Abstract
Objective
SLITRK family proteins control neurite outgrowth and regulate synaptic development. In mice, Slitrk6 plays a role in the survival and innervation of sensory neurons in the inner ear, vestibular apparatus, and retina, and also influences axial eye length. We provide the first detailed description of the auditory phenotype in humans with recessive SLITRK6 deficiency.
Study Design
Prospective observational case study.
Methods
Nine closely related Amish subjects from an endogamous Amish community of Pennsylvania underwent audiologic and vestibular testing. Single nucleotide polymorphism microarrays were used to map the chromosome locus and Sanger sequencing or high-resolution melt analysis were used to confirm the allelic variant.
Results
All 9 subjects were homozygous for a novel nonsense variant of SLITRK6 (c.1240C>T, p.Gln414Ter). Adult patients had high myopia. The 4 oldest SLITRK6 c.1240C>T homozygotes had absent ipsilateral middle ear muscle reflexes (MEMRs). Distortion product otoacoustic emissions (DPOAEs) were absent in all ears tested and the cochlear microphonic (CM) was increased in amplitude and duration in young patients and absent in the two oldest subjects. Auditory brainstem responses (ABRs) were dys-synchronised bilaterally with no reproducible waves I, III or V at high intensities. Hearing loss and speech reception threshold deteriortated symmetrically with age, resulting in severe-to-profound hearing impairment by early adulthood. Vestibular evoked myogenic potentials were normal in three ears and absent in one.
Conclusion
Homozygous SLITRK6 c.1240C>T (p.Gln414Ter) nonsense mutations are associated with high myopia, cochlear dysfunction attributed to outer hair cell disease, and progressive auditory neuropathy.
Keywords: auditory neuropathy, Amish, SLITRK6
INTRODUCTION
The SLITRK family consists of six neuronal transmembrane proteins that each have two N-terminal leucine-rich repeat (LRR) domains and a C-terminal region that shares homology with neurotrophin receptors. SLITRKs are found predominantly in neural tissue where they modulate neurite outgrowth and regulate synaptic development.1,2,3SLITRK6 is expressed in the auditory system during embryonic and postnatal life; expression is strongest in the inner ear, modest in the thalamus and lateral geniculate nucleus,4,5 and absent in cortex.1,2 Its expression in the inner ear promotes innervation and survival of sensory neurons.4
In Slitrk6-deficient (Slitrk6−/−) mice, organization of the organ of Corti is normal, but cochlear innervation density is decreased and many sensory neurons within spiral and vestibular ganglia die during development.4 Affected mice have a reduced first auditory brainstem response (ABR) wave, mid-frequency (8-16-kHz) hearing loss (HL), attenuated auditory startle and decreased vertical vestibulo-ocular reflex gains.6 Additionally, a recent study showed that Slitrk6/SLITRK6 deficiency affects cochlear and retinal innervation and axial eye length in mice and causes sensorineural deafness and high myopia in humans.7
We identified 9 Old Order Amish individuals who were homozygous for a nonsense mutation of SLITRK6 (c.1240C>T, p.Gln414Ter) and suffered progressive cochlear and auditory nerve dysfunction. As a complement to the ophthalmological phenotype described by Tekin et al.,7 here we focus on the longitudinal auditory phenotype of Amish SLITRK6-deficient patients.
METHODS
Patients
Nine subjects (mean age 15.3±13.9 years, range 0.3-36.8 years) from an endogamous Amish community of Pennsylvania were evaluated and cared for at the Clinic for Special Children. The study was approved by the Institutional Review Board of Lancaster General Hospital and all patients (or their parents) consented in writing to participate. All participants underwent thorough clinical examination, and no abnormal neurologic findings were identified outside of the auditory and visual systems. The four oldest SLITRK6 c.1240C>T homozygotes wore corrective lenses for high myopia.
Genetic Mapping and Genotyping
Single nucleotide polymorphism (SNP) genotyping and genetic mapping was performed with the GeneChip Mapping 10K Assay Kit (Affymetrix, Santa Clara, CA, USA) as previously described.8,9,10 Data were analyzed in Microsoft Excel spreadsheets (Microsoft Corporation, Redmond, WA, USA). SNP positions came from Affymetrix genome annotation files and genotype data came from the Affymetrix GeneChip Human Mapping 10K Xba 142 Arrays. Data analyses were designed for rapid identification of genomic regions demonstrating homozygous identity between all affected individuals (i.e. autozygosity). These analyses assumed mutation and locus homogeneity. Two-point lod scores were calculated for each genotyped SNP using an approach similar to Broman and Weber.11 Location scores for shared homozygous SNP blocks were calculated by summing the lod scores corresponding to the individual SNPs in the region. This provided a relative measure that a specific homozygous block harbored the disease gene. Genotype data from 100 healthy Amish females were used for allele frequency estimations.
To genotype Amish control samples, we developed a high resolution melt analysis using an unlabeled probe for the SLITRK6 variant on a LightScanner 32 System (BioFire Diagnostics, Salt Lake City,UT, USA). We validated the assay in patients, their parents and siblings of known genotype to demonstrate accurate allele discrimination and genotype calls. We then genotyped 571 randomly selected Lancaster Amish control samples. Autosomal recessive inheritance was assumed.
Auditory and Vestibular Testing
We tested tympanometry with a 226-Hz probe tone, measured ipsilateral middle ear muscle reflexes (MEMR) between 80-100dB HL at 0.5, 1, 2 and 4-kHz, and obtained distortion product otoacoustic emissions (DPOAEs) using the ILO (Otodynamic) “8 points/octave” function. 2f1-f2 were recorded for f2 varying from 842-Hz to 7996-Hz and intensities of the primaries were kept constant across the frequency range (f1=65dB SPL, f2=55dB SPL). The f1/f2 frequency ratio was 1.22.
