Abstract
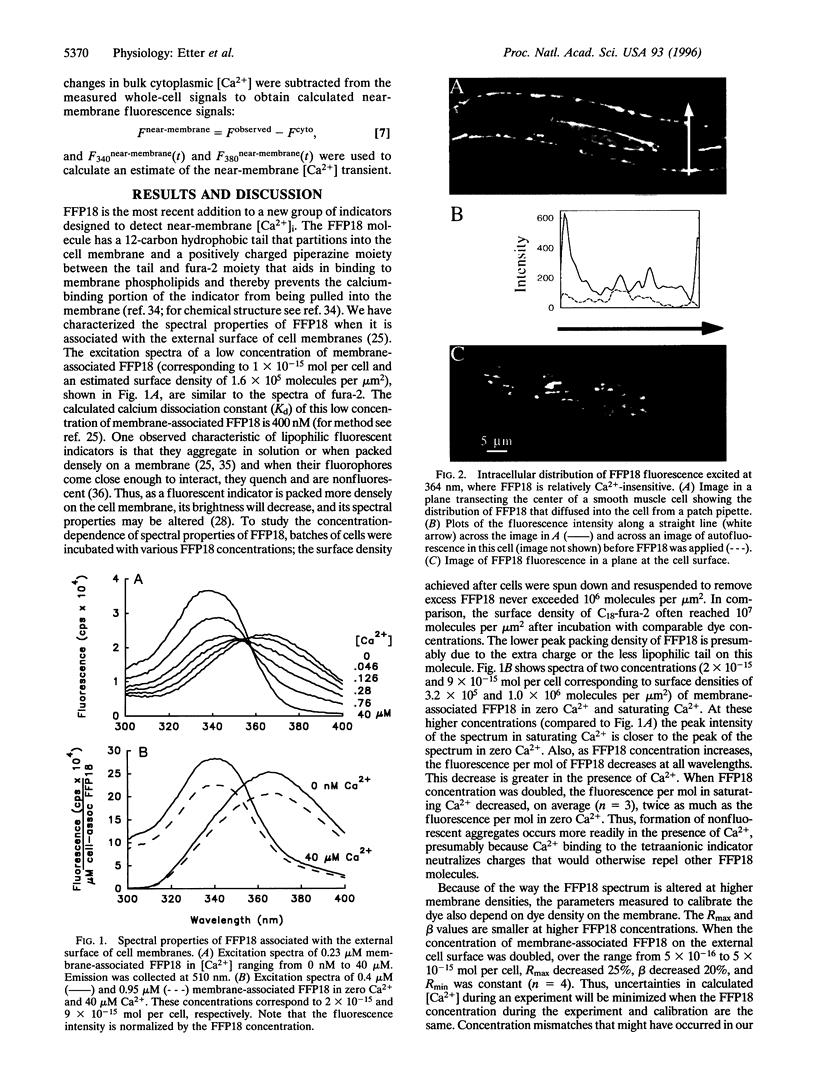
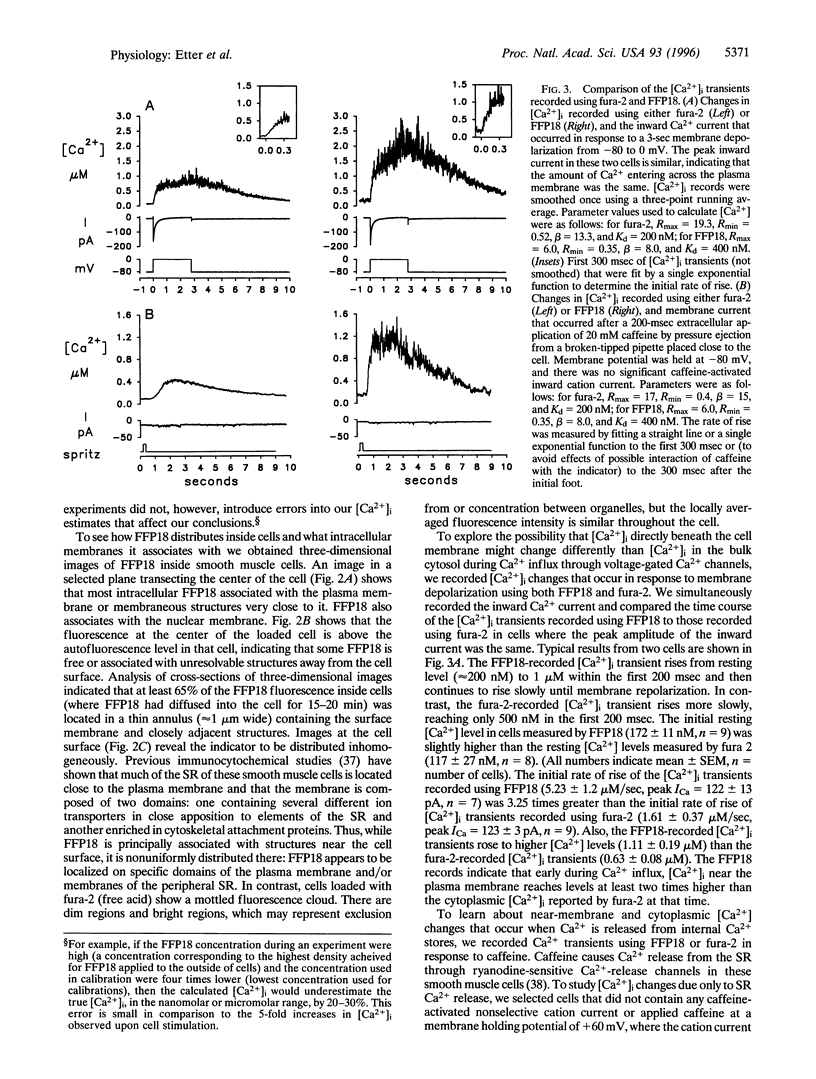
(Ca2+)-sensitive processes at cell membranes involved in contraction, secretion, and neurotransmitter release are activated in situ or in vitro by Ca2+ concentrations ([Ca2+]) 10-100 times higher than [Ca2+] measured during stimulation in intact cells. This paradox might be explained if the local [Ca2+] at the cell membrane is very different from that in the rest of the cell. Soluble Ca2+ indicators, which indicate spatially averaged cytoplasmic [Ca2+], cannot resolve these localized, near-membrane [Ca2+] signals. FFP18, the newest Ca2+ indicator designed to selectively monitor near-membrane [Ca2+], has a lower Ca2+ affinity and is more water soluble than previously used membrane-associating Ca2+ indicators. Images of the intracellular distribution of FFP18 show that >65% is located on or near the plasma membrane. [Ca2+] transients recorded using FFP18 during membrane depolarization-induced Ca2+ influx show that near-membrane [Ca2+] rises faster and reaches micromolar levels at early times when the cytoplasmic [Ca2+], recorded using fura-2, has risen to only a few hundred nanomolar. High-speed series of digital images of [Ca2+] show that near-membrane [Ca2+], reported by FFP18, rises within 20 msec, peaks at 50-100 msec, and then declines. [Ca2+] reported by fura-2 rose slowly and continuously throughout the time images were acquired. The existence of these large, rapid increases in [Ca2+] directly beneath the surface membrane may explain how numerous (Ca2+)-sensitive membrane processes are activated at times when bulk cytoplasmic [Ca2+] changes are too small to activate them.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allbritton N. L., Meyer T., Stryer L. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science. 1992 Dec 11;258(5089):1812–1815. doi: 10.1126/science.1465619. [DOI] [PubMed] [Google Scholar]
- Becker P. L., Fay F. S. Photobleaching of fura-2 and its effect on determination of calcium concentrations. Am J Physiol. 1987 Oct;253(4 Pt 1):C613–C618. doi: 10.1152/ajpcell.1987.253.4.C613. [DOI] [PubMed] [Google Scholar]
