Abstract
In 2007, the DYSCERNE pilot project funded by the European Commission Public Health Executive Agency (EU DG Sanco) aimed at setting up a network of expertise for patients with rare dysmorphic disorders. As part of DYSCERNE, a Dysmorphology Diagnostic System (DDS) was set up to enable clinicians throughout the EU to submit cases electronically for diagnosis using a secure, web-based interface, hosted at specified access points (Submitting nodes), in 26 different European countries. We report the outcome of this service for 200 cases submitted consecutively between January 2010 and 2012. Each case was reviewed by an average of five expert reviewers. An average of three possible syndromic diagnoses was suggested per case. In 22.5% of the cases, a consensus clinical diagnosis was reached. Genetic testing was suggested in 70.5% of the cases, whereas other laboratory investigations and diagnostic imaging were recommended in 35.5 and 26% of the cases, respectively. Further specialized opinions were suggested in 23.5% of the cases. Overall, a total of 181 very rare or extremely rare genetic syndromes were considered in the differential diagnosis of the 200 cases. In two cases, the reviewers suggested that the findings represented a new syndrome, and in one of these syndromes the underlying genetic cause was subsequently identified. Other benefits of the submission process included the possibility of directing the case submitters to specific centres for diagnostic testing or participation in research and educational benefit derived for both case submitters and reviewers.
Keywords: dysmorphic syndrome, digital service, clinical genetics
Introduction
It has been estimated that 1 in every 40 neonates (2.5%) are born with congenital malformations that are responsible for 20–30% of neonatal and 30–50% of infantile deaths.1 A study aimed at quantifying the impact of genetic disease on inpatient pediatrics and the health-care system showed that 71% of the admissions to a children's hospital had an underlying disorder with a significant genetic component.2 A dysmorphic syndrome is defined as a pattern of congenital anomalies that are observed in combination more frequently than they are statistically likely to have occurred together by chance. Individually, most of the 2500 recognised dysmorphic conditions are rare, but collectively they cause high morbidity; therefore it is important that patients are diagnosed correctly and promptly, and they receive appropriate care. Dysmorphology is the study of birth defects or malformations that constitute recognisable patterns of physical features, growth, development, and behaviour. An experienced clinical dysmorphologist can recognise and diagnose conditions based on these features.
There are relatively few experts in clinical dysmorphology, and Centres of Expertise (formerly, Centres of Reference) for patients with dysmorphic diseases have been established in some countries within the EU by designation or reputation. The rarity of these conditions means that even within these centres, experience may be limited, resulting in a delayed or uncertain diagnosis, reported to occur in 38% of the cases in a study by Moeschler et al3 Access to specialists in dysmorphology varies widely across the EU. To date, there has been no formal network for dysmorphology, and though there is considerable knowledge and experience within the existing European Centres of Expertise, the channels of communication between Centres are informal and inconsistent.
In 2007, the DYSCERNE pilot project funded by the European Commission Public Health Executive Agency (EU DG Sanco) aimed at setting up a network of expertise for patients with rare dysmorphic disorders. As part of DYSCERNE, a Dysmorphology Diagnostic System (DDS) was set up to enable clinicians throughout the EU to submit cases electronically for diagnosis using a secure, web-based interface hosted at specified access points. The aim was to facilitate diagnosis of rare syndromes associated with physical, growth and developmental problems.4 We demonstrate the outcome of this service by reporting 200 cases that have undergone consecutive review between January 2010 and 2012.