We elicited ABRs using 100 μsec air-conduction clicks and recorded from a two-channel four-electrode montage (mastoid-high forehead-mastoid). Responses were first obtained at 90dB normal hearing level (nHL) then at variable intensities (50-100dB nHL) based on individual responses. Condensation and rarefaction clicks were used to distinguish the cochlear microphonic (CM) from the compound action potential. The rate of stimulation was 27.7/s, low pass filter was 1500-Hz and high pass filter was 100-Hz, and gain was set at 20K. The presence of a wave was only established when at least 2 different recordings (for each polarity of the click) were available at the same or different intensity to verify reproducibility.
We measured pure tone audiograms and speech audiometry with insert earphones in a sound proof booth. Speech reception thresholds (SRT) and speech discrimination scores (SDS) were obtained using live voice. Because of age and a language barrier, SDS could not be established in the youngest children. Right-left symmetry and correlations of age with HL (dB) and SRT (dB) were tested using the non-parametric Spearman correlation coefficient (rs).
Vestibular evoked myogenic potentials (VEMP) recordings were obtained with two surface electrodes placed on the sternocleidomastoid muscles in 3 children. Each child contracted their sternocleidomastoid muscle by rotating their head contralaterally to the ear receiving sound stimulation. Recordings were first obtained at 95dB. In presence of a response, stimulus intensity was decreased to find the VEMP threshold. In the absence of a response, intensity was increased to 100dB. Each recording was repeated to ensure proper accuracy.
In Vitro Functional Studies
Human SLITRK6 (NM_032229.2) was amplified by polymerase chain reaction (PCR) using cDNA reverse transcribed (SuperScript II; Life Technologies, Carlsbad, CA) from human adherent retinal pigment eipthelium cells (ARPE-19; ATCC, Manassas, VA) and then ligated into pENTR/D-TOPO (Life Technologies). The c.1240C>T variant was introduced by site-directed mutagenesis (QuikChange II; Agilent Technologies, Santa Clara, CA) and constructs verified by Sanger sequencing were recombined into pcDNA3.2/V5-DEST (Life Technologies) for expression of C-terminal V5-tagged fusion proteins.
Verified expression clones were transfected into mouse UB/OC-2 auditory hair cell precursor cells (gift from M. Holley, Univ. of Sheffield) using FuGENE 6 (Promega, Madison, WI). Prior to transfection, UB/OC-2 cells were maintained at 33°C/5%CO2 in Eagle’s minimal essential medium (MEM) with GlutaMAX, 10% fetal bovine serum (FBS), and 50U/ml interferon-gamma (Life Technologies).4,6,12 For transfection, UB/OC-2 cells were transferred to MEM with GlutaMAX and 10% FBS (without interferon- and were cultured for 48h (post-transfection) at 37°C/5%CO2.
Following transfection, UB/OC-2 cells grown on 25-mm coverslips were prepared for immunofluorescence microscopy and imaged as described elsewhere.10 SLITRK6/C-terminal V5 fusion proteins were detected with mouse monoclonal anti-V5 (1:500) and AlexaFluor 488-conjugated goat-anti-mouse IgG2a (1:400) (Life Technologies). Nuclei were labeled with with DAPI (4′,6-diamidino-2-phenylindole – 1.5 mg/ml; Santa Cruz Biotechnology, Santa Cruz, CA).
For western blotting, UB/OC-2 cells grown to 100% confluence on 10-cm dishes were lysed in RIPA buffer (50 mM Tris-HCl, pH 8.0, with 150 mM NaCl, 1.0% Igepal CA-630, 0.5% sodium deoxycholate, and 0.1% sodium dodecyl sulfate) supplemented with 1mM EDTA, 2mM NaF, 1mM Na3VO4, and protease inhibitor cocktail (Roche, Indianaoplis, IN).4 Lysates were sonicated briefly on ice and cleared by centrifugation at 14,000 rpm/4°C for 10-15 min. SDS-PAGE and western blotting were performed as described elsewhere.10 SLITRK6/C-terminal V5 fusion proteins were detected with mouse monoclonal anti-V5 (1:5,000) and HRP-conjugated goat-anti-mouse IgG (1:1,500) (Cell Signaling Technologies, Beverly, MA). Immunoblots were developed using enhanced chemiluminescence (LumiGlo, Cell Signaling) and imaged with Kodak BioMax film (Carestream, Rochester, NY).
RESULTS
Genetic Mapping and Sequencing
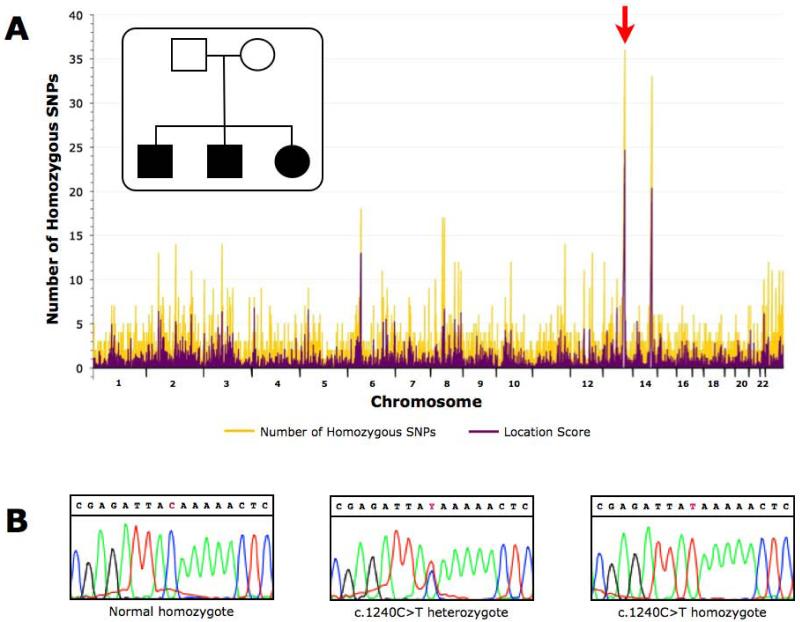
Using DNA samples from three affected children from a single sibship, we mapped a locus for non-syndromic deafness to chromosome 13q31 between SNP markers rs722023 and rs958373 (Fig.1A). The mapped region contained 33 known or putative genes, 19 of which were pseudogenes. Annotation of the remaining 14 genes revealed SLITRK6 as a strong candidate gene based on expression, function, and the phenotype of a Slitrk6 knockout mouse.4 Direct Sanger sequencing of the SLITRK6 gene in affected individuals identified homozygosity for a novel nonsense variant: c.1240C>T (p.Gln414Ter) (Fig.1B). Using high resolution melt analysis, we genotyped 571 Old Order Amish control samples and identified 27 carriers (27/571=4.7% carrier frequency). This carrier frequency is not unexpected within an endogamous study group such as the Amish that has pathogenic allele carrier frequencies as high as 6% (Ellis-van Creveld syndrome, EVC IVS13+5G>T) and 11% (Nemaline rod myopathy, TNNT1 c.505G>T).13
Figure 1. Gene discovery in autosomal recessive auditory neuropathy.
