Abstract
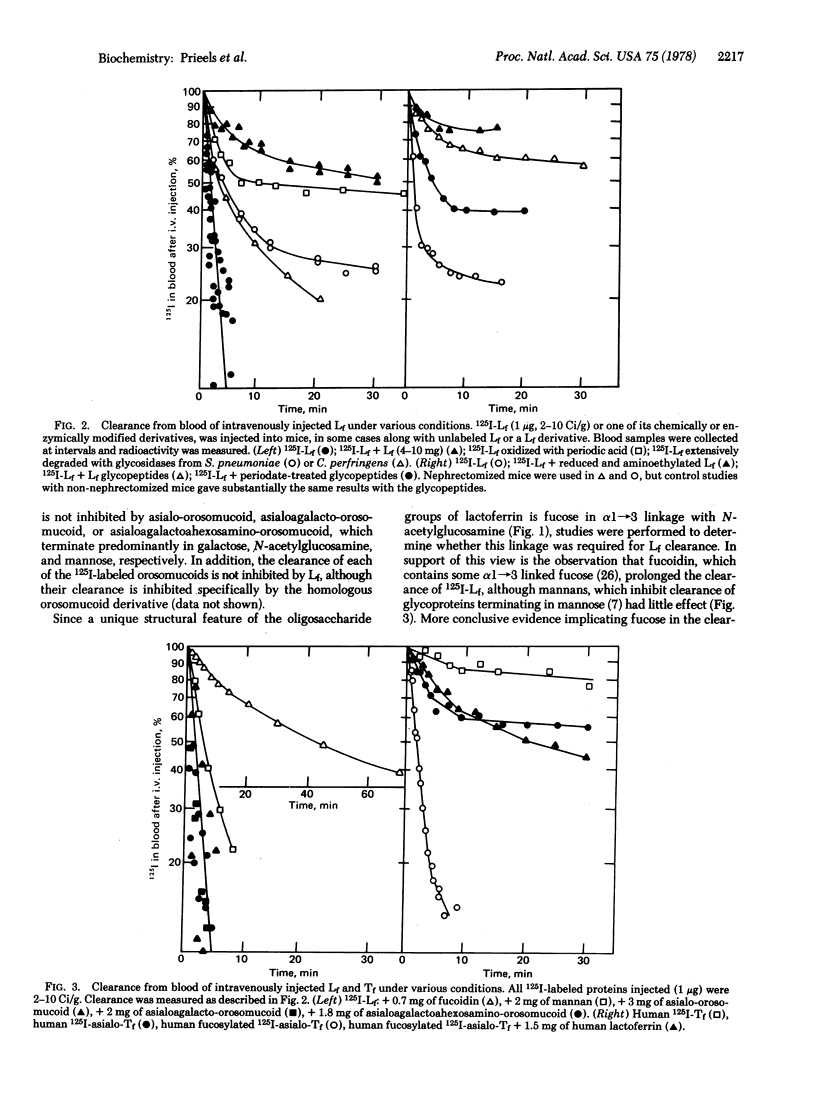
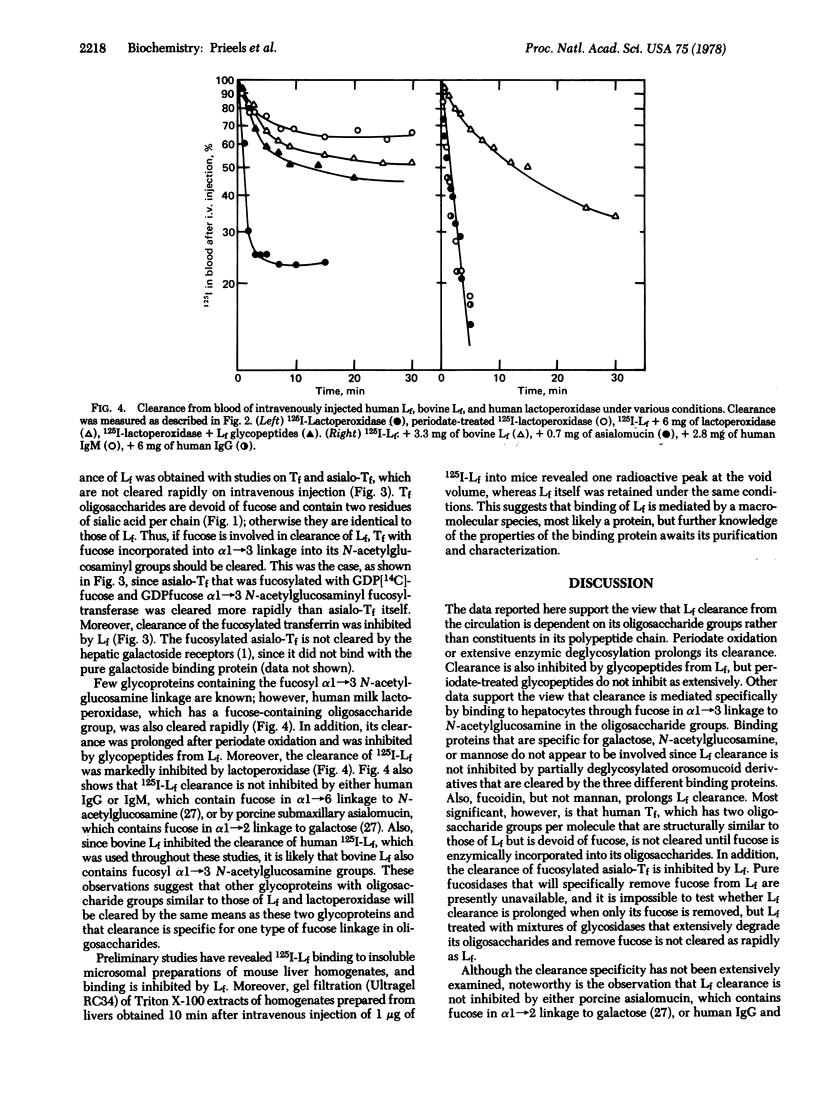
Evidence is presented suggesting that hepatocytes contain a receptor that binds glycoproteins specifically through fucose in α1→3 linkage to N-acetylglucosamine. Human lactoferrin, which contains this type of linkage, is rapidly cleared from the circulation of mice after intravenous injection, and greater than 90% of the injected material is found in hepatocytes. Binding of lactoferrin is mediated through its carbohydrate groups, since its clearance is prolonged after periodate oxidation or after its oligosaccharide groups are extensively degraded with glycosidases. In addition, glycopeptides from lactoferrin inhibit lactoferrin clearance. That lactoferrin clearance is mediated through binding to its fucosyl groups is suggested for several reasons. First, transferrin and asialotransferrin, whose oligosaccharide groups are essentially structurally identical to those of lactoferrin but devoid of fucose, are not cleared on intravenous injection. Second, when fucose is incorporated into asialotransferrin by α1→3 N-acetylglucosamine fucosyl transferase, the resulting fucosylated derivative is cleared rapidly. Neither mannan nor derivatives of orosomucoid that are cleared by binding to receptors for galactose, N-acetylglucosamine, or mannose, inhibit clearance of lactoferrin although clearance is inhibited by fucoidin. Finally, glycoproteins containing fucose in α1 → 2 linkage to galactose or α1 → 6 linkage to N-acetylglucosamine do not inhibit lactoferrin clerance by the liver. Since clearance of other glycoproteins, such as human lactoperoxidase, also appears to be mediated through binding to the same hepatocyte receptor as lactoferrin, it is concluded that the fucose-specific receptor studied here may fulfill other functions than binding lactoferrin. Preliminary studies with liver homogenates and detergent extracts of liver show binding in vitro.