Methods
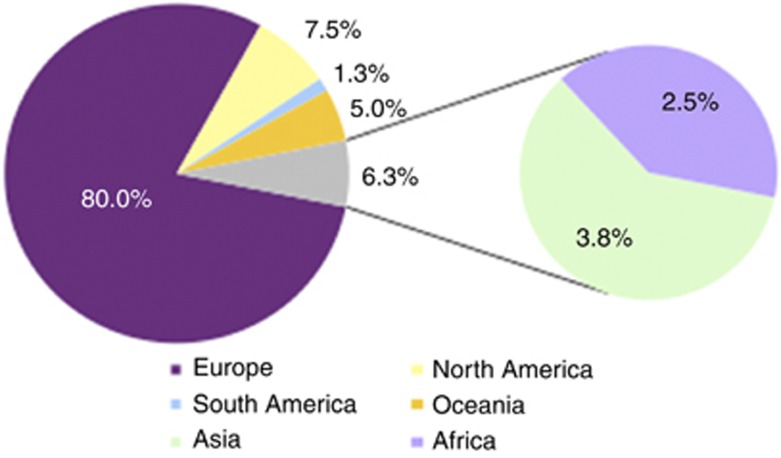
Six designated Centres of Expertise for Dysmorphology (UK, Belgium, France, Italy, The Netherlands, and Poland), coordinated by the lead partner, the University of Manchester, organised European clinical expertise and resources in dysmorphology to form a network of more than 100 individuals from 86 centres in 39 different countries (Supplementary Table 1). The breakdown of the medical specialities and positions of most of the registered users is illustrated in Table 1. As many EU countries as possible were covered (Figure 1).
Table 1. Medical specialties and positions of registered users.
| Medical specialities | Number | % |
|---|---|---|
| Clinical genetics | 46 | 57.5 |
| Pediatrics | 13 | 16.3 |
| Medical genetics | 12 | 15.0 |
| Pathology | 7 | 8.8 |
| Obstetrics and gynaecology | 3 | 3.8 |
| None given | 3 | 3.8 |
| Prenatal diagnosis | 2 | 2.5 |
| Molecular genetics | 2 | 2.5 |
| Neurogenetics | 1 | 1.3 |
| Neuropsychiatry | 1 | 1.3 |
| Internal medicine | 1 | 1.3 |
| Neuromuscular disorders | 1 | 1.3 |
| Haematology | 1 | 1.3 |
| Total | 93 |
| Position | Number | % |
|---|---|---|
| None given | 28 | 35.0 |
| Consultant | 12 | 15.0 |
| Resident | 10 | 12.5 |
| Head of Department | 8 | 10.0 |
| Trainee | 6 | 7.5 |
| Director | 5 | 6.3 |
| Associate Professor | 4 | 5.0 |
| Registrar | 2 | 2.5 |
| Professor | 2 | 2.5 |
| Clinical Fellow | 2 | 2.5 |
| Medical Officer | 1 | 1.3 |
| Total | 80 | 100.0 |
Figure 1.
Continent of origin for registered users.
The DDS was developed by the lead centres in association with a software manufacturer Certus Technologies (Exeter, UK) to enable clinicians throughout the EU to submit cases of rare and difficult-to-diagnose dysmorphic conditions. The case details are submitted electronically, using a web-based interface, hosted at 76 Submitting Nodes in 26 different European countries. Guidelines for submission and on-line proformas are provided to ensure that submissions fulfil a standard format, mirroring the dysmorphology-consultation procedure used in the clinical situation. Descriptive terms utilised in the Winter–Baraitser5 Dysmorphology Database are used for case submissions, as this provided a means of describing and standardising phenotypes, which case submitters were familiar with. The DDS allows clinicians to upload photographic images and results of investigations including imaging studies to a secure, searchable archive. On-line proformas standardise the level of consent granted by the family/patient. There is the possibility within the system for clinicians to submit their own diagnostic considerations. Thus, the DDS creates a secure forum for expert discussion and incorporates an archive of on-line consultations and clinical conclusions.
A process of gatekeeping or internal review follows the upload of cases. A clinical fellow checks the submitted proformas for the existence and level of consent granted by the patients, the content and relevance of the clinical information provided the anonymity and informativity of photographs provided, and the terminology used. Suitable cases with all the required information are accepted onto the DDS for review by the Expert Panel; unsuitable cases are returned to the case submitter with an explanation that the case is not suitable for submission to the DDS; where further information is required, the case is returned to the case submitter with a request that they resubmit the case with the required further information. Resubmitted cases are reviewed again by the DYSCERNE clinical fellow and accepted onto the DDS if appropriate. At the end of the process of internal review, a brief clinical summary is created and the expert panel is notified, through an automated e-mail, that there is a new case to review. Every panel member receives each case to review.