We mapped and identified the gene using a single sibship which in isolated populations such as the Old Order Amish can be accomplished with as few as 2 affected individuals, then subsequently identified additional affected individuals from separate sibships. A) We performed a genome-wide homozygosity mapping study using Affymetrix GeneChip Mapping 10K SNP Arrays with a single Old Order Amish sibship with three affected. Two large, shared blocks of homozygosity were identified on chromosomes 13 and 14. However, the location score was highest for the chromosome 13q31 region and a subsequent affected child in the family was not homozygous for the chromosome 14 SNP markers. The common homozygous segment was flanked by SNPs rs722023 and rs958373, spanned 9.3 Mb and contained 33 known or predicted genes based on NCBI annotations. B) Sanger sequencing of the SLITRK6 gene revealed homozygosity for a nonsense variant in exon 2 in all affected individuals (c.1240C>T). The three panels depict sequencing of a normal homozygote, a carrier, and a mutation homozygote, respectively.
Audiological and Vestibular Testing
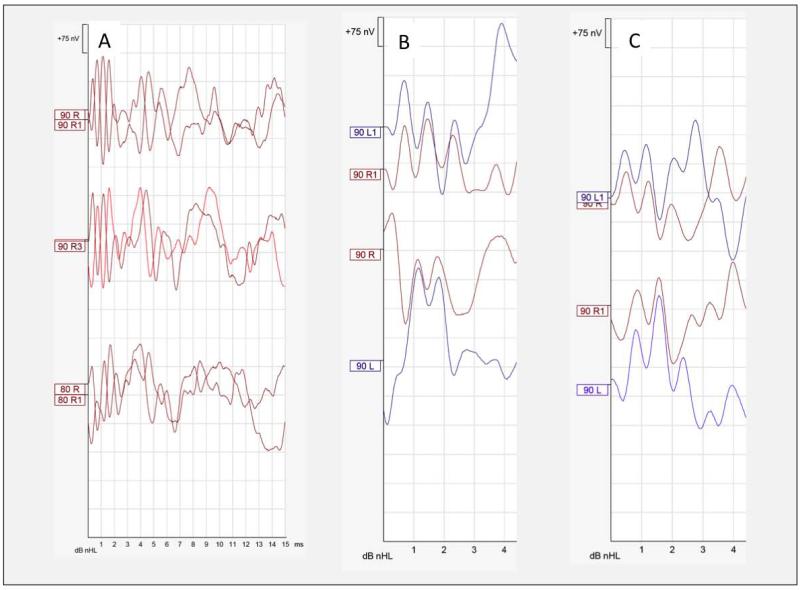
Audiological data are summarized in Table 1. In the 7 patients who could be tested for MEMRs, an ipsilateral response was present at 100dB (4-kHz) bilaterally in one child, in the left ear in two children, and absent bilaterally at maximal 100dB intensity (0.5, 1,and 2-kHz) in the four oldest subjects. Distortion product otoacoustic emissions were absent in all 7 testable subjects (Table 1). A CM was absent in the two oldest patients (ages 32.3 and 36.8 years), but was of increased amplitude and duration in all but one child’s ears (Fig.2).
Table 1. Summary of Audiologic Data from Nine SLITRK6-deficient Patients.
| MEMR |
DPOAE |
CM (duration in ms) |
Waves |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Subject ID | Age (years) | Left | Right | Left | Right | Left | Right | Left | Right |
| 1 | 0.3 | nt | nt | nt | nt | + (1.0) | + (3.0) | - | - |
| 2 | 1.5 | nt | nt | - | - | + (2.0) | + (2.0) | I | I, II |
| 3 | 5.6 | 100 dB/4 kHz | - | - | - | + (5.0) | + (5.0) | I | I |
| 4 | 6.2 | 100 dB/4 kHz | - | - | - | + (2.5) | + (2.5) | I | I |
| 5 | 11.9 | 100 dB/4 kHz | 100 dB/4 kHz | - | - | + (3.0) | + (2.0) | I, V* | I |
| 6 | 13.9 | - | - | - | - | + (3.0) | + (2.5) | I | I |
| 7 | 29.1 | - | - | nt | nt | - | + (0.5) | - | - |
| 8 | 32.3 | - | - | - | - | - | - | - | - |
| 9 | 36.8 | - | - | - | - | - | - | - | - |
No latency/intensity shift. Abbreviations: CM, cochlear microphonic; DPOAE, distortion product otoacoustic emission; MEMR, ipsilateral middle ear muscle reflex; nt, not tested; “-”, absent response.
Fig. 2. Cochlear Microphonics and Auditory brainstem responses to condensation and rarefaction clicks.
A) Right ear from subject 2. The CM is robust and continue over 3 milliseconds B) Waveforms from right and left ears from subject 4 are superposed for each stimulus polarity to show ear symmetry of the CM. C) Same as B) for subject 3.