- Becker P. L., Singer J. J., Walsh J. V., Jr, Fay F. S. Regulation of calcium concentration in voltage-clamped smooth muscle cells. Science. 1989 Apr 14;244(4901):211–214. doi: 10.1126/science.2704996. [DOI] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B. Spontaneous transient outward currents in single visceral and vascular smooth muscle cells of the rabbit. J Physiol. 1986 Dec;381:385–406. doi: 10.1113/jphysiol.1986.sp016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etter E. F., Kuhn M. A., Fay F. S. Detection of changes in near-membrane Ca2+ concentration using a novel membrane-associated Ca2+ indicator. J Biol Chem. 1994 Apr 1;269(13):10141–10149. [PubMed] [Google Scholar]
- Fay F. S., Hoffmann R., Leclair S., Merriam P. Preparation of individual smooth muscle cells from the stomach of Bufo marinus. Methods Enzymol. 1982;85(Pt B):284–292. doi: 10.1016/0076-6879(82)85027-1. [DOI] [PubMed] [Google Scholar]
- Fogelson A. L., Zucker R. S. Presynaptic calcium diffusion from various arrays of single channels. Implications for transmitter release and synaptic facilitation. Biophys J. 1985 Dec;48(6):1003–1017. doi: 10.1016/S0006-3495(85)83863-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Guerrero A., Fay F. S., Singer J. J. Caffeine activates a Ca(2+)-permeable, nonselective cation channel in smooth muscle cells. J Gen Physiol. 1994 Aug;104(2):375–394. doi: 10.1085/jgp.104.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero A., Singer J. J., Fay F. S. Simultaneous measurement of Ca2+ release and influx into smooth muscle cells in response to caffeine. A novel approach for calculating the fraction of current carried by calcium. J Gen Physiol. 1994 Aug;104(2):395–422. doi: 10.1085/jgp.104.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter T. E., Pfeiffer D. R. Mechanisms by which mitochondria transport calcium. Am J Physiol. 1990 May;258(5 Pt 1):C755–C786. doi: 10.1152/ajpcell.1990.258.5.C755. [DOI] [PubMed] [Google Scholar]
- Gurney A. M., Tsien R. Y., Lester H. A. Activation of a potassium current by rapid photochemically generated step increases of intracellular calcium in rat sympathetic neurons. Proc Natl Acad Sci U S A. 1987 May;84(10):3496–3500. doi: 10.1073/pnas.84.10.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Cruz A., Sala F., Adams P. R. Subcellular calcium transients visualized by confocal microscopy in a voltage-clamped vertebrate neuron. Science. 1990 Feb 16;247(4944):858–862. doi: 10.1126/science.2154851. [DOI] [PubMed] [Google Scholar]
- Herrmann-Frank A., Darling E., Meissner G. Functional characterization of the Ca(2+)-gated Ca2+ release channel of vascular smooth muscle sarcoplasmic reticulum. Pflugers Arch. 1991 May;418(4):353–359. doi: 10.1007/BF00550873. [DOI] [PubMed] [Google Scholar]
