Abstract
Beauveria bassiana strain 04/01-Tip, obtained from a larva of the opium poppy stem gall wasp Iraella luteipes (Hymenoptera; Cynipidae), endophytically colonizes opium poppy (Papaver somniferum L.) plants and protects them against this pest. The goal of this study was to monitor the dynamics of endophytic colonization of opium poppy by B. bassiana after the fungus was applied to the seed and to ascertain whether the fungus is transmitted vertically via seeds. Using a species-specific nested PCR protocol and DNA extracted from surface-sterilised leaf pieces or seeds of B. bassiana-inoculated opium poppy plants, the fungus was detected within the plant beginning at the growth stage of rosette building and them throughout the entire plant growth cycle (about 120–140 days after sowing). The fungus was also detected in seeds from 50% of the capsules sampled. Seeds that showed positive amplification for B. bassiana were planted in sterile soil and the endophyte was again detected in more than 42% of the plants sampled during all plant growth stages. Beauveria bassiana was transmitted to seeds in 25% of the plants from the second generation that formed a mature capsule. These results demonstrate for the first time the vertical transmission of an entomopathogenic fungus from endophytically colonised maternal plants. This information is crucial to better understand the ecological role of entomopathogenic fungi as plant endophytes and may allow development of a sustainable and cost effective strategy for I. luteipes management in P. somniferum.
Introduction
The symbiosis between plants and entomopathogenic Ascomycetes such as Beauveria bassiana (Bals.) Vuill., Isaria sp., Lecanicillium spp. (Ascomycota: Hypocreales) etc., has become an important area of study in crop protection, agronomy and ecology over recent years [1]. This interest has been triggered not only because endophytic behaviour is a newly discovered aspect of the life style of arthropod pathogenic fungi, but also because entomopathogenic Ascomycetes symbioses can positively impact plant growth, resistance against invertebrate pests and fungal pathogens [1] [2] [3] [4].
We have described such an endophytic association between B. bassiana and opium poppy Papaver somniferum L., one of the oldest medicinal plants that is today the commercial source of important narcotic analgesics such as morphine [5] [6]. Beauveria bassiana strain EABb 04/01-Tip was originally isolated from naturally infected larvae of the stem gall wasp Iraella luteipes (Thompson) (Hymenoptera: Cynipidae) that were found dead within opium poppy stems. Iraella luteipes is a serious pest of opium poppy throughout most of the insect’s range of distribution [5]. Subsequently, it was shown that B. bassiana strain EABb 04/01-Tip can become an endophyte in the plant and systemically protects opium poppy against the stem gall wasp [5] [6]. We recently developed a two-step nested PCR protocol for specific identification and detection of B. bassiana in opium poppy tissues [7]; the assay is highly sensitive and detects as low as 10 fg of B. bassiana. This molecular tool was used to demonstrate that B. bassiana strain EABb 04/01-Tip, when applied to leaves as a conidial suspension, effectively colonized aerial tissues of opium poppy plants mainly through intercellular spaces and even leaf trichomes [7].
Until now, most studies of symbioses between plants and entomopathogenic Ascomycetes have focused on documenting the benefits of symbiosis to plant (crop) host fitness and tolerance to biotic factors, mainly pest and diseases, with very few studies focusing on the direct interaction between endophytic entomopathogenic Ascomycetes and their host plant [4]. Understanding the dynamics of fungal colonization of plant tissues and the mode of transmission of entomopathogenic Ascomycetes, if any, is important to identifying the incidence of an endophytic entomopathogenic Ascomycete in the host crop. It has been proposed that transmission of Clavicipitaceous endophytes, Class 1 endophytes [8], is primarily vertical, with maternal plants passing fungi on to offspring via seed infections [9]. Nonetheless, to our knowledge, no such information exists on the mechanism(s) of transmission of endophytic entomopathogenic Ascomycetes in a host.