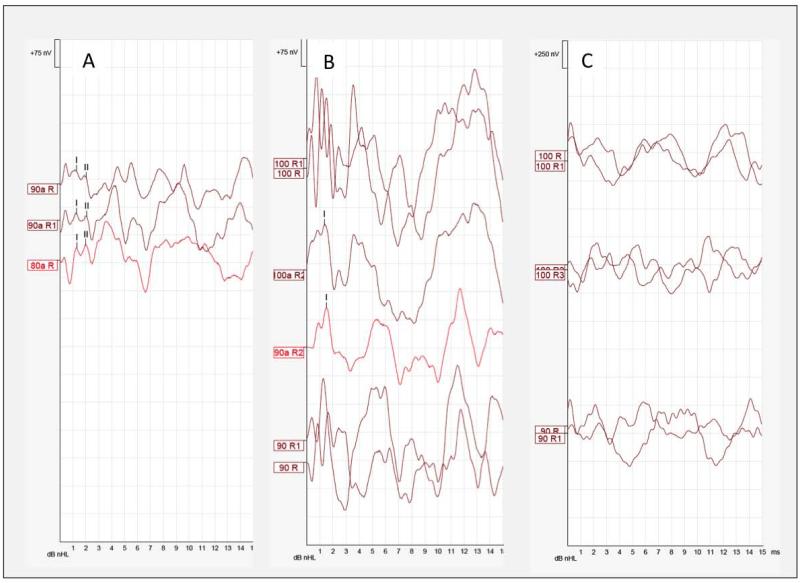
In both children and adults, ABRs were markedly dys-synchronised bilaterally (Table 1, Fig.3). When comparing condensation and rarefaction responses, no reproducible waves I, III or V were observed at high intensities. However, adding both polarities together revealed some synchrony in several ears. Figure 3A is an ABR tracing from the right ear of Subject 2 that depicts the presence of wave I and II obscured by the large ringing CM. Figure 3B shows wave I in the right ear of Subject 6. Only one of all ears tested showed the presence of wave V, which did not shift in latency when click intensity was decreased. In the oldest subjects tested, CM and neural synchrony were absent (Fig.3C).
Fig. 3. Auditory Brainstem Responses.
A) ABR tracing from the right ear of Subject 2 that depicts the presence of wave I and II obscured by the large ringing CM. The waves are revealed by adding both condensation and rarefaction waveforms B) shows wave I in the right ear of Subject 6 when both condensation and rarefaction waveforms are added together. C) CM and neural synchrony were absent in subject 8.
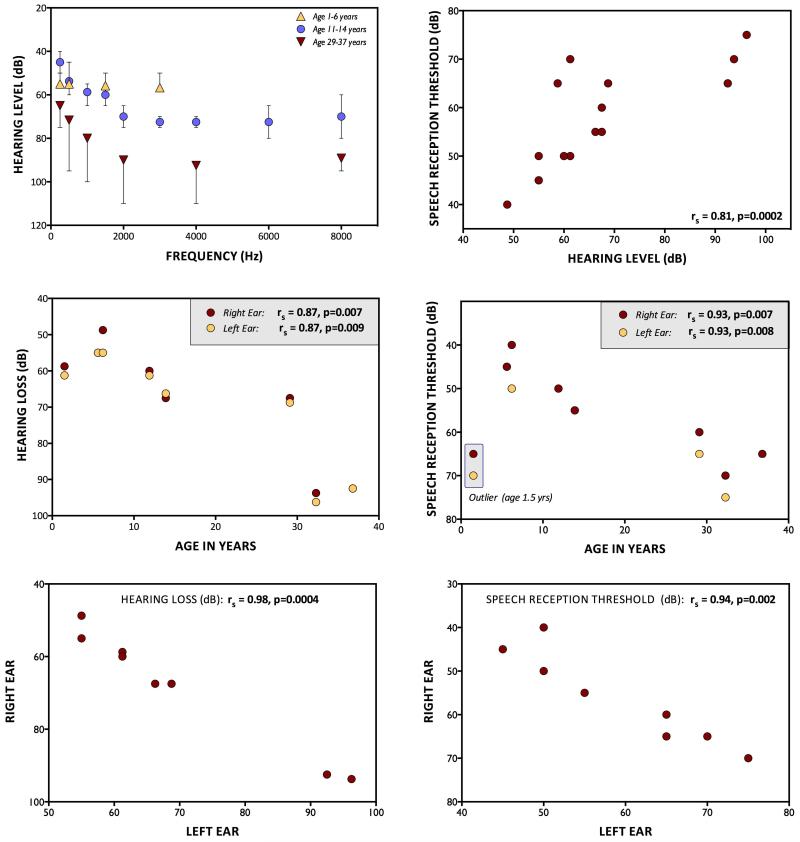
Among the 8 subjects who could be tested, behavioral audiograms revealed strong correlations between age and both HL (rs=0.87, p=0.007) and SRT (rs=0.93, p=0.007)(Fig.4), which in turn were closely correlated (rs=0.81, p=0.0002). Both the CMs and degree of HL showed striking right-left symmetry (right versus left ear rs=0.98, p=0.0004)(Figs.2,4). Vestibular evoked myogenic potentials were tested in four ears from three subjects and were normal in three ears and absent in one.
Fig. 4. Behavioral Audiology.
A) Averaged pure tone audiograms for 3 different age groups. B) Speech reception thresholds as a function of pure tone average (0.5, 1, 2, 4 kHz). C) Pure tone average as a function of age. D) Speech reception thresholds as a function of age. E) Pure tone average correlation between right and left ears. F) Correlation of speech reception thresholds between right and left ears.
