, Tulsa, OK).
Results
Subjects’ preferred running speeds ranged from 2.4 to 3.8 m/s (mean ± SD = 2.81 ± 0.38 m/s) and preferred step rates ranged from 156–192 steps per minute (174 ± 9).
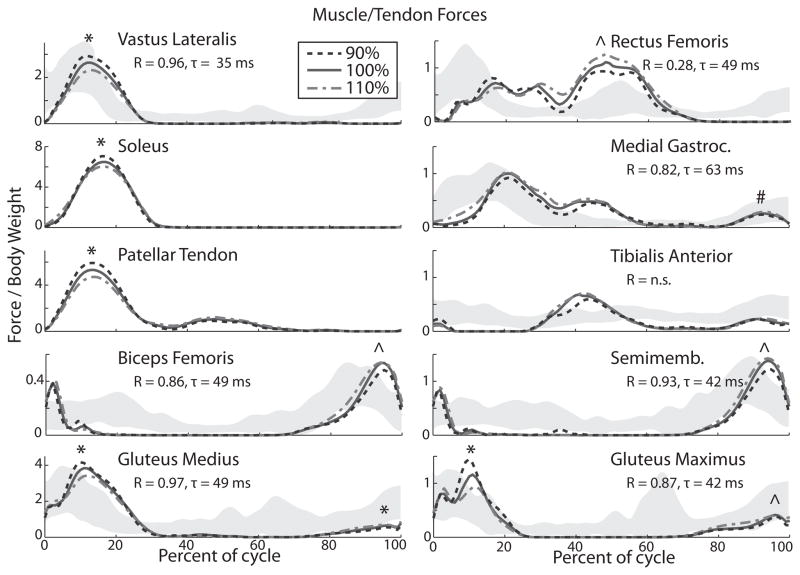
Model-based estimates of muscle force patterns generally agreed well with EMG data over the gait cycle (4) (Fig. 1). Specifically, there was good temporal agreement between EMG bursts and the phasing of peak muscle forces in the vastus lateralis, gastrocnemius, and gluteal muscles during stance, with the rectus femoris during early swing and with the hamstring muscles during late swing. After accounting for electromechanical delays, the correlation between average EMG and force patterns were greatest for the vastus lateralis, medial gastrocnemius, biceps femoris, semimembranosus, gluteus medius and gluteus maximus muscles (R=0.82–0.97 at a preferred step rate, p<0.0001). A lower, but significant, correlation was seen for the rectus femoris (R=0.28), where the model predicted greatest loading in early swing while the EMG data indicates greater activation during early and mid-stance. Correlations between estimated force and EMG patterns were not significant for the tibialis anterior muscle.
Figure 1.
Average muscle force trajectories per unit body weight across a gait cycle for each of the step rate conditions. The grey shaded regions reflect the EMG (mean ± 1 s.d.) patterns measured by Chumanov et al. at a preferred step rate (4). The peak correlations (R) and associated lag (τ) between EMG and force data are given for the average preferred step rate. All listed correlations are significant to p≤0.005 (n.s. = not significant). Significant step rate effects on peak muscle forces are denoted (* = all conditions are significantly different from one another, p<0.05: ^ = 90% vs. 100% and 90% vs. 110% are significantly different, p<0.05: # = 110% vs. 90% significantly different from one another, p<0.05)
Step rate did not substantially alter the temporal patterns of lower extremity muscle forces, but did modulate muscle force magnitudes (Fig. 1, Table 1). Notably, increasing step rate led to significant reductions in vasti, gluteal, soleus, and patellar tendon forces during stance (all conditions, p<0.01). In late stance/early swing, increasing step rate (from the 90% condition) led to higher rectus femoris forces (90 vs. 100%, and 90 vs. 110%: p<0.05). In late swing, increasing step rate resulted in higher peak gastrocnemius (90% vs. 110%: p<0.001), hamstring (90 vs. 100%, and 90 vs. 110%: p<0.0005), and gluteal muscle forces (medius: all comparisons, p<0.005, maximus: 90 vs. 100%, and 90 vs. 110%: p<0.001). Patellar tendon force also changed with step rate. The increased (110%) step rate induced an 11% lower peak patellar tendon force in midstance than the preferred (100%) condition (4.87 vs. 5.49 BW, p<0.0005), while the decreased step rate (90%) resulted in a 12% higher peak patellar tendon force than preferred (6.13 vs. 5.49 BW, p<0.0005).
