Abstract
Immune homeostasis in peripheral tissues is achieved by maintaining a balance between pathogenic effector T cells (Teff) and protective Foxp3+ regulatory T cells (Treg). Using a mouse model of an inducible tissue-antigen we demonstrate that antigen (Ag) persistence is a major determinant of the relative frequencies of Teff and Treg cells. Encounter of transferred naïve CD4+ T cells with transiently expressed tissue-Ag leads to generation of cytokine-producing Teff cells and peripheral Treg cells. Persistent expression of Ag, a mimic of self Ag, leads to functional inactivation and loss of the Teff cells with preservation of Treg in the target tissue. The inactivation of Teff cells by persistent Ag is associated with reduced ERK phosphorylation (pERK), whereas Treg cells show less reduction in pERK and are relatively resistant to ERK inhibition. Our studies reveal a crucial role for Ag in maintaining appropriate ratios of Ag-specific Teff to Treg cells in tissues.
INTRODUCTION
The choice between tolerance and autoimmunity is determined, to a significant extent, by the relative generation and maintenance of Teff and Treg cells specific for self-Ags. An imbalance in this ratio is thought to underlie inflammatory and autoimmune disorders. Therefore, defining the variables that control these cell populations is critical for understanding the pathogenesis of autoimmune disease and for developing rational therapeutic approaches. Emerging treatments for both systemic and tissue-specific autoimmunity focus on inhibiting the activation of pathogenic cells and augmenting the pathways that suppress these cells and boost Treg function (1). It is also becoming increasingly clear that many of the dominant mechanisms of immune regulation occur in peripheral tissues and not in lymphoid organs, highlighting the importance of studying immune responses in tissues (2)(3).
In order to analyze the control of Teff and Treg cells in tissues, we have developed an experimental model in which a self-Ag is inducibly expressed in the skin (4). Studies with this model have shown that Ag recognition generates cytokine-producing Teff cells that induce an inflammatory skin disease, followed by the activation of Foxp3+ Treg cells that mediate disease resolution. A fraction of the Treg cells survive in an IL-7 dependent manner (5) in the skin in the absence of continuous Ag expression, and these “memory Treg” cells suppress subsequent inflammatory reactions in the tissue.
To further elucidate the mechanisms responsible for controlling the Teff/Treg cell balance, we utilize an adoptive transfer approach in which a single bolus of Ag-specific T cells is given to mice in which expression of tissue Ag can be turned on and off. An advantage of this system is the ability to follow Teff and peripheral Treg (pTreg) cells developing in the same animal from a single naïve cell population. Additionally, both the target tissue and lymphoid organs can be accessed and analyzed. For these reasons, our model is uniquely amenable to dissection of the mechanisms underlying the Teff/Treg cell balance in tissues. We have found that the duration of Ag exposure is a major determinant of the Teff/Treg cell balance in the Ag-expressing tissue, and that there are striking differences in the responses of these cells to transient or persistent Ag recognition. The implications of these results for self-tolerance and for therapeutic tolerance induction strategies are discussed.
MATERIALS and METHODS
Mice
All animal studies were performed in compliance with institutional guidelines in a specific pathogen-free facility. K5/rTA and TGO (TRE-Ova) mice were crossed and designated K5/TGO mice as described previously. (4) The double-transgenic K5/TGO mouse line was crossed onto TCRα−/− mice on Balb/c background. (6) DO11.10 TCR-transgenic mice (7) were crossed with a Rag2−/−/CD90.1+ (“WT”) or Rag2−/−/CD90.2+ mice carrying a mutation if Foxp3 (“scurfy”) mutation (8).
Adoptive transfer of T cells
Single cell suspensions from all lymph nodes from DO11.10/Rag2−/−/CD90.1+ were prepared by mechanical disruption of LN. 3–8×105 LN cells were adoptively transferred i.v. into sex-matched K5/TGO/TCRα−/− recipient mice.
Skin disease
To induce expression of the TGO transgene in the skin, K5/TGO/TCRα−/− mice were maintained on 1g/kg doxycycline chow (Bio-Serv, Frenchtown, NJ). A 15-point clinical scoring scale was utilized to quantify skin disease. The clinical parameters of scaling, alopecia, erythema, level of activity, and peri-ocular inflammation were each given a score of 0 – 3. Scores for individual clinical parameters were summed for each mouse.