In Vitro Functional Studies
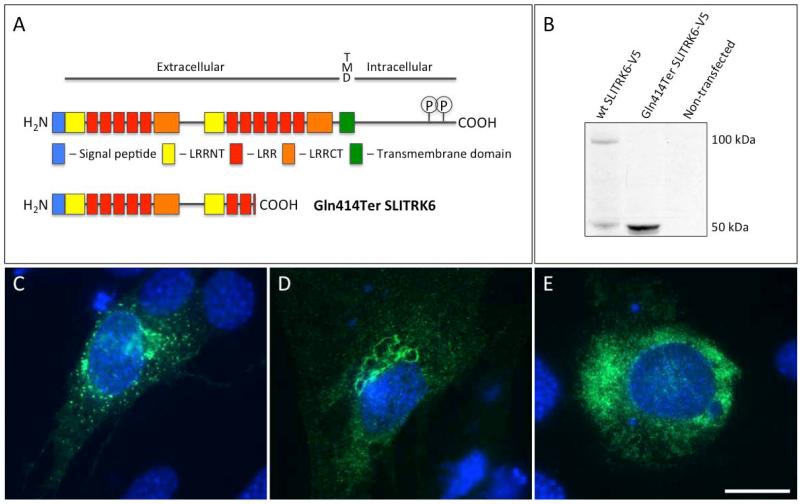
SLITRK6 is an 841 amino acid protein with a structure as shown in Figure 5A.14 Heterologous overexpression of wild-type human SLITRK6 in mouse UB/OC-2 auditory hair cell precursors resulted in the production of an approximately 100-kDa protein that trafficked to the plasma membrane and collected in abundant membrane puncta, consistent with the behavior of other SLITRK family proteins (Fig. 5B and 5C).3 Overexpression of c.1240C>T SLITRK6/V5 produced a 50-kDa protein that distributed uniformly throughout the cytosol and did not produce puncta (Fig.5B,5E).
Fig. 5. In Vitro Expression and Localization.
A) SLITRK6 has an extracellular N-terminal extension that contains two SLIT-like leucine-rich repeat domains and an intracellular C-terminus with two conserved tyrosine residues that are subject to phosphorylation in a sequence homologous to the Ntrk neurotrophin receptors. The c.1240C>T (p.Gln414Ter) change introduces a premature termination codon predicted to truncate the protein after Leu413 Conserved domains in human wild-type SLITRK6 (top) (Uniprot Q9H5Y7) and SLITRK6 Gln414Ter (bottom). LRRNT – leucine–rich repeat N-terminal domain, LRR – leucine-rich repeat, LRRCT – leucine-rich repeat C-terminal domain, TMD – transmembrane domain. C-terminal tyrosine residues 820 and 833 are phosphotyrosine residues in a conserved Ntrk-like domain.12B) Western blot of lysates of UB/OC-2 cells overexpressing human SLITRK6 C-terminal V5 epitope-tagged constructs. SLITRK6 Gln414Ter is stably expressed as an approximately 50-kDa cytosolic protein. The increased abundance of SLITRK6 Gln414Ter relative to wild-type SLITRK6 despite equal total protein loading may result from lower efficiency in extracting wild-type SLITRK6 from the membrane relative to cytosolic SLITRK6 Gln414Ter. C & D) Heterologous overexpression of wild-type SLITRK6 in UB/OC-2 cells suggests that SLITRK6 traffics normally through the major mFembrane system to the plasma membrane. By contrast, SLITRK6 Gln414Ter appears to be relatively uniformly distributed throughout the cytosol without the membrane puncta characteristic of SLITRK family expression.3 Scale bar in E = 10 μm in C-E. Data in B are representative of 6 independent transfections. Data in C-E are representative of 4 independent transfections.
DISCUSSION
The Expanding Spectrum of Auditory Neuropathy
Homozygous nonsense mutations of human SLITRK6 (c.1240C>T) are associated with an auditory neuropathy spectrum disorder (ANSD) characterized by absent MEMRs and OAEs, large and prolonged CMs, dys-synchronized ABRs, and pure tone HL that progresses with age 15,16,17,18 In ANSD patients, CMs can be quite large and continue over several milliseconds which may reflect a lack of time-locked neural activity.16 A large CM is especially common in ANSD subjects less than 10 years of age.19 In the absence of middle ear conductive HL, OAEs are often present in patients with ANSD, but can decline or disappear over time while CMs remain intact.19
ANSD is historically defined as a disorder of inner hair cells (IHCs) or the auditory nerve in the presence of normal outer hair cells (OHCs), but there is now evidence that some types also involve loss of OHC function. Although the preservation of OAEs and CMs has been considered evidence of normal OHCs, an especially large CM that persists for several milliseconds after a transient click stimulus suggest OHC dysfunction in SLITRK6-associated and other forms of ANSD. Moreover, ANSDs can be progressive as shown by abnormal OAEs, a loss of OAEs over time, a progression in the extent of hearing loss, or development of peripheral neuropathy.20,21,22,23 In one retrospective of 260 ANSD patients, one-fourth of patients had either partial or absent OAEs.24
In Vitro Functional Studies
Our observations corroborate the recent report of an association between autosomal recessive HL and another SLITRK6 nonsense mutation (c.890C>A, p.S297X)13,25, and suggest that the SLITRK6 protein plays a critical role in the formation and retention of auditory and visual sensory circuits.
The entire coding sequence for SLITRK6 is contained within a single exon (exon 2) of the mature transcript and thus SLITRK6 Gln414Ter is expected to escape nonsense-mediated decay. The SLITRK6 c.1240C>T change truncates the C-terminal transmembrane domain that normally localizes the protein to the plasma membrane and anchors the N-terminal LRR directed to the extracellular space. Accordingly, SLITRK6 Gln414Ter is expressed in mouse UB/OC-2 auditory hair cell precursors, but fails to localize to the membrane.
The preservation of a signal peptide and signal peptidase cleavage site in the N-terminus of SLITRK6 Gln414Ter suggests that the truncated protein might be secreted from the cell via the lumen of trafficking membrane vesicles. However, we were unable to detect SLITRK6 Gln414Ter in concentrated medium from UB/OC-2 cells overexpressing SLITRK6 c.1240C>T cDNA (data not shown), and our in vitro studies indicate that SLITRK6 Gln414Ter is a soluble protein fragment homogenously distributed throughout the cytosol.
Similarities between the human SLITRK6 c.1240C>T and murine Slitrk6−/− phenotype suggest that the c.1240C>T variant results in loss of SLITRK6 function,4,6 but we did not exclude the possibility that a toxic gain of function associated with SLITRK6 Gln414Ter results from misdirection of leucine-rich repeat domains to the intracellular space or loss of the intracellular Ntrk-like C-terminal domain (Fig. 5). The impact of SLITRK6 Gln414Ter on neurotrophin and neurotrophin receptor expression and modulation of neurite outgrowth remains to be characterized. Of note, none of the affected subjects exhibited signs or symptoms of generalized neurologic disease that could account for their auditory neuropathy.