Accepted case submissions are reviewed by a core group of 33 experts from 28 Centres of Expertise in dysmorphology. As not all EU countries have designated national Centres of Expertise, centres and reviewers are considered as experts based on the number of patients with dysmorphic syndromes they have seen each year (>1000 per centre) and their track records in research, dysmorphology teaching, and publication record. The experts provide recommendations and opinions on possible diagnosis and may suggest further investigations and/or management strategies by entering comments in the secure DDS forum. The consensus is ‘a posteriori', as each reviewer who enters the web-based forum is, if they wish, able to see all other expert comments and discussions. After a period of time, aiming for 4 weeks, the comments are collated into a DYSCERNE Expert Case Report (DECR) that is sent back electronically to the submitting clinician. At any point in time, only the submitting clinician, the coordinating clinician and the expert panel can see a particular case.
We reviewed systematically 200 DECRs, generated consecutively between January 2010 and 2012, in an effort to measure the outcomes of this digital diagnostic service. All data were recorded in an Excel spreadsheet and analysed using simple frequency analysis to identify common findings across the whole group.
Results
The results are summarised in Table 2. The age of cases submitted ranged from neonates to adults. Each case underwent review by an average of five expert reviewers. A DECR was produced for all cases within an average of 36 days.
Table 2. DYSCERNE Clinical Genetics Digital Service.
| New, consensus clinical diagnosis | 45/200 (22.5%) |
| Consensus recurrence risk | 34/200 (17%) |
| Consensus diagnosis with available genetic test | 28/200 (14%) |
| Consensus diagnosis of unknown genetic cause | 10/200 (5%) |
| Confirmation of suggested diagnosis | 12/200 (26%) |
| Refuted suggested diagnosis | 34/200 (17%) |
| New syndrome | 2/200 (1%) |
| Differential diagnosis offered | 181 |
| Genetic investigations suggested | 141/200 (70.5%) |
| Other laboratory investigations | 71/200 (35.5%) |
| Imaging suggested | 52/200 (26%) |
| Other specialised opinion suggested | 47/200 (23.5%) |
| Average number of expert reviews | 5 |
| Average turn-around-time of diagnosis | 36 Days |
Diagnoses were suggested in 100% of cases, with an average of 3.0 diagnoses per case. A total of 181 very rare or extremely rare genetic syndromes and 23 different groups of syndromic conditions were considered in the differential diagnosis (Supplementary Table 2). A new consensus diagnosis was formulated in 22.5% of the accepted cases. The consensus diagnoses included 36 very rare, distinct conditions as illustrated in Table 3. Each consensus diagnosis was suggested in just a single case with the exception of a mucopolysaccharidosis disorder, Kabuki syndrome and conditions within the group of Ras-MAPK group of disorders that were diagnosed in 2, 3 and 5 cases, respectively. The latter group included suggestions for the subtypes of the condition in question, for example, Noonan syndrome with loose, anagen hair. In one instance, prenatal exposure to alcohol was considered the most appropriate diagnosis in a submission regarding an 11-year-old girl with undiagnosed learning difficulties, dysmorphic features and carpal coalition syndrome. The expert panel supported a clinical diagnosis of Mowat–Wilson syndrome in a case with negative testing for this disorder. The fastest consensus diagnosis was achieved in 30 min following acceptance on the system in a 10-year-old boy with Börjeson–Forssman–Lehmann syndrome. In some instances, reviewers suggested that the diagnosis fell within a group of disorders (congenital myopathy, mucopolysaccharidosis), thus allowing the targeting of diagnostic testing. A consensus recurrence risk was given in 34 instances (17%). Forty-six cases were submitted with an existing diagnostic suspicion that was confirmed by reviewers in 26% of the cases. In 5% of the cases, the consensus opinion was that the patient had an entity of unknown genetic cause. In two cases, reviewers suggested that the findings represented a new syndrome and in one of these syndromes the underlying genetic cause has subsequently been found.6
Table 3. DYSCERNE consensus diagnoses.