- Hume J. R., Leblanc N. Macroscopic K+ currents in single smooth muscle cells of the rabbit portal vein. J Physiol. 1989 Jun;413:49–73. doi: 10.1113/jphysiol.1989.sp017641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M. Calcium-induced calcium release mechanism in guinea pig taenia caeci. J Gen Physiol. 1989 Aug;94(2):363–383. doi: 10.1085/jgp.94.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kargacin G. J. Calcium signaling in restricted diffusion spaces. Biophys J. 1994 Jul;67(1):262–272. doi: 10.1016/S0006-3495(94)80477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer W. J., Niggli E., Hadley R. W. Sodium-calcium exchange in excitable cells: fuzzy space. Science. 1990 Apr 20;248(4953):283–283. doi: 10.1126/science.2326638. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Silver R. B. Microdomains of high calcium concentration in a presynaptic terminal. Science. 1992 May 1;256(5057):677–679. doi: 10.1126/science.1350109. [DOI] [PubMed] [Google Scholar]
- Loew L. M., Tuft R. A., Carrington W., Fay F. S. Imaging in five dimensions: time-dependent membrane potentials in individual mitochondria. Biophys J. 1993 Dec;65(6):2396–2407. doi: 10.1016/S0006-3495(93)81318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missiaen L., Wuytack F., Raeymaekers L., De Smedt H., Droogmans G., Declerck I., Casteels R. Ca2+ extrusion across plasma membrane and Ca2+ uptake by intracellular stores. Pharmacol Ther. 1991;50(2):191–232. doi: 10.1016/0163-7258(91)90014-d. [DOI] [PubMed] [Google Scholar]
- Moore E. D., Etter E. F., Philipson K. D., Carrington W. A., Fogarty K. E., Lifshitz L. M., Fay F. S. Coupling of the Na+/Ca2+ exchanger, Na+/K+ pump and sarcoplasmic reticulum in smooth muscle. Nature. 1993 Oct 14;365(6447):657–660. doi: 10.1038/365657a0. [DOI] [PubMed] [Google Scholar]
- Neher E., Augustine G. J. Calcium gradients and buffers in bovine chromaffin cells. J Physiol. 1992 May;450:273–301. doi: 10.1113/jphysiol.1992.sp019127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala F., Hernández-Cruz A. Calcium diffusion modeling in a spherical neuron. Relevance of buffering properties. Biophys J. 1990 Feb;57(2):313–324. doi: 10.1016/S0006-3495(90)82533-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon S. M., Llinás R. R. Compartmentalization of the submembrane calcium activity during calcium influx and its significance in transmitter release. Biophys J. 1985 Sep;48(3):485–498. doi: 10.1016/S0006-3495(85)83804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims P. J., Waggoner A. S., Wang C. H., Hoffman J. F. Studies on the mechanism by which cyanine dyes measure membrane potential in red blood cells and phosphatidylcholine vesicles. Biochemistry. 