Cochlear Structure and Function
In Slitrk6−/− mice, the organ of Corti and the cochlear sensory epithelium appear normal but type II spiral ganglion fibers that innervate OHCs are reduced in number.4 Wild-type Slitrk6 is expressed in the murine primitive neurosensory epithelium where hair cells are produced during embryogenesis1. Although OAEs have not been tested in Slitrk6−/− mice,6 they are absent in humans with the Gln414Ter nonsense mutation, suggesting abnormal number or function of OHCs.
Absent OAEs coupled to an especially prominent and prolonged CM is typical of ANSDs. 16,26,27 The CM is a field potential, believed to result from the vector sum of extracellular components of receptor potentials arising in outer and IHCs28 OHCs appear to be the major source of CMs,29 which arise primarily from basal regions of the cochlea.30 It is thus likely that the large CM in young SLITRK6 c.1240C>T homozygotes is generated by basal OHCs that still function at high frequencies (>10-kHz), are not tested by DPOAEs, and senesce by early adulthood.
The relationship between pure CM surface detection and normal OHC function is not fully understood. Cortical microphonics and OAEs are generated by distinct mechanisms; CMs have been recorded in the context of extensive OHC loss31,32,33 and in ANSD patients who have absent OAEs.22 Thus, the CM cannot be considered a reliable marker of OHC integrity and it is likely that SLITRK6 c.1240C>T homozygotes have some degree of OHC dysfunction that worsens with aging. In contrast, the absence of OAEs in patients with large CMs and wave I recordings at high intensities (at least in children) suggests that IHC function is relatively preserved. Consequently, most residual cochlear function in SLITRK6-deficient patients is probably mediated by IHCs.
Afferent and Efferent Auditory Nerve Function
In Slitrk6−/− mice, there are 50% fewer spiral ganglion neurons, fewer axon projections from the spiral ganglion to cochlear hair cells, and abnormal ABR thresholds and amplitudes.4,6 Among SLITRK6 c.1240C>T homozygotes, wave I can be generated in children at high stimulus intensity, but there is insufficient synchrony (except in one ear) to generate waves III and V in the brainstem. The absence of OAEs precluded testing of the medial efferent olivocochlear system, but abnormal efferent function is also typical of patients with ANSD,34 and may be reflected by increased duration of the CM.23,34,35 The auditory neuropathy associated with SLITRK6 c.1240C>T in humans is more severe than that observed in Slitrk6−/− mice. This may reflect a more prominent role of SLITRK6 in human versus murine auditory development.
Distribution, Symmetry, and Progression of Hearing Loss
Afferent and efferent innervation deficits are distributed evenly throughout all turns of the cochlea in Slitrk6−/− mice4 and in SLITRK6 c.1240C>T patients, HL tends to be flat across frequencies (more in children than adults) and symmetric between right and left ears. Moreover, both HL and the CM (when present) are quite symmetrical, a pattern that is atypical for ANSD.35
Moderate HL associated with SLITRK6 c.1240C>T during childhood appears to progress to severe or profound HL by early adulthood, with better preservation of low versus medium and high frequencies. We did not perform the serial auditory exams on single individuals that would be necessary to verify this, but there are strong inverse correlations between age and both pure-tone audiograms and SRTs in subjects homozygous for SLITRK6 c.1240C>T. As previously noted in ANSD patients, these behavioral changes correlate with decreases in the presence, amplitude, and duration of the CM.23 The disappearance of the CM (and increase in behavioral thresholds) in older subjects is probably a sign of IHC death.
Auditory Behavior and Speech and Language Development
Like most ANSD patients, SLITRK6-deficient subjects present with better behavioral thresholds than their ABR would indicate. Speech reception thresholds correlate with pure-tone audiograms, but speech perception is impaired out of proportion to the pure-tone threshold. Despite OHC dysfunction and auditory dys-synchrony at the level of the brainstem, affected subjects heard well enough in their early years to develop speech and language without specific intervention. They become good lip readers, and use visual cues to compensate for HL. By adulthood, however, understanding speech becomes difficult and adults increasingly rely on lip reading. The two oldest SLITRK6 c.1240C>T patients became hearing aid users but with limited benefit. This is not surprising as the amplification of sounds used to compensate for OHC dysfunction cannot overcome the auditory neuropathy.
None of our SLITRK6-deficient subjects used cochlear implants (CIs). The efficacy of CIs cannot be predicted solely on our results, but outcomes after implantation in other ANSD patients suggest that they could be of some benefit. A recent study of 35 pediatric subjects with ANSD showed that speech and language outcomes after CI were indistinguishable from those of an equal number of age-matched, implanted children with non-ANSD sensorineural HL.36 Thus detection of affected individuals during infancy holds promise for better and more timely interventions that should improve long-term speech and language outcomes.
Conclusions
Homozygous nonsense mutations of SLITRK6 (c.1240C>T, p.Gln414Ter) is associated with high myopia and ANSD. Recessive, non-syndromic, progressive HL is the first human phenotype associated with a variant of the SLITRK protein family.
Acknowledgments
Financial Disclosures: Drs. Jinks, Puffenberger, and Strauss were supported by HHMI undergraduate science education awards 52006294 and 52007538. Dr. Jinks was also supported by the Center for Research on Women and Newborn Health, and by ConnectCare3. Dr. Morlet is supported by NIH grant 8P20GM103464. The remaining authors have no financial disclosures.
Presentations: This work was presented at the Annual COSM meeting in Orlando, Florida on April 10-14, 2013. Manuscript #448.
Footnotes
Conflict of Interests: none.