| Consensus syndromic diagnosis | Estimated prevalence | Transmission | Genetic cause |
|---|---|---|---|
| Acro-cardio-facial | <1/1 000 000 | AR | Unknown |
| Association of constriction rings and malformations | Unknown | Unknown | Unknown |
| Bohring–Opitz | <1/1 000 000 | AD | Known |
| Börjeson–Forssman–Lehman | Unknown | XR | Known |
| Bosma arhinia | <1/1 000 001 | Unknown | Unknown |
| Brachydactyly–mental retardation | <1/1 000 000 | Sporadic | Known |
| BRWD3 mental retardation | Unknown | XR | Known |
| Cerebro-Oculo-Facial | <1/1 000 000 | AR | Known |
| Chromosome abnormality | Unknown | AD | Known |
| Coffin–Lowry | 1-9/100 000 | XD | Known |
| Congenital myopathy | Unknown | heterogeneous | Heterogeneous |
| Cornelia De Lange | 1-9/100 000 | heterogeneous | Heterogeneous |
| Encephalocraniocutaneous Lipomatosis | <1/1 000 000 | AD | Unknown |
| Fetal alcohol | Unknown | — | Known |
| Gingival overgrowth, hypertrichosis, mental retardation, epilepsy | Unknown | heterogeneous | Unknown |
| Gomez–Lopez–Hernandez | Unknown | Unknown | Unknown |
| Kabuki | 1–9/100 000 | Heterogeneous | Heterogeneous |
| Kleefstra | Unknown | AD | Known |
| Lamin A/C deficiency | <1/1 000 000 | AD | Known |
| Macrocephaly-Cutis Marmorata Telangectasia Congenita (M-CMTC) | <1/1 000 000 | AD | Unknown |
| Meier–Gorlin | <1/1 000 000 | AR | Heterogeneous |
| Mowat–Wilson | <1/1 000 000 | AD | Known |
| Mucopolysaccharidosis | Unknown | AR | Heterogeneous |
| MULIBREY | <1/1 000 000 | AR | Known |
| Multiple sulphatase deficiency | <1/1 000 000 | AR | Known |
| Myhre | <1/1 000 000 | AD | Known |
| New syndrome | Unknown | AR | unknown |
| Noonan-like syndrome with loose anagen hair | <1/1 000 000 | AD | Known |
| RAS-Mapk disorder | Unknown | AD | Known |
| Robinow | Unknown | AD | Heterogeneous |
| Rothmund–Thomson | <1/1 000 000 | AR | Known |
| SAPHO | Unknown | Unknown | unknown |
| Say–Barber–Biesecker variant of blepharophimosis/mental retardation | <1/1 000 000 | AD | Heterogeneous |
| Sensenbrenner | Unknown | AR | Known |
| SHORT | <1/1 000 000 | AD | unknown |
| Townes–Brocks | 1–9/1 000 000 | AD | Known |
| Trichotiodystrophy | Unknown | AR | Heterogeneous |
| Weaver | <1/1 000 001 | AD | Known |
| Zimmermann–Laband | Unknown | AD | unknown |
Abbreviations: AD, autosomal dominant; AR, autosomal recessive; XD, X-linked dominant; XR, X-linked recessive.
A genetic test was suggested in 70.5% of the cases, whereas other types of laboratory investigations and diagnostic imaging were recommended in 35.5 and 26% of the cases, respectively. In 23.5% of the cases, the panel of reviewers suggested that a further specialised opinion would be of help. We sought feedback on testing of the suggested diagnosis in 40 randomly selected cases and the results are shown in Table 4. In three cases (7.5%), we received no feedback from the clinicians, in six instances (15%) the proposed diagnosis was not tested and six more cases (15%) were still under investigation. The difficulty of the family to pay for array studies in a country where this is not routinely funded by the national health system was the most frequent reason of not testing in this small series. In 40% of the cases, the suggested diagnosis had been confirmed on testing, whereas in 17.5% of the cases it was refuted. The refuted diagnoses included a case of Mowat–Wilson syndrome that was, however, clinically typical.