Cell isolation from skin
To isolate skin-infiltrating cells, shaved dorsal and ventral trunk skin, and ears were harvested, minced and digested for 40 mins with 2.0 mg/ml collagenase from Clostridium histolyticum (Sigma,) 0.5 mg/ml hyaluronidase (Sigma), and 0.1 mg/ml DNAse (Sigma). Single cells were filtered, washed with tissue culture medium and stained for flow cytometry or cultured for intracellular cytokine staining.
Restimulation for intracellular cytokine staining
Skin draining lymph nodes (sdLNs) or skin single cell suspensions were cultured overnight with LPS/Ova-pulsed BM-DCs (4:1 skin or LN cells: DC ratio) in supplemented RPMI-1640 tissue culture medium. Cultures were then treated with Brefeldin A for 3h and stained for FACS analysis.
In vitro culture of T cells
Single cell suspensions of lymph nodes and spleens of Balb/c mice were labeled with 1 µM CFSE and cultured for 6 days in 24 well plates coated with α-CD3 and α-CD28 (clones 2C11 and PV1, 1 ug/ml each). PD0325901 (Selleckchem), a selective and non ATP-competitive MEK inhibitor that inhibits the phosphorylation of ERK1 and ERK2, was added at 0 – 10 nM.
Statistics
Statistical analysis was done using GraphPad Prism software (GraphPad). p values were calculated using a two-tailed unpaired or paired (Fig. 4D, right panel) t test. Nomenclature: * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001
FIGURE 4.
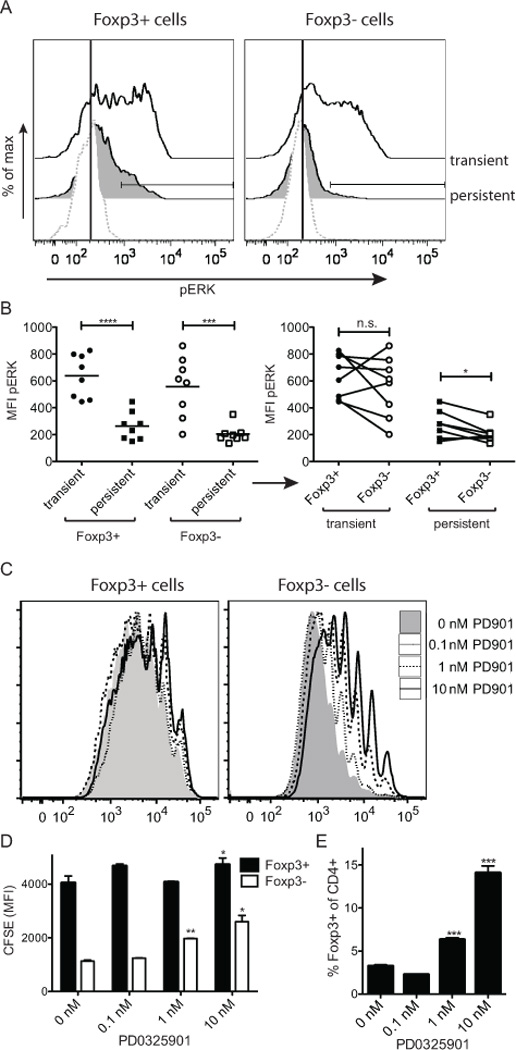
Persistent Ag expression results in reduced phospho-ERK induction. DO11/Rag−/− cells were adoptively transferred into K5/TGO/TCRα−/− recipients. Recipient mice were treated as in Fig. 3 (i.e. transient or persistent Ag expression). (A, B) 50–70 days post-transfer mice were i.v. injected with 86 µg Ova peptide and 17 min later sdLN cells were fixed with 1.6% paraformaldehyde (Electron Microscopy Sciences), permeabilized with ice-cold methanol and stained with p-p44/42 MAPK (Erk1/2) (Thr202/Tyr204, 197G2; Cell Signaling Technologies) for analysis by flow cytometry. (A) Representative histograms showing phospho-ERK in sdLN. (B) MFI of phospho-ERK signal of DO11 T cells in sdLN gated on either the Foxp3+ or Foxp3− population (left). Same data as left but Foxp3+ or Foxp3− population within individual mice paired (right). Data pooled from two independent experiments. (C – E) CFSE labeled cells were continuously stimulated in vitro with plate bound α-CD3/CD28 for 6 days in the presence of increasing concentrations of the MEK inhibitor PD0325901. (C) Representative FACS plots of CFSE signals of T cells gated on CD4+Foxp3+ and CD4+Foxp3−. (D) MFI of CFSE of Foxp3+CD4+ and Foxp3−CD4+ cells are summarized. (E) Relative enrichment of Foxp3+ cells with increasing concentrations of PD0325901 is quantified. Each data point measured in triplicates; one representative experiment of two.