1974 Jul 30;13(16):3315–3330. doi: 10.1021/bi00713a022. [DOI] [PubMed] [Google Scholar]
- Singer J. J., Walsh J. V., Jr Characterization of calcium-activated potassium channels in single smooth muscle cells using the patch-clamp technique. Pflugers Arch. 1987 Feb;408(2):98–111. doi: 10.1007/BF00581337. [DOI] [PubMed] [Google Scholar]
- Somlyo A. V., Bond M., Somlyo A. P., Scarpa A. Inositol trisphosphate-induced calcium release and contraction in vascular smooth muscle. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5231–5235. doi: 10.1073/pnas.82.15.5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M. D. Buffering of calcium in the vicinity of a channel pore. Cell Calcium. 1992 Mar;13(3):183–192. doi: 10.1016/0143-4160(92)90046-u. [DOI] [PubMed] [Google Scholar]
- Vivaudou M. B., Singer J. J., Walsh J. V., Jr Multiple types of Ca2+ channels in visceral smooth muscle cells. Pflugers Arch. 1991 Mar;418(1-2):144–152. doi: 10.1007/BF00370463. [DOI] [PubMed] [Google Scholar]
- Vorndran C., Minta A., Poenie M. New fluorescent calcium indicators designed for cytosolic retention or measuring calcium near membranes. Biophys J. 1995 Nov;69(5):2112–2124. doi: 10.1016/S0006-3495(95)80082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wier W. G., Egan T. M., López-López J. R., Balke C. W. Local control of excitation-contraction coupling in rat heart cells. J Physiol. 1994 Feb 1;474(3):463–471. doi: 10.1113/jphysiol.1994.sp020037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Lai F. A., Cohn A., Etter E., Guerrero A., Fay F. S., Meissner G. Evidence for a Ca(2+)-gated ryanodine-sensitive Ca2+ release channel in visceral smooth muscle. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3294–3298. doi: 10.1073/pnas.91.8.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi S., Becker P. L., Fay F. S. Relationship between force and Ca2+ concentration in smooth muscle as revealed by measurements on single cells. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4109–4113. doi: 10.1073/pnas.85.11.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]