Abstract
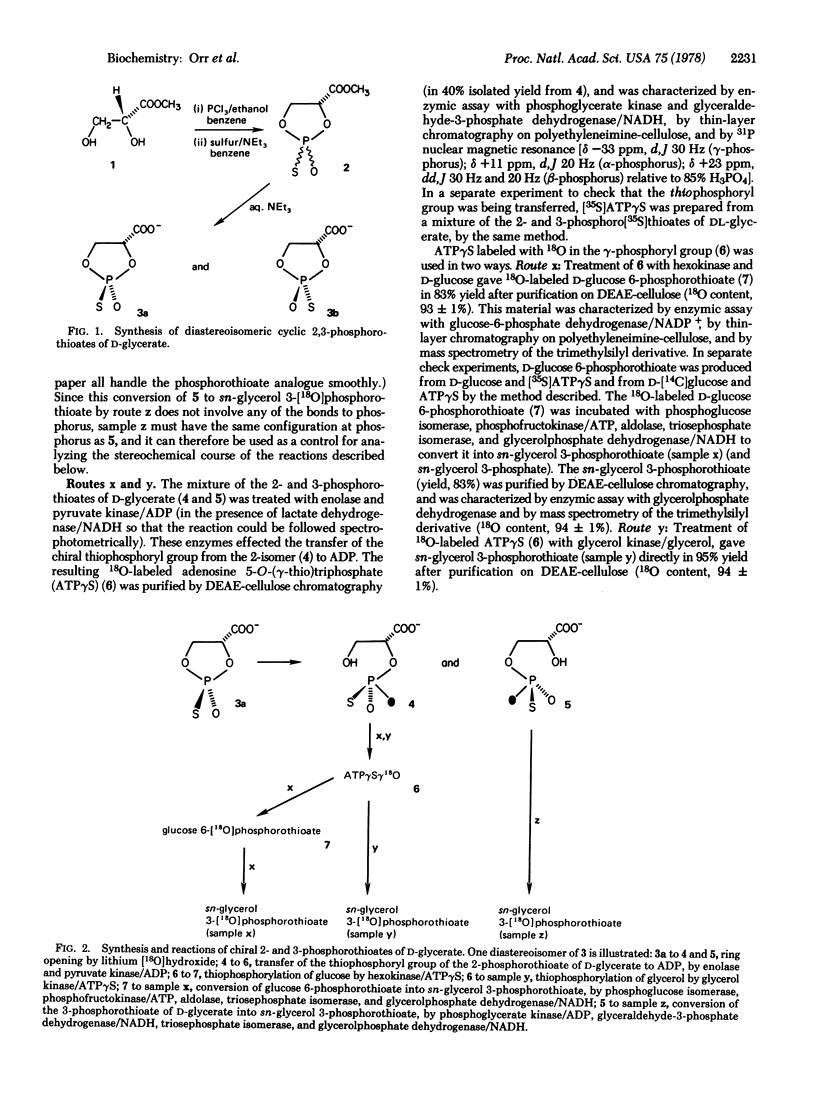
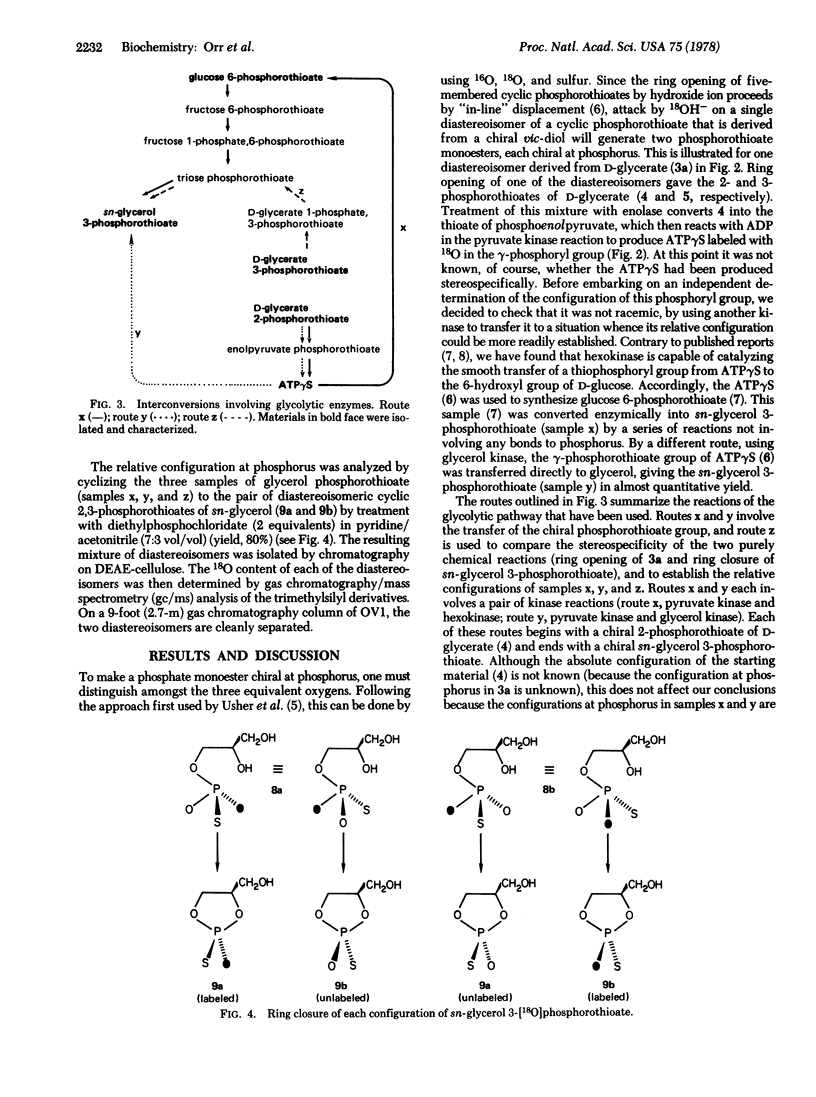
The 2-[18O]phosphorothioate of D-glycerate, chiral at phosphorus, was prepared. The chiral phosphoryl group was transferred enzymically to ADP [by using enolase and pyruvate kinase (ATP:pyruvate 2-O-phosphotransferase; EC 2.7.1.40)] resulting in the synthesis of adenosine 5'-O-([gamma-18O],gamma-thio)triphosphate. This labeled ATP was used as a thiophosphoryl group donor in the reactions catalyzed by glycerol kinase (ATP:glycerol 3-phosphotransferase; EC 2.7.1.30) and by hexokinase (ATP:D-hexose 6-phosphotransferase; EC 2.7.1.1). The product from the latter (glucose 6-phosphorothioate) was converted enzymically into glycerol phosphorothioate. Determination of the relative configurations and diastereoisomeric purities of the samples of glycerol phosphorothioate demonstrates that all three phosphokinases (pyruvate kinase, glycerol kinase, and hexokinase) transfer the thiophosphoryl group with complete stereospecificity, and further shows that these reactions follow an identical stereochemical course.