In the present study, molecular tools were used to: (1) monitor the dynamics of colonization of B. bassiana strain EABb 04/01-Tip in opium poppy tissues during various stages of plant phenological development after applying the strain to the seed and; (2) determine whether B. bassiana strain EABb 04/01-Tip is transmitted to progeny from endophytically colonised maternal plants.
Results and Discussion
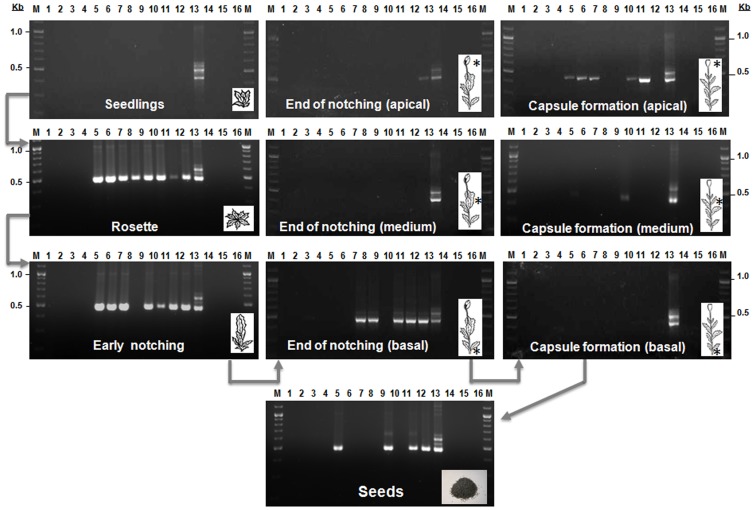
Our first objective was to ascertain whether B. bassiana strain EABb 04/01-Tip can effectively establish as an endophyte and colonize different opium poppy tissues at various stages of plant growth after spores were applied as a seed treatment. Using our PCR protocol and DNA extracted from surface-sterilised leaf pieces of B. bassiana seed-inoculated opium poppy plants, we detected the endophyte in 100% (8/8 plants), 87.5% (7/8 plants), 62.5% (5/8 plants) and 62.5% (5/8 plants) during the growth stages of rosette, early notching, end of notching, and capsule formation, respectively (Figure 1). Interestingly, at the end of notching, B. bassiana was detected mainly at the basal part of the plant, whereas at capsule formation it was detected inside the leaves at the plant tip, indicating that the B. bassiana strain had adapted its growth strategy to reach the reproductive tissues, ensuring transmission to progeny from endophytically colonised maternal plants (Figure 1). The endophyte was detected in seeds from 50% (4/8 plants) of the capsules sampled (Figure 1). We ensured that the colonization of B. bassiana was indeed endophytic by analysing with the specific PCR assay aliquots of the washing water used for surface sterilisation of the leaves. In all samples of the washings B. bassiana inoculum was absent (data not shown). We also confirmed that the amplified products were identical to the expected ITS rDNA sequence of B. bassiana strain EABb 04/01-Tip (GenBank accession numbers DQ364698 and DQ 364699) by sequencing six PCR amplification products from randomly selected plants (Figure 1). In a previous study, similar or higher percentages of detection in leaves occurred shortly after the strain was sprayed onto the leaves (i.e., 2 to 5 days after inoculation); however, the ability to detect B. bassiana decreased in within a few days although fungal hyphae still remained present in the leaf tissues [7]. It is notable that in this study, the strain could be monitored within the plant from early stages of rosette (about 35 days) through the entire plant cycle (i.e., up to capsule drying, about 120–140 days). A long association (60 days) of a GFP-tagged strain of the entomopathogen Metarhizium robertsii and haricot beans has been reported, in which the fungus endophytically colonized cortical cells of roots; however the fungus did not move vertically throughout the plant [4].
Figure 1. Monitoring of endophytic colonization by Beauveria bassiana strain EABb 04/01-Tip of opium poppy plants at different stages of growth.