Table 1.
Peak muscle forces per unit body weight, mean (standard deviation) across all subjects. Percentages denoted for medial gastrocnemius and gluteal muscles represent the windows in the gait cycle where the peak was analyzed.
| Muscle | 90% | 100% | 110% | |
|---|---|---|---|---|
| Vastus Lateralis | 3.03 (0.38)* | 2.71 (0.40) | 2.39 (0.43)*^ | |
| Rectus Femoris | 1.14 (0.27)* | 1.32 (0.51) | 1.41 (0.32)^ | |
| Soleus | 7.30 (1.26)* | 6.65 (1.24) | 6.18 (1.28)*^ | |
| Medial Gastrocnemius | 0–40% | 1.05 (0.34) | 1.16 (0.40) | 1.11 (0.31) |
| 80–99% | 0.28 (0.09) | 0.30 (0.06) | 0.32 (0.08)^ | |
| Patellar Tendon | 6.13 (0.88)* | 5.49 (0.88) | 4.87 (0.91)*^ | |
| Tibialis Anterior | 0.65 (0.18) | 0.80 (0.60) | 0.75 (.20) | |
| Biceps Femoris | 0.51 (0.10)* | 0.57 (0.11) | 0.57 (0.10)^ | |
| Semimembranosus | 1.29 (0.25)* | 1.44 (0.25) | 1.49 (0.24)^ | |
| Gluteus Medius | 0–40% | 4.31 (0.69)* | 3.99 (0.47) | 3.57 (0.45)*^ |
| 80–99% | 0.65 (0.20)* | 0.76 (0.24) | 0.84 (0.26)*^ | |
| Gluteus Maximus | 0–40% | 1.52 (0.29)* | 1.30 (0.27) | 1.16 (0.21)*^ |
| 80–99% | 0.43 (0.12)* | 0.48 (0.13) | 0.50 (0.14)^ | |
= condition significantly different than preferred (100%) with p<0.05,
= 110% condition is significantly different than 90% condition with p<0.05.
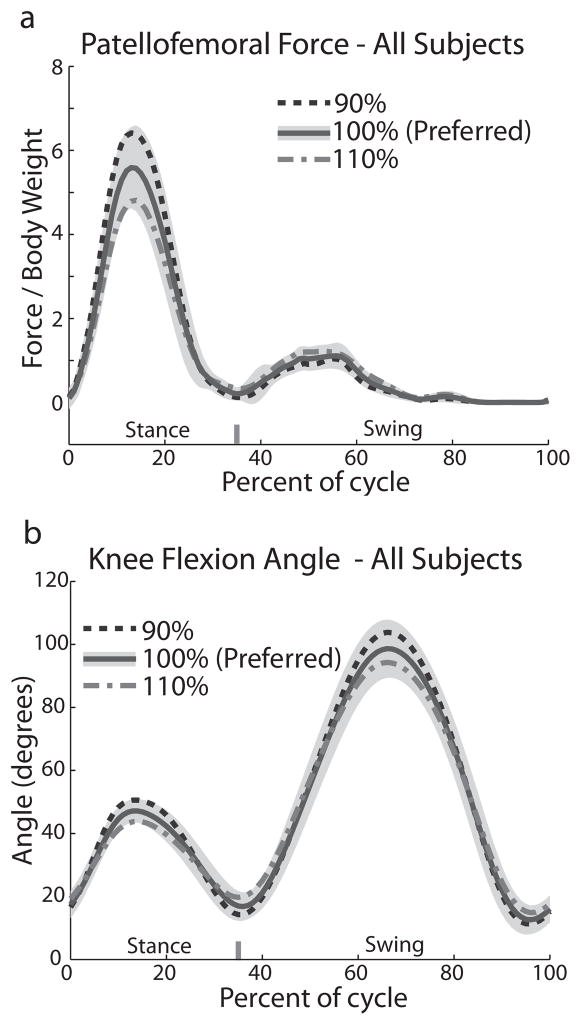
Peak patellofemoral force occurred in mid-stance, at 14.5 (± 1.9) percent of the overall gait cycle (Fig. 2) which is well aligned with the time of peak stance phase knee flexion (14.8 ± 1.6% of the gait cycle). A second smaller peak in patellofemoral loading occurred just after toe-off, and corresponded with rectus femoris loading during initial swing. Peak patellofemoral force magnitude was inversely proportional to step rate, with the highest step rate (110% of preferred) having the lowest patellofemoral force (Fig. 3). The predicted net joint force at the preferred step rate condition averaged 5.76 ± 1.02 times body weight (BW). The 110% condition has a peak force that was 14% lower (4.96 ± 1.05 BW, p<0.0005), while the 90% step rate condition has a peak force that was 15% higher (6.64 ± 1.06 BW, p<0.0005). Patellofemoral loading rate and