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gratecos D., Fischer E. H. Adenosine 5'-O(3-thiotriphosphate) in the control of phosphorylase activity. Biochem Biophys Res Commun. 1974 Jun 18;58(4):960–967. doi: 10.1016/s0006-291x(74)80237-8. [DOI] [PubMed] [Google Scholar]
- Morrison J. F., Heyde E. Enzymic phosphoryl group transfer. Annu Rev Biochem. 1972;41(10):29–54. doi: 10.1146/annurev.bi.41.070172.000333. [DOI] [PubMed] [Google Scholar]
- Schlimme E., Lamprecht W., Eckstein F., Goody R. S. Thiophosphate-analogues and 1-N-oxides of ATP and ADP in mitochondrial translocation and phosphoryl-transfer reactions. Eur J Biochem. 1973 Dec 17;40(2):485–491. doi: 10.1111/j.1432-1033.1973.tb03217.x. [DOI] [PubMed] [Google Scholar]
- Usher D. A., Erenrich E. S., Eckstein F. Geometry of the first step in the action of ribonuclease-A (in-line geometry-uridine2',3'-cyclic thiophosphate- 31 P NMR). Proc Natl Acad Sci U S A. 1972 Jan;69(1):115–118. doi: 10.1073/pnas.69.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usher D. A., Richardson D. I., Jr, Eckstein F. Absolute stereochemistry of the second step of ribonuclease action. Nature. 1970 Nov 14;228(5272):663–665. doi: 10.1038/228663a0. [DOI] [PubMed] [Google Scholar]