RESULTS and DISCUSSION
CD4+ T cells induce a spontaneously resolving inflammatory disease following encounter with cutaneous antigen
The model of inducible Ag we have developed (4) involves two transgenes. The ‘TGO’ transgene expresses a membrane-associated form of ovalbumin (Ova) under the control of a tetracycline-response element, and the ‘K5’ transgene expresses the tetracycline transactivator under the control of the keratin K5 promoter. In mice expressing both constructs (i.e., K5/TGO), expression of the Ag in the skin is dependent on exposure to the tetracycline analog, doxycycline (dox) (4).
To define the consequences of Ag recognition on the generation and maintenance of Teff and Treg in a peripheral tissue, we transferred Ova-specific CD4+ T cells from DO11/Rag−/− donors (DO11 T cells expressing a single TCR) into K5/TGO recipients crossed onto a TCRα−/− background. Upon transfer of DO11 cells and induction of cutaneous Ag expression, K5/TGO/TCRα−/− mice developed a severe inflammatory dermatitis within 2 weeks, associated with weight loss and death of ~30% of the recipients (Fig. 1A–D). Disease was associated with expansion of the DO11 population in the skin draining lymph nodes (sdLNs), production of the effector cytokine IFN-γ, and infiltration and persistence of these cells in the skin (Fig. 1E, F). Importantly, the production of IL-2 and IFN-γ by skin infiltrating Teff cells was transient and appreciable levels of cytokines were only detected in the first two weeks after transfer.
FIGURE 1.
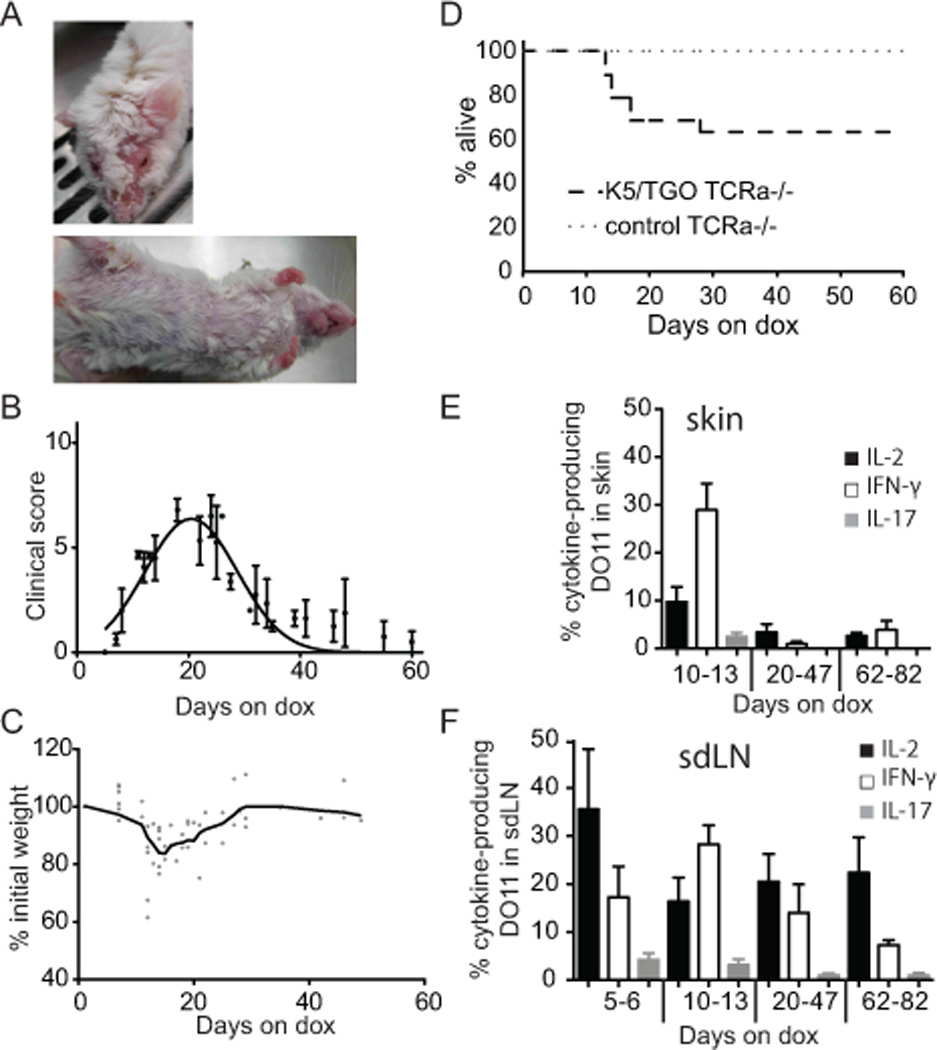