impulse also decreased with a higher step rate. At the 110% condition, peak loading rate was 11% lower than the preferred condition (0.96 BW/s vs. 1.08 BW/s, p<0.005) and stance phase impulse was 20% lower (0.51 BW*s vs. 0.63 BW*s, p<0.0005). Conversely, the 90% condition led to a 6.4% increase in peak loading rate compared to the preferred condition (1.15 BW/s vs. 1.08 BW/s, p<0.01) and a 27% increase in stance phase impulse (0.81 BW*s vs. 0.63 BW*s, p<0.0005).
Figure 2.
a) Patellofemoral force magnitude and b) knee flexion angle across one running stride, averaged across all subjects. Lines represent the mean of all strides and the shaded region represents the standard deviation of all strides for the preferred (100%) condition. Standard deviation magnitude and profile was similar among conditions (not shown).
Figure 3.
a) Peak patellofemoral force, b) loading rate, c) stance-phase knee flexion angle and d) patellofemoral stance-phase impulse. Mean (connected dots) and standard deviation (error bars). * = p<0.01
Kinematic changes were also observed, with an increase in step rate from 100 to 110% preferred leading to an average 3.3 degree (7.0%) decrease in stance phase peak knee flexion, a 2.5 degree (8.4%) decrease in ankle dorsiflexion at mid-stance, and a 1.8 degree (10.6%) increase in knee flexion at initial contact (all variables, p<0.005 - data not shown). Peak vertical ground reaction forces decreased by 2.6% when increasing step rate from 100 to 110% of preferred (p<0.005). Anterior ground reaction force also changed with step rate, with the increased step rate leading to a 5.5% decrease in the maximum magnitude (p<0.005).
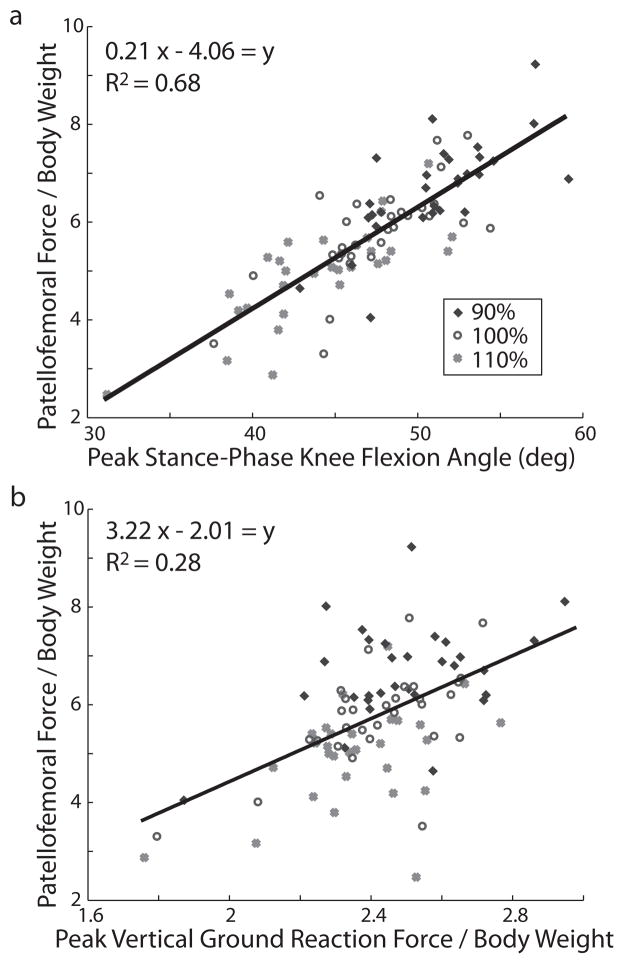
Linear regression revealed that knee flexion angle was the univariate predictor most closely associated with patellofemoral force, with 68% of the variance in peak patellofemoral force being explained by the maximum knee flexion angle during stance (Fig. 4, p<0.001). A one degree increase in peak knee flexion led to a 0.21 BW increase in patellofemoral force. Peak vertical ground reaction forces were less associated with the peak patellofemoral force (R2=0.28, p<0.001).