Keywords: fucose, lactoferrin, glycoprotein, lectin
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisen P., Leibman A. Lactoferrin and transferrin: a comparative study. Biochim Biophys Acta. 1972 Feb 29;257(2):314–323. doi: 10.1016/0005-2795(72)90283-8. [DOI] [PubMed] [Google Scholar]
- Arnold R. R., Cole M. F., McGhee J. R. A bactericidal effect for human lactoferrin. Science. 1977 Jul 15;197(4300):263–265. doi: 10.1126/science.327545. [DOI] [PubMed] [Google Scholar]
- Ashwell G., Morell A. G. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):99–128. doi: 10.1002/9780470122860.ch3. [DOI] [PubMed] [Google Scholar]
- Castellino F. J., Fish W. W., Mann K. G. Structural studies on bovine lactoferrin. J Biol Chem. 1970 Sep 10;245(17):4269–4275. [PubMed] [Google Scholar]
- Den H., Malinzak D. A. Isolation and properties of beta-D-galactoside-specific lectin from chick embryo thigh muscle. J Biol Chem. 1977 Aug 10;252(15):5444–5448. [PubMed] [Google Scholar]
- Dorland L., Haverkamp J., Schut B. L., Vliegenthart J. F. The structure of the asialo-carbohydrate units of human serotransferrin as proven by 360 MHz proton magnetic resonance spectroscopy. FEBS Lett. 1977 May 1;77(1):15–20. doi: 10.1016/0014-5793(77)80183-x. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow L. R., Paulson J. C., Hill R. L. Systematic purification of five glycosidases from Streptococcus (Diplococcus) pneumoniae. J Biol Chem. 1977 Dec 10;252(23):8615–8623. [PubMed] [Google Scholar]
- Hill H. D., Jr, Reynolds J. A., Hill R. L. Purification, composition, molecular weight, and subunit structure of ovine submaxillary mucin. J Biol Chem. 1977 Jun 10;252(11):3791–3798. [PubMed] [Google Scholar]
- Kaplan A., Achord D. T., Sly W. S. Phosphohexosyl components of a lysosomal enzyme are recognized by pinocytosis receptors on human fibroblasts. Proc Natl Acad Sci U S A. 1977 May;74(5):2026–2030. doi: 10.1073/pnas.74.5.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T., Ashwell G. Carbohydrate structure of glycopeptides isolated from an hepatic membrane-binding protein specific for asialoglycoproteins. J Biol Chem. 1976 Sep 10;251(17):5292–5299. [PubMed] [Google Scholar]
- Kawasaki T., Ashwell G. Isolation and characterization of an avian hepatic binding protein specific for N-acetylglucosamine-terminated glycoproteins. J Biol Chem. 1977 Sep 25;252(18):6536–6543. [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Comparative aspects of glycoprotein structure. Annu Rev Biochem. 1976;45:217–237. doi: 10.1146/annurev.bi.45.070176.001245. [DOI] [PubMed] [Google Scholar]
- Paulson J. C., Rearick J. I., Hill R. L. Enzymatic properties of beta-D-galactoside alpha2 leads to 6 sialytransferase from bovine colostrum. J Biol Chem. 1977 Apr 10;252(7):2363–2371. [PubMed] [Google Scholar]
- Prieels J. P., Beyers T., Hill R. L. Human milk fucosyltransferases [proceedings]. Biochem Soc Trans. 1977;5(4):838–839. doi: 10.1042/bst0050838. [DOI] [PubMed] [Google Scholar]
- Querinjean P., Masson P. L., Heremans J. F. Molecular weight, single-chain structure and amino acid composition of human lactoferrin. Eur J Biochem. 1971 Jun 11;20(3):420–425. doi: 10.1111/j.1432-1033.1971.tb01408.x. [DOI] [PubMed] [Google Scholar]
- Schwyzer M., Hill R. L. Porcine A blood group-specific N-acetylgalactosaminyltransferase. J Biol Chem. 1977 Apr 10;252(7):2346–2355. [PubMed] [Google Scholar]
- Seglen P. O. Preparation of rat liver cells. 3. Enzymatic requirements for tissue dispersion. Exp Cell Res. 1973 Dec;82(2):391–398. doi: 10.1016/0014-4827(73)90357-1. [DOI] [PubMed] [Google Scholar]
- Teichberg V. I., Silman I., Beitsch D. D., Resheff G. A beta-D-galactoside binding protein from electric organ tissue of Electrophorus electricus. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1383–1387. doi: 10.1073/pnas.72.4.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wincek T. J., Hupka A. L., Sweat F. W. Stimulation of adenylate cyclase from isolated hepatocytes and Kupffer cells. J Biol Chem. 1975 Nov 25;250(22):8863–8873. [PubMed] [Google Scholar]
- de Waard A., Hickman S., Kornfeld S. Isolation and properties of beta-galactoside binding lectins of calf heart and lung. J Biol Chem. 1976 Dec 10;251(23):7581–7587. [PubMed] [Google Scholar]