Author Contributions: Conceived and designed the experiments: TM, MRR, RCO, RNJ, EGP, KAS. Performed the experiments: TM, MRR, LRL, TR, LAG, EAS, NA, AZ, EY, RCR, RNJ, EGP, AH, KAS. Analyzed the data: TM, MRR, RCO, RNJ, EGP, KAS. Wrote the paper: TM, MRR, RCO, RNJ, EGP, KAS. Delineation of phenotype, patient management, collection of samples, and summarization of clinical data: TM, MRR, RCO, EY, DHM, KAS.
REFERENCES
- 1.Aruga J. Slitrk6 expression profile in the mouse embryo and its relationship to that of Nlrr3. Gene Expression Patterns. 2003;3:727–733. doi: 10.1016/s1567-133x(03)00141-8. [DOI] [PubMed] [Google Scholar]
- 2.Aruga J, Yokota N, Mikoshiba K. Human SLITRK family genes: genomic organization and expression profiling in normal brain and brain tumor tissue. Gene. 2003;2(315):87–94. doi: 10.1016/s0378-1119(03)00715-7. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi H, Katayama K, Sohya K, Miyamoto H, Prasad T, Matsumoto Y, Ota M, Yasuda H, Tsumoto T, Aruga J, Craig AM. Selective control of inhibitory synapse development by Slitrk3-PTPdelta trans-synaptic interaction. Nat Neurosci. 2012;15:389–398. S381–382. doi: 10.1038/nn.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katayama K, Zine A, Ota M, Matsumoto Y, Inoue T, Fritzsch B, Aruga J. Disorganized innervation and neuronal loss in the inner ear of Slitrk6-deficient mice. PloS One. 2009;4(11):e7786. doi: 10.1371/journal.pone.0007786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beaubien F, Cloutier JF. Differential expression of Slitrk family members in the mouse nervous system. Dev Dyn. 2009 Dec;238(12):3285–96. doi: 10.1002/dvdy.22160. [DOI] [PubMed] [Google Scholar]
- 6.Matsumoto Y, Katayam K, Okamoto T, Yamada K, Takashima N, Nagao S, Aruga J. Impaired auditory-vestibular functions and behavioral abnormalities of Slitrk6-deficient mice. PloS One. 2011;6(1):e16497. doi: 10.1371/journal.pone.0016497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tekin M, Chioza BA, Matsumoto Y, Diaz-Horta O, Cross HE, Duman D, Kokotas H, Moore-Barton HL, Sakoori K, Ota M, Odaka YS, Foster J, 2nd, Cengiz FB, Tokgoz-Yilmaz S, Tekeli O, Grigoriadou M, Petersen MB, Sreekantan-Nair A, Gurtz K, Xia XJ, Pandya A, Patton MA, Young JI, Aruga J, Crosby AH. SLITRK6 mutations cause myopia and deafness in humans and mice. J Clin Invest. 2013 May 1;123(5):2094–102. doi: 10.1172/JCI65853. doi: 10.1172/JCI65853. Epub 2013 Apr 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strauss KA, Puffenberger EG, Huentelman MJ, Gottlieb S, Dobrin SE, Parod JM, Stephan DA, Morton DH. Recessive symptomatic focal epilepsy and mutant contactin-associated protein-like 2. N Engl J Med. 2006;354(13):1370–1377. doi: 10.1056/NEJMoa052773. [DOI] [PubMed] [Google Scholar]
- 9.Puffenberger EG, Strauss KA, Ramsey KE, Craig DW, Stephan DA, Robinson DL, Hendrickson CL, Gottlieb S, Ramsay DA, Siu VM, Heuer GG, Crino PB, et al. Polyhydramnios, megalencephaly and symptomatic epilepsy caused by a homozygous 7-kilobase deletion in LYK5. Brain. 2007;130(Pt 7):1929–1941. doi: 10.1093/brain/awm100. [DOI] [PubMed] [Google Scholar]
- 10.Puffenberger EG, Jinks RN, Sougnez C, Cibulskis K, Willert RA, Achilly NP, Cassidy RP, Fiorentini CJ, Heiken KF, Lawrence JJ, Mahoney MH, Miller CJ, et al. Genetic mapping and exome sequencing identify variants associated with five novel diseases. PLoS One. 2012;7(1):e28936. doi: 10.1371/journal.pone.0028936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Broman KW, Weber JL. Long homozygous chromosomal segments in reference families from the centre d’Etude du polymorphisme humain. Am J Hum Genet. 1999;65(6):1493–1500. doi: 10.1086/302661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rivolta MN, Grix N, Lawlor P, Ashmore JF, Jagger DJ, Holley MC. Auditory hair cell precursors immortalized from the mammalian inner ear. Proc Biol Sci. 1998;265:1595–1603. doi: 10.1098/rspb.1998.0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strauss KA, Puffenberger EG. Genetics, medicine, and the Plain people. Annu Rev Genomics Hum Genet. 2009;10:513–536. doi: 10.1146/annurev-genom-082908-150040. [DOI] [PubMed] [Google Scholar]
- 14.Aruga J, Mikoshiba K. Identification and characterization of Slitrk, a novel neuronal transmembrane protein family controlling neurite outgrowth. Mol Cell Neurosci. 2003;24:117–129. doi: 10.1016/s1044-7431(03)00129-5. [DOI] [PubMed] [Google Scholar]
- 15.Starr A, Picton TW, Sininger Y, Hood LJ, Berlin CI. Auditory neuropathy. Brain. 1996 Jun;119(Pt 3):741–53. doi: 10.1093/brain/119.3.741. [DOI] [PubMed] [Google Scholar]
- 16.Berlin CI, Bordelon J, St John P, Wilensky D, Hurley A, Kluka E, Hood LJ. Reversing click polarity may uncover auditory neuropathy in infants. Ear Hear. 1998;19:37–47. doi: 10.1097/00003446-199802000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Berlin CI, Morlet T, Hood LJ. Auditory neuropathy/dyssynchrony: its diagnosis and management. Pediatr Clin North Am. 2003 Apr;50(2):331–40. vii–viii. doi: 10.1016/s0031-3955(03)00031-2. [DOI] [PubMed] [Google Scholar]