Surfaced sterilised seeds were treated with the endophyte and sown in sterile soil. DNA was extracted from surface sterilised pieces of plant sampled at different growth stages (seedling, rosette, early notching, end of notching, capsule formation, and seeds). M, ÓGene Ruler DNA ladder mix (Fermentas international Inc., Burlington, Ontario, Canada ); lanes: 1 to 4, Control plants (not inoculated); lanes 5 to 12, seed-dressed inoculated plants; lane 13, positive control (B. bassiana DNA); lane 14, negative control (water, 1st-PCR round); lane 15, negative control (water, 2nd-PCR round); lane 16 (empty lane). (*) Area sampled. Lane numbers for the different growth stages do not refer to the same plants, each plant was sampled only once.
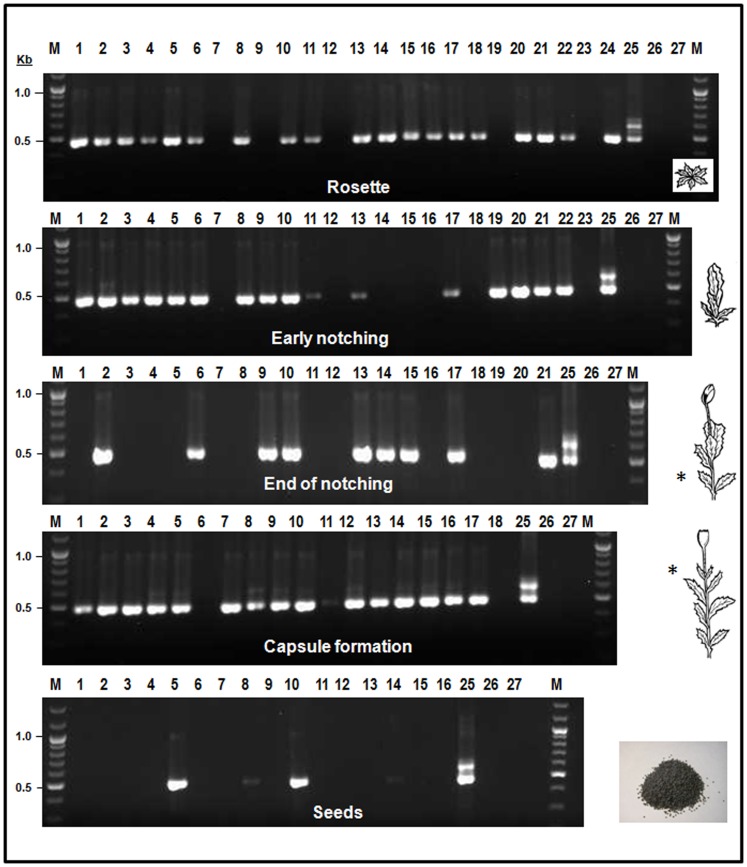
Our second objective was to determine whether B. bassiana strain EABb 04/01-Tip is transmitted to progeny from endophytically colonised maternal plants. For that purpose, seeds (approximately 20 seeds per capsule) obtained from three of the four capsules from the previous experiment that showed positive amplification for B. bassiana (Figure 1), were surface-sterilised before planting them into a sterile soil mixture. Of the surface-sterilised seeds, 24 germinated, 23 plants grew up to notching, and of those, 16 formed a mature capsule with seeds (Figure 2). We demonstrated vertical spread of the endophyte (as indicated by a positive PCR amplification) in 79.2% (19/24 plants), 69.6% (16/23 plants), 42.9% (9/21 plants) and 88.9% (16/18 plants) of plants from the growth stages rosette, early notching, end of notching, and capsule formation, respectively (Figure 2). More importantly, B. bassiana was transmitted to seeds in 25% (4 positive PCR samples) of 16 plants from the second generation that formed a mature capsule (Figure 2).
Figure 2. Monitoring of vertical transmission of Beauveria bassiana strain EABb 04/01-Tip.