Figure 4.
Univariate regression analyses. Patellofemoral force is plotted vs. a) peak knee flexion angle and b) peak vertical ground reaction force. Each dot represents the average for each subject for a given condition. Line of best fit is also shown along with its equation and R2 value.
Discussion
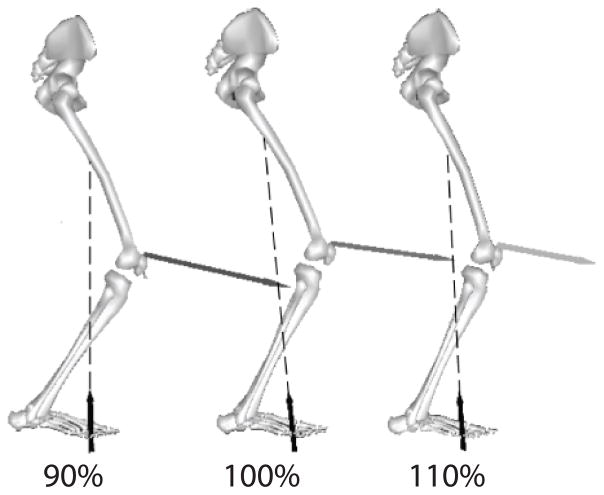
This study used a 3D modeling based approach to predict how muscle and patellofemoral joint forces change with step rate during running. The results support our hypothesis that increasing step rate, while maintaining speed, can substantially diminish patellofemoral joint loading (Fig. 5). The reduction in joint compressive load primarily arises from altered muscular coordination, which places the knee in a more extended posture during mid-stance. The strong relationship between patellofemoral load and knee flexion (Fig. 4) indicates that posture is an important feature associated with increasing or decreasing patellofemoral force. Hence, step rate manipulation could be an effective way of addressing patellofemoral pain that arises from excessive joint loading.
Figure 5.
Representative subject with depiction of limb position, ground reactions and patellofemoral joint force at the 90% (left), preferred (middle), and 110% (right) step rate conditions, shown at the time of peak patellofemoral force.
Our estimates of patellofemoral joint loading during running are comparable to values reported by others. Several studies have used sagittal plane models to estimate patellofemoral forces during running, with peak forces ranging anywhere from 4.3 to 7.6 BW (10, 27, 29). Our average patellofemoral force estimates ranged from 5.0 (110%) to 6.6 BW (90%) across a fairly broad range of running speeds (2.4–3.8 m/s) and step rates (140–211 steps per minute). A recent 3D modeling study estimated peak patellofemoral forces of 5.9 BW in runners at a preferred cadence and speed (2), which is very close to our estimate of the mean force at the preferred step rate of 5.8 BW. Notably, these patellofemoral loads are much higher than that seen in other locomotor activities such as walking (0.5–1.0 BW (2, 25)), stair climbing (3.3–3.5 BW (2, 25)), and backward running (3.0–3.4 BW (10, 27)).