- 18.Berlin CI, Hood LJ, Morlet T, Wilensky D, Brashears S, St John P, Montgomery L, Thibodeaux M. Absent or elevated middle ear muscle reflexes in the presence of normal otoacoustic emissions: a universal finding in 136 cases of auditory neuropathy-dys-synchrony. J Am Acad Audiol. 2005;16:546–553. doi: 10.3766/jaaa.16.8.3. [DOI] [PubMed] [Google Scholar]
- 19.Hood L, Morlet T. Current issues in auditory neuropathy spectrum Disorder. In: Tremblay Kelly, Burkard Robert., editors. Translational Perspectives in Auditory Neurosciences. Special Topics. Plural Publishing; 2012. pp. 35–68. [Google Scholar]
- 20.Deltenre P, Mansbach AL, Bozet C, Christiaens F, Barthelemy P, Paulissen D, Renglet T. Auditory neuropathy with preserved cochlear microphonics and secondary loss of otoacoustic emissions. Audiology. 1999;38:187–195. doi: 10.3109/00206099909073022. [DOI] [PubMed] [Google Scholar]
- 21.Kovach MJ, Campbell KCM, Herman K, Waggoner B, Gelber D, Hughes LF, Kimonis VE. Anticipation in a unique family with Charcot-Marie-Tooth syndrome and deafness: Delineation of the clinical features and review of the literature. American Journal of Medical Genetics. 2002;108:295–303. doi: 10.1002/ajmg.10223. [DOI] [PubMed] [Google Scholar]
- 22.Starr A, Sininger YS, Pratt H. The varieties of auditory neuropathy. Journal of Basic and Clinical Physiology and Pharmacology. 2000;11:215–230. doi: 10.1515/jbcpp.2000.11.3.215. [DOI] [PubMed] [Google Scholar]
- 23.Starr A, Sininger Y, Nguyen T, Michalewski HJ, Oba S, Abdala C. Cochlear receptor (microphonic and summating potentials, otoacoustic emissions) and auditory pathway (auditory brain stem potentials) activity in auditory neuropathy. Ear and Hearing. 2001;22:91–99. doi: 10.1097/00003446-200104000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Berlin CI, Hood LJ, Morlet T, Wilensky D, Li L, Rose-Mattingly K, Frisch SA. Multi-site diagnosis and management of 260 patients with Auditory Neuropathy/Dys-synchrony (Auditory Neuropathy Spectrum Disorder) International Journal of Audiology. 2010;49:30–43. doi: 10.3109/14992020903160892. [DOI] [PubMed] [Google Scholar]
- 25.Tekin M, Diaz-Horta O, Duman D, Foster J, II, Sirmaci A, Gonzalez M, Mahdieh N, Bonyadi M, Cengiz FB, Ulloa R, Zuchner S, Blanton S. Whole-exome sequencing for autosomal recessive non-syndromic deafness: 93% of known genes covered and OTOGL and SLITRK6 are novel genes. http://www.ashg.org/2012meeting/abstracts/fulltext/f120122160.htm.
- 26.Deltenre P, Mansbach AL, Bozet C, Clercx A, Hecox KE. Temporal distortion products (kernel slices) evoked by maximum-length-sequences in auditory neuropathy; Evidence for a cochlear pre-synaptic origin. Electroencephalography Clinical Neurophysiology. 1997;104:10–16. doi: 10.1016/s0168-5597(96)96535-1. [DOI] [PubMed] [Google Scholar]
- 27.Starr A, McPherson J, Patterson J, Don M, Luxford W/, Shannon R, Sininger Y, Tonakawa L. Waring M. Absence of both auditory evoked potentials and auditory percepts dependent on timing cues. Brain. 1991;114:1157–1180. doi: 10.1093/brain/114.3.1157. [DOI] [PubMed] [Google Scholar]
- 28.Dallos P, Cheatham MA. Production of cochlear potentials by inner and outer hair cells. Journal of Acoustical Society of America. 1976;60:510–512. doi: 10.1121/1.381086. [DOI] [PubMed] [Google Scholar]
- 29.Dallos P. Some electrical circuit properties of the organ of Corti. I. Analysis without reactive elements. Hear. Res. 1983;12:89–120. doi: 10.1016/0378-5955(83)90120-x. [DOI] [PubMed] [Google Scholar]
- 30.Withnell RH. Brief Report: The cochlear microphonic as an indication of outer hair cell function. Ear Hear. 2001;22:75–77. doi: 10.1097/00003446-200102000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Aran JM, Charlet de Sauvage R, Ruben RJ. Clinical value of cochlear microphonic recordings. In: Elberling C, Salomon G, editors. Electrocochleography. University Park Press; Baltimore: 1976. pp. 55–65. [Google Scholar]
- 32.Charlet de Sauvage R, Aran JM. [The normal electrocochleogram] Rev Laryngol Otol Rhinol (Bord) 1973 Mar-Apr;94(3):93–107. [PubMed] [Google Scholar]
- 33.Schoonhoven R, Lamore PJJ, De Laat JAPM, Grote JJ. The prognostic value of electrocochleography in severely hearing-impared infants. Audiology. 1999;38:141–154. doi: 10.3109/00206099909073016. [DOI] [PubMed] [Google Scholar]
- 34.Hood LJ, Berlin CI, Bordelon J, Rose K. Patients with auditory neuropathy/dys-synchrony lack efferent suppression of transient evoked otoacoustic emissions. J Am Acad Audiol. 2003 Aug;14(6):302–13. [PubMed] [Google Scholar]
- 35.Santarelli R, Scimemi P, Dal Monte E, Arslan E. Cochlear microphonic potential recorded by transtympanic electrocochleography in normally-hearing and hearing-impaired ears. Acta Otorhinolaryngol Ital. 2006;26:78–95. [PMC free article] [PubMed] [Google Scholar]
- 36.Breneman AI, Gifford RH, Dejong MD. Cochlear implantation in children with auditory neuropathy spectrum disorder: long-term outcomes. J Am Acad Audiol. 2012 Jan;23(1):5–17. doi: 10.3766/jaaa.23.1.2. [DOI] [PubMed] [Google Scholar]