T cells induce a spontaneously resolving inflammatory disease following encounter with cutaneous Ag. DO11/Rag−/− cells were adoptively transferred i.v. into K5/TGO/TCRα−/− recipients. Mice were fed with dox chow from the time of T cell transfer (=day 0). (A) Representative pictures of clinical appearance of mice on day 19. (B) Course of clinical skin disease. (Error bars show SEM). (C) Weight loss during the disease course. (D) Survival of K5/TGO/TCRα−/− recipients. Single-transgenic TCRα−/− recipients served as controls. (n = 19) (E,F) Skin or sdLN cells were stimulated overnight with Ova-pulsed BM-DCs and stained for expression of IL-2, IFN-γ, and IL-17A. FACS data are gated on live donor CD4+ KJ-126+ Foxp3− CD90.1+ DO11 cells. Cytokine production within the Foxp3− DO11 population in (E) skin and (F) sdLNs over the course of disease is shown. (Cumulative data from four experiments, n = 3–5 mice/time point. Error bars show SEM).
Frequency of Treg increases progressively and Foxp3+ cells are required for resolution of disease
One of the striking features of this acutely induced inflammatory reaction was that it resolved spontaneously, despite the fact that mice were maintained on dox and Ag was continuously expressed, and Ag specific T cells remained in the skin (Fig. 2A). Resolution was associated with the generation and progressive accumulation of Foxp3+ Treg cells. Because the starting population of DO11/Rag−/− T cells does not contain Foxp3-expressing Treg (9), all Treg cells observed in this system are pTreg. Approximately 40 days after Ag induction there was a reversal of the predominant cell population in the skin from Foxp3− effector cells to Foxp3+ Treg cells (Fig. 2B). To test whether T cell lymphopenia affected the generation and maintenance of pTreg cells in our model, we adoptively transferred about an 8-fold excess of WT polyclonal T cells into K5/TGO/TCRα−/− recipients prior to DO11 T cell transfer and found that the generation of Ag-specific Foxp3+ T cells was unaffected by the presence of irrelevant T cells (data not shown).
FIGURE 2.