In addition to these patellofemoral changes, our results suggest that increasing step rate leads to decreased extensor muscle forces during stance, an increase in rectus femoris forces in early swing, and an increase in gastrocnemius, hamstring, and gluteus maximus forces in late swing. The decrease extensor loading in stance is likely related to the more extended limb posture adopted at higher step rates. The increase in rectus femoris and hamstring forces presumably result from the greater inertial forces involved with initiating and braking swing limb motion at higher step rates (23). The step rate influences on muscle force patterns generally mirror the EMG results reported by Chumanov et al. (Fig. 1 - same running speed) (4) and Swanson et. al. (faster running speeds) (31), which increases our confidence that the step rate modulation effects are an accurate representation of changes in the runners’ muscle coordination. Also, patellar tendon forces paralleled the patellofemoral forces, with the maximum force occurring in mid-stance and at the lowest step rate condition. Moreover, the pattern and magnitude of the patellar tendon force demonstrated here is similar to what has been predicted by others (29), further supporting our results. While these findings give insight into muscular activation/force changes at different step rates, future work is needed to determine how individual muscles contribute to patellofemoral force (21).
This study focused on step rate as a method of modifying running form. We recognize that varying step rate has an inverse effect on step length when speed is kept constant, and thus step length could alternatively be considered predictive of the biomechanical changes seen in this study. However, it is challenging to train and subsequently maintain a desired step length, especially when subjects are fatigued. Alternatively, a simple audible metronome can be used to quickly and easily vary step rate (or cadence) by small increments. This metronome, in combination with a watch or GPS to monitor overall running pace, could be easily implemented to reproduce the experimental conditions.
A byproduct of step rate manipulation is a decrease in stance duration and increase in loading cycles, if running speed and distance are both maintained constant. The decrease in stance duration would act to increase patellofemoral loading rate. However, we found that increasing step rate by 10% resulted in a 11% reduction in peak patellofemoral joint loading rate, primarily due to the 14% decrease in force magnitude. To assess the influence of increased number of cycles, one can consider the net impulse accumulated over time. When we did such analyses, we found that the cumulative patellofemoral load impulse would be 5.5% lower in the 110% step rate condition, if the same distance was traversed. Hence, we conclude that patellofemoral force magnitude, loading rate, and impulse are all diminished when increasing running step rate by 10%.
While we have shown that we can use musculoskeletal models to estimate patellofemoral forces during running, practically it would be beneficial to have a simpler way of doing so. Our regression analyses showed that in fact peak knee flexion angle at mid-stance is a good predictor of patellofemoral force. This implies that knee flexion could be a simple metric to monitor clinically to indirectly assess patellofemoral joint loading that exists in a runner. An increase in patellofemoral force with increasing knee flexion during activity has been described previously (7, 30, 33), however the implications in running analysis were unknown. Future work will be done to determine if this result extends beyond healthy runners to those with patellofemoral pain. Interestingly, it has been noted that those with patellofemoral pain do exhibit lower peak knee flexion during stance, and is thought to represent a compensation technique (6).
Results of this study have potential clinical relevance in treating runners with patellofemoral pain. High loads have long been suggested as a possible cause of anterior knee pain (11, 15), with the belief that high net loads give rise to large cartilage stress. Indeed, a recent biomechanical modeling study found that patellofemoral stress estimates were elevated in individuals reporting patellofemoral pain (8). Therefore, reducing patellofemoral load by modulating step rate might be an effective way to mitigate cartilage compressive stress, and hence diminish pain symptoms while maintaining the ability to run. Future work will determine if increasing step rate has the same affect on step rate in those with patellofemoral pain, and if it is successful at reducing symptoms.
While there are novel insights that can be gained from this study, we realize that there are some limitations to the work. One limitation of this study is the use of a one degree of freedom patellofemoral joint. Some theories of anterior knee pain include a maltracking patella which would require a more sophisticated patellofemoral contact model to address. Further, the lack of a cartilage contact model inhibits us from commenting on variations in cartilage tissue stress and contact area through the running cycle. Changes in contact area may be important in stress alteration (36), and possibly pain reduction. Also, we assumed that the patellar tendon was inextensible, which ignores the variations in patellar tendon line of action that can occur with tendon stretch. The patellar tendon is estimated to stretch 5–10% with maximal quadriceps loading (12, 22, 24), such that small changes in quadriceps loading with step rate (12%) would likely have a small effect on patella position between step rate conditions. We performed a sensitivity study of the influence of patellar tendon length on results for a representative subject. These analyses revealed that absolute patellofemoral loads vary with patellar tendon length, but the percent difference in patellofemoral force between step rate conditions varied less than 2% for assumed patellar tendon lengths ranging from 4.5 to 6.5 cm. This result suggests that step rate variations in patellofemoral loading are relatively insensitive to the pre-assumed patellar tendon length. Results may also have been affected by data collection methods. For convenience, all running analyses were performed on a treadmill rather than overground (9, 26, 35). Finally, it should be made explicit that these analyses were completed using healthy individuals and further work is needed to ascertain if similar effects are seen in individuals with existing patellofemoral pain.