Seeds that showed a positive amplification for B. bassiana from Figure 1 were sown in a sterile soil. DNA was extracted from surface sterilised pieces of plant sampled at different stages of growth (rosette, early notching, end of notching, capsule formation, and seeds). M, ÓGene Ruler DNA ladder mix (Fermentas international Inc., Burlington, Ontario, Canada ); lanes: 1 to 24, plants grown from seed lots that showed positive amplification; lane 25, positive control (B. bassiana DNA); lane 26, negative control (water, 1st-PCR round); lane 27, negative control (water, 2nd-PCR round). (*) Area sampled. Lane numbers for the different growth stages do not necessarily refer to the same plant.
Most studies on the mode of transmission of fungal endophytes have focused on systemic fungal endophytes (Clavicipitaceae) of grasses [10]. These fungi can be transmitted either horizontally by sexual spores from infected individuals in the population or vertically from infected plants to offspring via seeds [10] [11]. Studies on vertically transmitted endophytes from seed are very scarce [12]. Neotyphodium endophytes and some Epichloë species (e.g. E. festucae Leuchtm., Schardl and M.R. Siegel, and E. sylvatica Leuchtm. and Schardl) are vertically transmitted to host progeny from infected seeds. Close to 100% of the seeds produced by an infected plant contained fungal mycelium near the embryo and in the aleurone layer [10], and these seeds gave rise to asymptomatic infected plants [10].
Results from our study have shown for the first time that B. bassiana EABb 04/01-Tip strain can be vertically transmitted through maternal plants. Although we have not elucidated yet which internal seed tissues are colonized by the fungus, nonetheless, to the best of our knowledge, no such information on this transmission mechanism in endophytic entomopathogens has been published to date. Additionally, the potential role of insect pests in disseminating endophytic fungi to their host plants has been recently highlighted [13]. In this context, the possible role of I. luteipes in disseminating the endophytic B. bassiana strain EABb 04/01-Tip in natural and cultivated populations of opium poppy and even in natural populations of P. rhoeas remains to be elucidated. Nevertheless, results from this study have provided key insights to formulate a sustainable and cost effective strategy for I. luteipes management in P. somniferum.
Materials and Methods
Fungal Isolate
The B. bassiana strain EABb 04/01-Tip isolated from a dead Iraella luteipes larva collected from a field in Carmona (Seville) and shown to behave as an endophytic strain [5] when inoculated into opium plant, was used throughout the study. This strain was deposited at the C.R.A.F. University of Cordoba Entomopathogenic Fungi Collection, Cordoba, Spain, and at the Spanish Collection of Culture Types (CECT), University of Valencia, with accession n°. CECT 20744. B. bassiana strain was routinely grown on slants of Malt Agar (MA; Biocult, Madrid, Spain) at 25°C in the dark and stored at 4°C.
Plant Material
Seeds of commercial opium poppy cv. Nigrum provided by ALCALIBER S.A. (Carmona, Sevilla, Spain) were used throughout the study. Seeds were surface-sterilised in 1% NaOCl for 5 min, rinsed three times with sterile, ultrapure water, dried on sterile filter paper in a laminar flow hood for 30 min and treated with the B. bassiana strain. To ensure that the surface sterilisation was effective, some of the sterilised seeds were plated onto Sabouraud Dextrose Chloramphenicol Agar (SDCA; Biocult, Madrid, Spain) medium and incubated as described above. No microbial growth was observed after two weeks of incubation.
DNA Extraction and Quantification
For positive PCR amplification controls DNA was extracted from actively growing cultures of the B. bassiana strain EABb 04/01-Tip grown onto a film of sterile cellophane layered over a plate of SDCA. Inoculated plates were incubated for 5 to 7 days at 25°C in the dark. Mycelium grown on the cellophane surface was removed by scraping the surface with a sterile scalpel, lyophilized, and stored at –20°C until used. Genomic DNA of the fungal strain was purified from 100 mg of lyophilized mycelium [7].
Genomic DNA of opium poppy seeds and leaves was extracted using the G-SpinTM IIp Plant Genomic DNA extraction kit (Intron Biotechnology, Korea) and the Fast Prep System Bio 101 (Qbiogene) as described in our previous studies [7] [14].