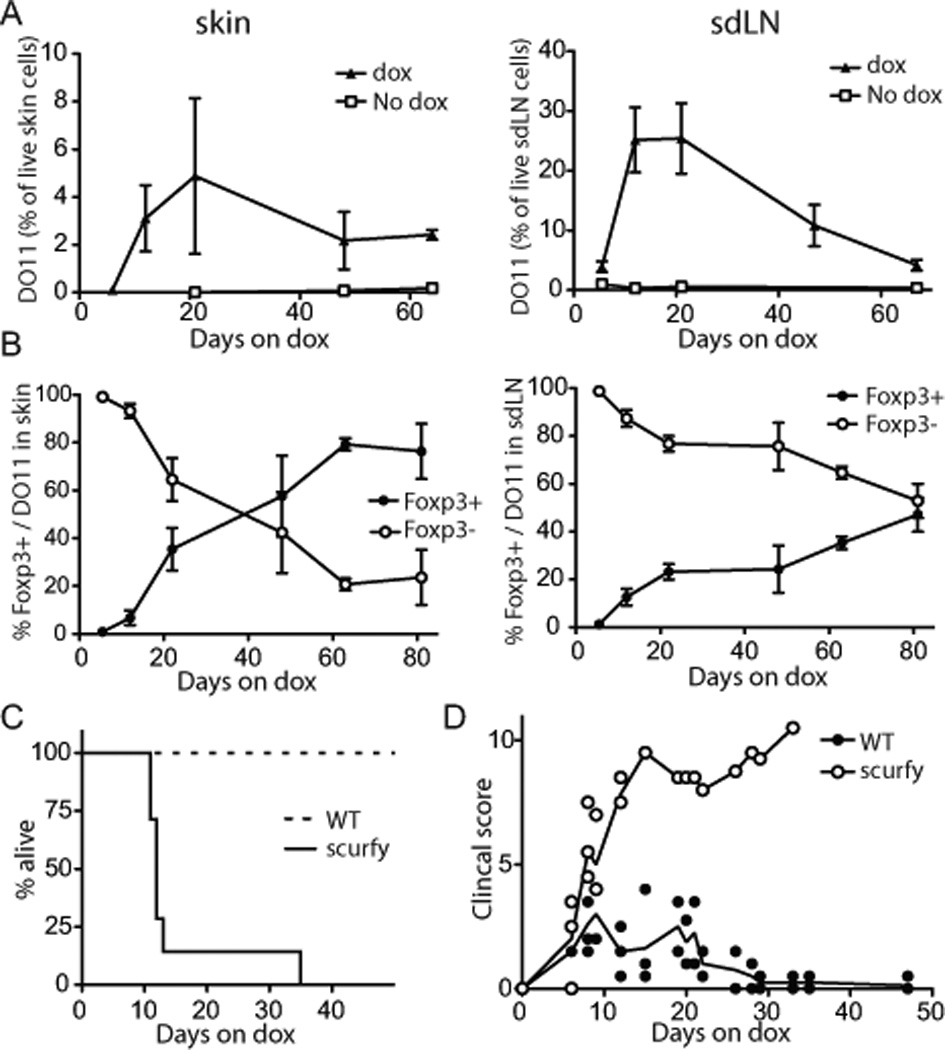
The frequency of Foxp3+ Treg increases progressively. DO11/Rag−/−/CD90.1+ LN cells were adoptively transferred into K5/TGO/TCRα−/−. (A) Skin (left) and sdLN (right) single cell suspensions from recipients over the course of disease were stained for CD90.1, CD4, KJ1-26 (specific for the DO11 TCR) and analyzed by flow cytometry, excluding dead cells. (Error bars show SEM; n = 2–8/time point) (B) % Foxp3− Teff cells and Foxp3+ Treg cells within the skin infiltrating (left) and sdLNs (right) DO11.10 population over the course of disease. (Error bars show SEM; cumulative data from 10 independent experiments, n = 2–8/time point). (C, D) 0.45×106 DO11/Rag−/− (WT) or DO11/scurfy/Rag−/− (scurfy) LN cells were adoptively transferred. (D) Survival of K5/TGO/TCRα−/− recipients. (E) Course of clinical skin disease. (scurfy: n = 7; WT: n = 5)
To determine if Treg were required for disease resolution, we used CD4+ T cells that lack functional Foxp3+ Treg cells due to the scurfy-mutation in Foxp3 (8). These cells induced non-resolving disease that led to death in 100% of the animals (Fig. 2C, D), which indicated that potential Teff intrinsic tolerance mechanisms (e.g. exhaustion or deletion) were not sufficient for disease resolution but that pTreg were required to induce resolution.