It is pertinent to note that the patellofemoral force estimates cannot be directly validated due to a lack of direct empirical measures of this variable. However, the numerical optimization approach did generate muscle force trajectory estimates that generally agreed well with EMG patterns of the major hip and knee extensors including the vastus lateralis, gluteus maximus and hamstring muscles (Fig. 1). EMG-force correlation for the rectus femoris was lower (R=0.28), but still significant (p=0.005). The lower correlation was a result of the rectus femoris EMG data exhibiting a greater burst in early stance, while the model predicted relatively greater rectus femoris force at toe-off. There is potential for EMG cross-talk from the vastii onto the rectus femoris EMG recording when using surface electrodes, which could have contributed to this result. There was also no significant agreement between the tibialis anterior EMG pattern and its estimated force trajectory, but the tibialis anterior is not a major contributor to knee extensor loading such that this likely did not have a major effect on the patellofemoral loading results.
In conclusion, we have shown that increasing step rate alters running form in a way that that can significantly reduce the magnitude and rate of patellofemoral loading. This implies that a prescribed increase in step rate may be a simple strategy to attempt to mitigate patellofemoral pain that arises from excessive force.
Acknowledgments
The project described was supported by the Clinical and Translational Science Award (CTSA) program, previously through the National Center for Research Resources (NCRR) grant 1UL1RR025011, and now by the National Center for Advancing Translational Sciences (NCATS), grant 9U54TR000021 and UL1TR000427. The authors would also like to thank the NSF (grant 0966535), UW Institute for Clinical and Translational Research, UW Medical Scientist Training Program, and UW Sports Medicine Classic for their support.
The results of the present study do not constitute endorsement by the American College of Sports Medicine.
Footnotes
Conflict of Interest:
The authors have no conflicts of interest to disclose.
Disclosure of Funding:
The project described was supported by the Clinical and Translational Science Award (CTSA) program, previously through the National Center for Research Resources (NCRR) grant 1UL1RR025011, and now by the National Center for Advancing Translational Sciences (NCATS), grant 9U54TR000021 and UL1TR000427. The authors would also like to thank the NSF (grant 0966535), and UW Sports Medicine Classic for their support.
References
- 1.Arnold EM, Ward SR, Lieber RL, Delp SL. A model of the lower limb for analysis of human movement. Ann Biomed Eng. 2010;38(2):269–79. doi: 10.1007/s10439-009-9852-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen YJ, Scher I, Powers CM. Quantification of patellofemoral joint reaction forces during functional activities using a subject-specific three-dimensional model. J Appl Biomech. 2010;26(4):415–23. doi: 10.1123/jab.26.4.415. [DOI] [PubMed] [Google Scholar]
- 3.Cheung R, Davis IS. Landing pattern modification to improve patellofemoral pain in runners: a case series. J Orthop Sports Phys Ther. 2011;41(12):914–9. doi: 10.2519/jospt.2011.3771. [DOI] [PubMed] [Google Scholar]
- 4.Chumanov ES, Wille CM, Michalski MP, Heiderscheit BC. Changes in muscle activation patterns when running step rate is increased. Gait Posture. 2012;36(2):231–5. doi: 10.1016/j.gaitpost.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delp SL, Loan JP, Hoy MG, Zajac FE, Topp EL, Rosen JM. An interactive graphics-based model of the lower extremity to study orthopaedic surgical procedures. IEEE Trans Biomed Eng. 1990;37(8):757–67. doi: 10.1109/10.102791. [DOI] [PubMed] [Google Scholar]