All DNA samples were quantified using the Quant-iT DNA Assay Kit Broad Range fluorometric assay (Molecular Probes Inc., Leiden, The Netherlands) and a Tecan Safire fluorospectrometer (Tecan Spain, Barcelona, Spain) [14]. Genomic DNA was diluted with sterile ultrapure water as appropriate.
B. bassiana Specific Nested PCR Protocol
A two-step nested PCR protocol [7] using primers ITS1-F/ITS-4 and Bb.fw/Bb.rv, in the first and second round, respectively was used to detect endophytic colonization of the B. bassiana strain. PCR conditions for the first round were (final volume of 25 µl) 2.5 µl of 10× reaction buffer, 1.0 µM of each ITS-1F/ITS-4 primer, 50 µM of each dNTP, 2 units of FIREPol polymerase (Solis BioDyne, Tartu, Estonia), 1.5 mM MgCl2, and 1 µl of template DNA (20 ng of DNA). The cycling program included an initial denaturation step of 4 min at 95°C, followed by 40 cycles of 1 min denaturation at 94°C, 1 min annealing at 61°C, and 1 min extension at 72°C and a final 10 min extension step at 72°C followed by a 4°C soak. PCR conditions for the second round PCR were (final volume of 25 µl) 2.5 µl of 10× reaction buffer, 1.0 µM of each BB.fw/BB.rv primer, 50 µM of each dNTP, 1.5 units of DNA Polymerase, 1.5 mM MgCl2 and 1 µl of the template DNA of the first PCR product. The cycling program was similar to that of the first round PCR with 2 min at 95°C of initial denaturalization and 1 min annealing at 65°C. All PCR reactions included a positive control (B. bassiana DNA) and negative controls (no DNA) in the first and second reaction.
Amplification products were separated by electrophoresis in 1.5% agarose gels in 1× TAE buffer for 60 min at 80 V, stained with SafeView nucleic acid stain (NBS Biologicals Ltd, Cambridgeshire, UK) and visualized under UV light. The Gene-ruler™ DNA ladder mix (Fermentas, St Leon-Rot, Germany) was used for electrophoresis.
Seed Treatment with the Endophytic B. bassiana Strain
To obtain a spore suspension, the B. bassiana strain EABb 04/01-Tip was grown on Petri plates on MA for 15 days at 25°C in the dark. Conidial suspensions were prepared by scraping conidia from Petri plates into an aqueous sterile solution of 0.002% Tween 80. The conidial suspensions were filtered through several layers of cheesecloth to remove mycelium mats, and sonicated for 10 minutes to homogenise the inoculum. Concentrations of viable conidia used for inoculation were determined using a haematocytometer and diluted in 0.01% Tween 80 to obtain a suspension of 1×108 spores/ml. Viability of conidia was checked before preparation of suspensions by germination tests in liquid Czapek-Dox broth plus 1% (w/v) yeast extract medium. In all experiments, germination rates were higher than 90%.
Surfaced sterilized seeds were immersed in the conidial suspension for 30 minutes on a rotatory carrousel. Seeds were dried on sterile filter paper in a laminar flow hood for 30 min. Control seeds were treated with 0.01% Tween 80 only. The number of viable conidia of B. bassiana strain EABb 04/01-Tip on opium poppy seeds was estimated by placing three sets of 25 treated seeds in tubes with 10 ml of sterile distilled water, sonicating the suspension for 10 min, and vortexing for 1 min. Serial dilutions of the suspensions were plated onto SDCA. Plates were incubated for 10 days under the same conditions described before, andthe mean population density of B. bassiana was of 1.7±0.5×104 cfu/seed.
Monitoring of endophytic opium poppy colonization and vertical transmission of B. bassiana strain EABb 04/01-Tip via nested PCR protocol.