Persistent exposure to self-antigen leads to loss of Teff cells and relative preservation of Treg
To test the hypothesis that the duration of Ag exposure may influence the Teff/Treg balance, DO11 T cells were transferred into K5/TGO/TCRα−/− recipients and dox was administered either for 7 days only (transient Ag expression) or 60–80 days (persistent Ag expression), and all mice were examined on day 60–80 after cell transfer. The initial skin inflammation was comparable in both groups. The proportion of Foxp3+ and Foxp3− T cells, however, was markedly different, especially in the skin. When Ag expression was persistent, ~80% of the DO11 cells that accumulated in the skin were Foxp3+ Treg. In contrast, following transient (7-day) Ag expression, the vast majority of the DO11 cells in the skin were Foxp3− cells (Fig. 3A). Differences in the ratio of Foxp3+ and Foxp3− T cells were due to the loss of Foxp3− T cells with persistent Ag expression, as the absolute number of Foxp3+ T cells was virtually unchanged (Fig. 3B). The reduction in Foxp3− cells was more pronounced in the skin than in the skin draining lymph nodes. Initially, both populations underwent a rapid burst of proliferation upon induction of Ag expression (as measured by expression of Ki67; Fig. 3C, D). After transient Ag exposure, Foxp3− cells continued to proliferate in the absence of Ag, which is expected for Teff memory cells (10)(11), whereas persistent Ag expression led to a marked reduction in the proliferation of Foxp3− cells (Fig. 3C). In contrast, with either persistent antigen or transient antigen, 30% of antigen specific Foxp3+ Treg within the skin draining LN were proliferating at day 66 – 82 (Fig. 3D).
FIGURE 3.
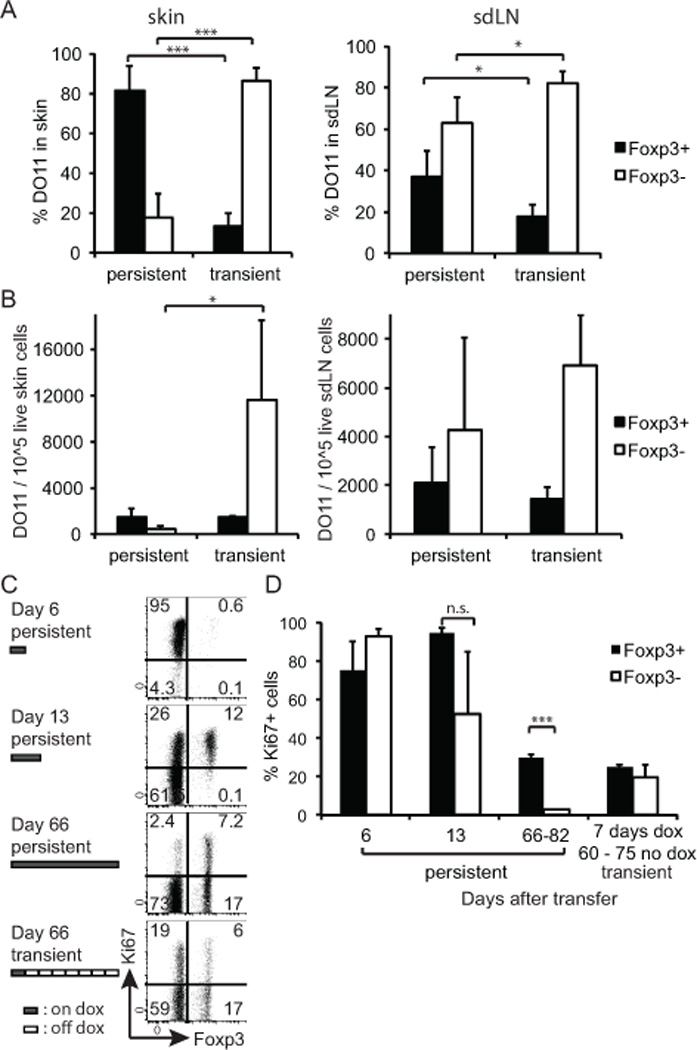
Persistent Ag expression leads to a specific loss of Teff cells in the periphery and relative preservation of Treg. (A) DO11/Rag−/− cells were adoptively transferred to K5/TGO/TCRα−/− and recipient mice were either fed with dox for 7 days and then switched back to regular feed (transient expression) or kept on dox feed for the entire time of the experiment (persistent expression). Single cell suspensions of skin (left) and sdLNs (right) were prepared between days 60–82 post transfer and analyzed by FACS. Percentage of Foxp3+ and Foxp3− T cells within the skin-infiltrating DO11 T cell population. Data pooled from three independent experiments. (Total number of mice analyzed n = 3–5/condition; error bars show SD) (B) As (A), showing the absolute number of Foxp3+ and Foxp3− DO11 T cells/105 live cells in the skin (left) or sdLN (right). (C) sdLN cells were analyzed for expression of Foxp3 and Ki67 on indicated days after DO11 transfer. Data are gated on live donor CD4+, CD90.1+ cells. (D) The percentage of Ki67-expressing LN DO11 cells within the Fopx3+ and Foxp3− population, respectively, was calculated. (Error bars show SD; n = 2–3/condition and time point.)