- 6.Dierks TA, Manal KT, Hamill J, Davis IS. Proximal and distal influences on hip and knee kinematics in runners with patellofemoral pain during a prolonged run. J Orthop Sports Phys Ther. 2008;38(8):448–56. doi: 10.2519/jospt.2008.2490. [DOI] [PubMed] [Google Scholar]
- 7.Escamilla RF, Zheng N, MacLeod TD, Edwards WB, Hreljac A, Fleisig GS, Wilk KE, Moorman CT, Imamura R. Patellofemoral compressive force and stress during forward and side lunges with and without a stride. Clin Biomech. 2008;23(8):1026–37. doi: 10.1016/j.clinbiomech.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Farrokhi S, Keyak JH, Powers CM. Individuals with patellofemoral pain exhibit greater patellofemoral joint stress: a finite element analysis study. Osteoarthritis Cartilage. 2011;19(3):287–94. doi: 10.1016/j.joca.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fellin RE, Manal K, Davis IS. Comparison of lower extremity kinematic curves during overground and treadmill running. J Appl Biomech. 2010;26(4):407–14. doi: 10.1123/jab.26.4.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flynn T, Soutas-Little R. Patellofemoral joint compressive forces in forward and backward running. J Orthop Sports Phys Ther. 1995;21(5):277–82. doi: 10.2519/jospt.1995.21.5.277. [DOI] [PubMed] [Google Scholar]
- 11.Fredericson M, Powers CM. Practical management of patellofemoral pain. Clin J Sport Med. 2002;12(1):36–8. doi: 10.1097/00042752-200201000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Hansen P, Bojsen-Moller J, Aagaard P, Kjær M, Magnusson SP. Mechanical properties of the human patellar tendon, in vivo. Clin Biomech. 2006;21(1):54–8. doi: 10.1016/j.clinbiomech.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Happee R. Inverse dynamic optimization including muscular dynamics, a new simulation method applied to goal directed movements. J Biomech. 1994;27(7):953–60. doi: 10.1016/0021-9290(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 14.Heiderscheit BC, Chumanov ES, Michalski MP, Wille CM, Ryan MB. Effects of step rate manipulation on joint mechanics during running. Med Sci Sports Exerc. 2011;43(2):296–302. doi: 10.1249/MSS.0b013e3181ebedf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juhn M. Patellofemoral pain syndrome: a review and guidelines for treatment. Am Fam Physician. 1999;60(7):2012–8. [PubMed] [Google Scholar]
- 16.Leardini A, Cappozzo A, Catani F, Toksvig-Larsen S, Petitto A, Sforza V, Cassanelli G, Giannini S. Validation of a functional method for the estimation of hip joint centre location. J Biomech. 1999;32(1):99–103. doi: 10.1016/s0021-9290(98)00148-1. [DOI] [PubMed] [Google Scholar]
- 17.Lu T, O’connor J. Bone position estimation from skin marker co-ordinates using global optimisation with joint constraints. J Biomech. 1999;32(2):129–34. doi: 10.1016/s0021-9290(98)00158-4. [DOI] [PubMed] [Google Scholar]
- 18.Lun V, Meeuwisse W, Stergiou P, Stefanyshyn D. Relation between running injury and static lower limb alignment in recreational runners. British journal of sports medicine. 2004;38(5):576–80. doi: 10.1136/bjsm.2003.005488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pal S, Besier TF, Beaupre GS, Fredericson M, Delp SL, Gold GE. Patellar maltracking is prevalent among patellofemoral pain subjects with patella alta: An upright, weightbearing MRI study. Journal of Orthopaedic Research. 2013;31(3):448–57. doi: 10.1002/jor.22256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pal S, Draper CE, Fredericson M, Gold GE, Delp SL, Beaupre GS, Besier TF. Patellar maltracking correlates with vastus medialis activation delay in patellofemoral pain patients. Am J Sports Med. 2011;39(3):590–8. doi: 10.1177/0363546510384233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pandy MG, Andriacchi TP. Muscle and Joint Function in Human Locomotion. Annu Rev of Biomed Eng. 2010;12:401–33. doi: 10.1146/annurev-bioeng-070909-105259. [DOI] [PubMed] [Google Scholar]