Beauveria bassiana treated and untreated seeds were sown in a sterile (120°C, 45 min, 2 cycles) soil mixture (clay loam/peat, 2∶1, vol/vol) in 350 cm3 pots and incubated in a growth chamber (Sanyo MLR-350 H, Sanyo Electric Co., Ltd. Japan) at 23°C±1, 50/90% relative humidity (RH) and a 12-h photoperiod of fluorescent light at 360 µE·m–2·s–1. Water was provided daily as needed and sterile 50% Hoagland solution (Hoagland & Arnon, 1950) was added weekly to the pots. There were 50 or 25 replicated pots (one plant per pot) for the inoculated or control seeds, respectively.
Colonisation of plants by B. bassiana strain EABb 04/01-Tip was determined at different plant growth stages (GS) including seedling (GS1), rosette (GS2), early notching (GS3), end of notching (GS4), capsule formation (GS5) and from seeds formed in the capsules (GS6). At each sampling time, a series of eight inoculated plants or four control plants (not inoculated) were randomly selected. Each plant (pot) was sampled only once. Plants from GS1 were destructively sampled due to small size. For GS2 and GS3 the leaf base close to the stem was preferentially sampled from randomly selected leaves. For stages GS4 and GS5 three leaves from different parts of the plant were sampled including basal, medium and apical leaves. For GS6 a small amount of seeds was used for PCR detection to ensure enough viable germinating seeds for the next generation.
All leaves were surface-sterilised with 1% sodium hypochlorite for 2 min, rinsed twice in sterile distilled water, and dried on sterile filter paper. Pieces of leaves (each approximately 2 cm2) were cut with a sterile scalpel and immediately frozen at −20°C. Frozen samples were lyophilized and ground before DNA extraction as previously described [7]. B. bassiana treated seeds or seeds from second-generation plants were not surfaced sterilized and processed directly for DNA extraction as described above. DNA isolated from plant samples were processed using the two-step nested PCR protocol as described above. The universal primers ITS-5 and ITS-4 [15] were used as described above as an internal positive control for successful PCR amplification and to test for the presence of PCR inhibitors.
To demonstrate the vertical transmission of B. bassiana strain EABb 04/01-Tip, at least 20 surface sterilised seeds from each of three capsules (namely 5, 9 and 11) that showed a positive PCR amplification signal for B. bassiana were planted in pots containing sterile soil (one seed per pot), and incubated and grown as described above. Leaf and seed samples from the same plant from growth stages GS2, GS3, GS4, GS5 and GS6 were sampled and processed as described above for PCR detection of the B. bassiana strain using the two-step nested specific-PCR protocol [7]. Opium poppy seeds showed 50% germination with 28 plants reaching the rosette stage. Seedlings (GS1) were not sampled because at this growth stage sampling would have meant destroying the plant; therefore, making impossible the inspection of further growth stages and the demonstration of vertical transmission.
Sequencing of the ITS Region of B. bassiana Strain EABb 04/01-Tip
To demonstrate that the amplified product from second-round PCR corresponded to the B. bassiana strain EABb 04/01-Tip, six PCR products were randomly chosen from second-round PCR and purified using a gel extraction kit (FavorPrepTM Gel/PCR Purification, Favorgen, Taiwan), quantified with the Quant-iT DNA Assay Kit Broad Range fluorometric assay as described before, and used for direct DNA sequencing with primers Bb.fw/Bb.rv in both directionsusing a terminator cycle sequencing ready reaction kit (BigDye, Perkin-Elmer Applied Biosystems, Madrid, Spain) according to the manufacturer’s instructions at the STABVIDA sequencing facilities (Caparica, Portugal).
Acknowledgments
We are grateful to Miguel Montes-Borrego and Javier Muñoz-Ledesma (ALCALIBER I+D+i S.L.U) for useful suggestions and technical advice and David M. Weller (USDA-ARS, Pullman, WA) for critically reviewing and editing the manuscript prior to final submission. We also thank Carlos Campos, Guillermo León and Sandra Castuera for technical support.
Contributions
Conceived and designed the experiments: EQM BBL. Performed the experiments: CLD. Analyzed the data: EQM BBL. Contributed reagents/materials/analysis tools: EQM. Wrote the paper: EQM BBL.