In addition to reduced proliferation of Foxp3− T cells, persistent Ag expression resulted in a diminution of both IL-2 and IFN-γ expression in Foxp3− cells upon restimulation with Ova when compared with the same population transiently exposed to Ag (Supplemental Fig. 1). Again, this effect was most pronounced in the skin compared to sdLNs. Foxp3+ Treg did not produce IL-2, IFN-γ or IL17 under any condition.
Signaling pathways influenced by duration of antigen exposure
Numerous studies have suggested differences in signaling pathways between Teff and Treg cells (12)(13)(14). Using phosphoflow analyses after ex vivo restimulation, we found that both populations in sdLNs showed reduced phospho-ERK (pERK) induction upon ex vivo restimulation following prolonged Ag exposure in vivo (Supplemental Fig. 2A). Similar results were seen in skin-infiltrating cells, although the percentage of cells in which pERK was induced was less than in sdLNs (Supplemental Fig. 2B). In vivo stimulation with Ag also resulted in reduced pERK in both populations after prolonged antigen exposure (Fig 4A). However, the proportion of pERK+ cells was lower in Foxp3− subset with the persistence of antigen than in the Foxp3+ subset when restimulated with intravenous Ova (Fig. 4B, right). To further ask whether ERK phosphorylation differentially impacts the proliferation of Treg and Teff we inhibited ERK in vitro using the MEK inhibitor PD0325901. This treatment inhibited the proliferation of Foxp3− cells at a ten-fold lower concentration than that of Foxp3+ cells, which were only mildly affected at the highest dose (Fig. 4C,D). Increasing doses of inhibitor thus resulted in a relative enrichment of Foxp3+ cells in the culture (Fig. 4E). These data indicate that the proliferation of Foxp3+ cells is relatively independent of ERK phosphorylation compared to Foxp3− cells and down-modulation of ERK-signals in response to chronic TCR stimuli might be a means to control the balance of self-reactive Foxp3+ and Foxp3− cells.
These studies highlight the essential role of peripherally generated Treg in regulating pathologic immune responses directed at tissue Ag, and the importance of chronicity of Ag exposure in regulating the Teff to Treg cell balance in the target tissue. Persistent Ag expression resulted in tolerance, manifested by loss and functional inactivation of Foxp3− effector cells and relative preservation of suppressive Foxp3+ Treg. Treg continued to proliferate even in the face of reduced pERK, likely because Treg proliferation is less dependent on pERK than Teff cell proliferation. In fact, other studies have shown that Treg cells do not require many signals thought to be essential for the responses of Teff cells, such as Akt and mTOR (15). These results suggest that Treg are more resistant to tolerance than Teff cells, which allows Treg to function in the face of constant encounter with a self-Ag. Elucidating the mechanisms that determine the strikingly different reactions of Teff and Treg cells to tissue Ag in vivo may reveal the critical pathways that control the Teff/Treg balance and provide insight into the pathogenesis of tissue-specific autoimmunity.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Carlos Benitez (University of California, San Francisco [UCSF], CA) for assistance in animal husbandry, Sara Isakson (UCSF, CA) for genotyping, Dr. Katya Ravid and Greg Martin (UCSF, CA) for derivation of TRE-TGO transgenic mice, and Haopeng Wang (UCSF, CA) and Bill O’Gorman (Stanford University, Stanford, CA) for advice with TCR signaling experiments. We thank Dr. Steven F. Ziegler (Benaroya Research Institute, Seattle, WA) for K5-rTA transgenic mice.