- 22.Pearson SJ, Burgess K, Onambele GNL. Creep and the in vivo assessment of human patellar tendon mechanical properties. Clin Biomech. 2007;22(6):712–7. doi: 10.1016/j.clinbiomech.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Prilutsky BI, Gregor RJ. Swing-and support-related muscle actions differentially trigger human walk–run and run–walk transitions. J Exp Biol. 2001;204(13):2277–87. doi: 10.1242/jeb.204.13.2277. [DOI] [PubMed] [Google Scholar]
- 24.Reeves ND, Maganaris CN, Narici MV. Effect of strength training on human patella tendon mechanical properties of older individuals. J Physiol. 2003;548(3):971–81. doi: 10.1113/jphysiol.2002.035576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reilly DT, Martens M. Experimental analysis of the quadriceps muscle force and patello-femoral joint reaction force for various activities. Acta Orthop Scand. 1972;43(2):126–37. doi: 10.3109/17453677208991251. [DOI] [PubMed] [Google Scholar]
- 26.Riley PO, Dicharry J, Franz J, Croce U, Wilder RP, Kerrigan DC. A kinematics and kinetic comparison of overground and treadmill running. Med Sci Sports Exerc. 2008;40(6):1093–100. doi: 10.1249/MSS.0b013e3181677530. [DOI] [PubMed] [Google Scholar]
- 27.Roos PE, Barton N, van Deursen RRW. Patellofemoral joint compression forces in backward and forward running. J Biomech. 2012;45(9):1656–60. doi: 10.1016/j.jbiomech.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Running USA. 2012 State of the Sport – Part III: U.S. Road Race Trends. 2012 [Google Scholar]
- 29.Scott SH, Winter DA. Internal forces of chronic running injury sites. Med Sci Sports Exerc. 1990;22(3):357–69. [PubMed] [Google Scholar]
- 30.Sharma A, Leszko F, Komistek RD, Scuderi GR, Cates HE, Liu F. In vivo patellofemoral forces in high flexion total knee arthroplasty. J Biomech. 2008;41(3):642–8. doi: 10.1016/j.jbiomech.2007.09.027. [DOI] [PubMed] [Google Scholar]
- 31.Swanson SC, Caldwell GE. An integrated biomechanical analysis of high speed incline and level treadmill running. Med Sci Sports Exerc. 2000;32(6):1146–55. doi: 10.1097/00005768-200006000-00018. [DOI] [PubMed] [Google Scholar]
- 32.Taunton J, Ryan M, Clement D, McKenzie D, Lloyd-Smith D, Zumbo B. A retrospective case-control analysis of 2002 running injuries. Br J Sports Med. 2002;36(2):95–101. doi: 10.1136/bjsm.36.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trepczynski A, Kutzner I, Kornaropoulos E, Taylor WR, Duda GN, Bergmann G, Heller MO. Patellofemoral joint contact forces during activities with high knee flexion. J Orthop Res. 2012;30(3):408–15. doi: 10.1002/jor.21540. [DOI] [PubMed] [Google Scholar]
- 34.Van Gent R, Siem D, van Middelkoop M, Van Os A, Bierma-Zeinstra S, Koes B. Incidence and determinants of lower extremity running injuries in long distance runners: a systematic review. British journal of sports medicine. 2007;41(8):469–80. doi: 10.1136/bjsm.2006.033548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wank V, Frick U, Schmidtbleicher D. Kinematics and electromyography of lower limb muscles in overground and treadmill running. Int J Sports Med. 1998;19(7):455–61. doi: 10.1055/s-2007-971944. [DOI] [PubMed] [Google Scholar]
- 36.Ward SR, Powers CM. The influence of patella alta on patellofemoral joint stress during normal and fast walking. Clin Biomech. 2004;19(10):1040–7. doi: 10.1016/j.clinbiomech.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 37.Wille CM, Chumanov ES, Schubert A, Kempf J, Heiderscheit BC. Running step rate modification to reduce anterior knee pain in runners. J Orthop Sports Phys Ther. 2013;43(1):A119. [Google Scholar]
- 38.Woltring HJ. A FORTRAN package for generalized, cross-validatory spline smoothing and differentiation. Adv Eng Software. 1986;8(2):104–13. [Google Scholar]
- 39.Yamaguchi GT, Zajac FE. A planar model of the knee joint to characterize the knee extensor mechanism. J Biomech. 1989;22(1):1–10. doi: 10.1016/0021-9290(89)90179-6. [DOI] [PubMed] [Google Scholar]