Funding Statement
This research was supported by project AGR-7681 from the Consejería de Innovación, Ciencia y Empresa de la Junta de Andalucía, Spain. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Vega FE, Goettel MS, Blackwell M, Chandler D, Jackson MA, et al. (2009) Fungal entomopathogens: new insights on their ecology. Fungal Ecol 2 149–159. [Google Scholar]
- 2. Jaber LR, Vidal S (2010) Fungal endophyte negative effects on herbivory are enhanced on intact plants and maintained in a subsequent generation. Ecol Entomol 35 25–36. [Google Scholar]
- 3. Ownley BH, Gwinn KD, Vega FE (2010) Endophytic fungal entomopathogens with activity against plant pathogens: ecology and evolution. BioControl 55 113–128. [Google Scholar]
- 4. Sasan RK, Bidochka MJ (2012) The insect-pathogenic fungus Metarhizium robertsii (Clavicipitaceae ) is also an endophyte that stimulates plant root development. Am J Bot 99 101–107. [DOI] [PubMed] [Google Scholar]
- 5. Quesada Moraga E, Landa BB, Muñoz-Ledesma FJ, Jiménez-Díaz RM, Santiago-Álvarez C (2006) Endophytic colonisation of opium poppy, Papaver somniferum, by an entomopathogenic Beauveria bassiana strain. Mycopathologia 161 323–329. [DOI] [PubMed] [Google Scholar]
- 6. Quesada-Moraga E, Muñoz-Ledesma FJ, Santiago-Álvarez C (2009) Systemic protection of Papaver somniferum L. against Iraella luteipes (Hymenoptera: Cynipidae) by an endophytic strain of Beauveria bassiana (Ascomycota: Hypocreales). Environ Entomol 38 723–730. [DOI] [PubMed] [Google Scholar]
- 7. Landa BB, López-Díaz C, Jiménez-Fernández D, Montes-Borrego M, Muñoz-Ledesma FJ, et al. (2013) In-planta detection and monitorization of endophytic colonization by a Beauveria bassiana strain using a new-developed nested and quantitative PCR-based assay and confocal laser scanning microscopy. J Invert Pathol 114 128–138. [DOI] [PubMed] [Google Scholar]
- 8. Rodriguez RJ, White JF, Arnold AE, Redman RS (2009) Fungal endophytes: diversity and functional roles. New Phytol 182 314–330. [DOI] [PubMed] [Google Scholar]
- 9. Saikkonen K, Ion D, Gyllenberg M (2002) The persistence of vertically transmitted fungi in grass metapopulations. Proc Biol Sci 269 1397–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zabalgogeazcoa I (2008) Fungal endophytes and their interaction with plant pathogen. Spa J Agric Res, n°Extra 1 138–146. [Google Scholar]
- 11. Ahlholm JU, Helander M, Lehtimäki S, Wäli P, Saikkonen K (2002) Vertically transmitted fungal endophytes: different responses of host-parasite systems to environmental conditions. Oikos 99 173–183. [Google Scholar]
- 12. Gallery RA, Dalling JW, Arnold AE (2007) Diversity, host affinity and distribution of seed-infecting fungi: a case study with Cecropia. . Ecol 88 582–588. [DOI] [PubMed] [Google Scholar]
- 13. Giordano L, Garbelotto M, Nicolotti G, Gonthier P (2012) Characterization of fungal communities associated with the bark beetle Ips typographus varies depending on detection method, location, and beetle population levels. Mycol Progress 12 127–140. [Google Scholar]
- 14. Landa BB, Montes-Borrego M, Muñoz-Ledesma FJ, Jiménez-Díaz RM (2007) Phylogenetic analysis of Downy Mildew pathogens of opium poppy and PCR-based in planta and seed detection of Peronospora arborescens . Phytopathology 97 1380–1390. [DOI] [PubMed] [Google Scholar]
- 15. White TJ, Bruns T, Lee S, Taylor J (1990) PCR protocols: a guide to methods and application, chap. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics, 315–322. Academic Press, Inc., San Diego, California. [Google Scholar]