Funding sources:
This work was funded in part by National Institute of Health grants R01 AI73656 and U19 AI56388 (to A.K.A.). I.K.G. was supported by an Erwin Schroedinger Fellowship from the Austrian Science Fund (FWF) (J2997-B13) and an NIH R03 grant (1R03AR064554-01). M.D.R. is supported by an NIH K08 grant (1K08AR062064-01), a Burroughs Wellcome Career Award for Medical Scientists (CAMS), the Scleroderma Research Foundation, and the UCSF Department of Dermatology.
REFERENCES
- 1.Rosenblum MD, Gratz IK, Paw JS, Abbas AK. Treating human autoimmunity: current practice and future prospects. Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3003504. 125sr1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li C-R, Deiro MF, Godebu E, Bradley LM. IL-7 uniquely maintains FoxP3(+) adaptive Treg cells that reverse diabetes in NOD mice via integrin-β7-dependent localization. J. Autoimmun. 2011;37:217–227. doi: 10.1016/j.jaut.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dudda JC, Perdue N, Bachtanian E, Campbell DJ. Foxp3+ regulatory T cells maintain immune homeostasis in the skin. J. Exp. Med. 2008;205:1559–1565. doi: 10.1084/jem.20072594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenblum MD, Gratz IK, Paw JS, Lee K, Marshak-Rothstein A, Abbas AK. Response to self antigen imprints regulatory memory in tissues. Nature. 2011;480:538–542. doi: 10.1038/nature10664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gratz IK, Truong H-A, Yang SH-Y, Maurano MM, Lee K, Abbas AK, Rosenblum MD. Cutting Edge: Memory Regulatory T Cells Require IL-7 and Not IL-2 for Their Maintenance in Peripheral Tissues. J. Immunol. 2013 doi: 10.4049/jimmunol.1300212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mombaerts P, Clarke AR, Rudnicki MA, Iacomini J, Itohara S, Lafaille JJ, Wang L, Ichikawa Y, Jaenisch R, Hooper ML. Mutations in T-cell antigen receptor genes alpha and beta block thymocyte development at different stages. Nature. 1992;360:225–231. doi: 10.1038/360225a0. [DOI] [PubMed] [Google Scholar]
- 7.Murphy KM, Heimberger AB, Loh DY. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 8.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat. Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 9.Knoechel B, Lohr J, Kahn E, Bluestone JA, Abbas AK. Sequential development of interleukin 2-dependent effector and regulatory T cells in response to endogenous systemic antigen. J. Exp. Med. 2005;202:1375–1386. doi: 10.1084/jem.20050855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stubbe M, Vanderheyde N, Goldman M, Marchant A. Antigen-specific central memory CD4+ T lymphocytes produce multiple cytokines and proliferate in vivo in humans. J. Immunol. 2006;177:8185–8190. doi: 10.4049/jimmunol.177.11.8185. [DOI] [PubMed] [Google Scholar]
- 11.Leignadier J, Hardy M-P, Cloutier M, Rooney J, Labrecque N. Memory T-lymphocyte survival does not require T-cell receptor expression. Proc. Natl. Acad. Sci. U.S.A. 2008;105:20440–20445. doi: 10.1073/pnas.0806289106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Au-Yeung BB, Levin SE, Zhang C, Hsu L-Y, Cheng DA, Killeen N, Shokat KM, Weiss A. A genetically selective inhibitor demonstrates a function for the kinase Zap70 in regulatory T cells independent of its catalytic activity. Nat. Immunol. 2010;11:1085–1092. doi: 10.1038/ni.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hickman SP, Yang J, Thomas RM, Wells AD, Turka LA. Defective activation of protein kinase C and Ras-ERK pathways limits IL-2 production and proliferation by CD4+CD25+ regulatory T cells. J. Immunol. 2006;177:2186–2194. doi: 10.4049/jimmunol.177.4.2186. [DOI] [PubMed] [Google Scholar]
- 14.Gavin MA, Clarke SR, Negrou E, Gallegos A, Rudensky A. Homeostasis and anergy of CD4(+)CD25(+) suppressor T cells in vivo. Nat. Immunol. 2002;3:33–41. doi: 10.1038/ni743. [DOI] [PubMed] [Google Scholar]
- 15.Josefowicz SZ, Lu L-F, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu. Rev. Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