Table 4. DYSCERNE feedback on genetic testing.
| Level of submitting node | DYSCERNE suggested diagnosis | Testing result |
|---|---|---|
| D | Blepharophimosis–epicanthus inversus | P |
| D | Bohring-Opitz | Due |
| E | Börjeson–Forssman–Lehman | Due |
| E | Brachydactyly–mental retardation | Due |
| D | BRWD3 mental retardation | N |
| D | Chromosomal disorder (aCGH) | Not tested |
| D | Chromosomal disorder (aCGH) | Not tested |
| D | Chromosomal disorder (aCGH) | Not tested |
| D | Chromosomal disorder (aCGH) | Not tested |
| E | Coffin–Lowry | P |
| E | COFS | P |
| D | Congenital myopathy | N |
| E | Cornelia de Lange | P |
| D | Kabuki | P |
| D | Kabuki | No feedback |
| E | Kleefstra | Not tested |
| D | Lamin A/C deficiency | P |
| E | Meier–Gorlin | P |
| E | Mowat–Wilson | N |
| D | Mucopolysaccharidosis | P |
| D | Mucopolysaccharidosis | No feedback |
| D | MULIBREY | Due |
| E | Multiple sulphatase deficiency | P |
| D | Myotonic dystrophy | N |
| E | New syndrome | P (exome) |
| E | Noonan with loose-anagen hair | P |
| D | Pallister–Killian | P |
| D | Ras-MAPK | N |
| D | Ras-MAPK | N |
| D | Ras-MAPK | P |
| D | Ras-MAPK | No feedback |
| D | Robinow | Due |
| D | Rothmund–Thomson | P |
| E | Say–Barber–Biesecker type | Due |
| D | Sensenbrenner | P |
| E | Townes–Brocks | P |
| D | Trichotiodystrophy | Not tested |
| E | Undiagnosed | P (exome) |
| E | Weaver | Not tested |
| D | X-linked inactivation studies | N |
Abbreviations: D, developing node; E, established node; P, positive, confirmation of diagnosis; N, negative, refuted diagnosis.
In several instances, submitting clinicians were directed to specific research groups working on the conditions recognised by the expert panel. The DDS forum, which takes the form of a dialogue with comments posted in a ‘notice-board' format, facilitated the sharing of clinical experiences between reviewers. Where a diagnosis is made, the DECRs summarise the dysmorphic features, differential diagnoses and relevant tests for the condition.
Discussion
With the wide availability of internet access, databases have become an integral aspect of practice in clinical genetics and dysmorphology. Available resources to date include, among others, the Online Mendelian Inheritance in Man7 (OMIM, Johns Hopkins University, Baltimore, MD, USA) and the European resource for information on rare disorders, Orphanet8 (Institut National de la Santé et de la Recherche medicale, Paris, France). However, dysmorphologists prefer specialised databases, such as the Winter–Baraitser Dysmorphology Database from the London Medical Databases and POSSUM, Pictures of Standard Syndromes and Undiagnosed Malformations, for their content. In particular, the reference images of the conditions and syndromes within these databases often trigger diagnostic insights to prompt diagnosis.5, 9 The diagnostic value of these resources has proven significant in clinical-genetic discussion groups and dysmorphology education.10 This study proves that it is possible for expert reviewers to make a clinical genetic diagnosis on the basis of web-organised, representative, consented, clinical photographs of patients and short clinical summaries.
The percentage of cases in which diagnoses were suggested by the DDS was 22.5%. Dysmorphologists have long recognised the value of peer review of their cases as an adjunct to making a diagnosis for patients and their families with rare genetic conditions. This is the first study that formally describes the clinical diagnostic rate of a dysmorphology discussion group and the types of diagnosis suggested. The rarity of these diseases highlights why a consensus expert opinion is so valuable. In an attempt to test whether there was any correlation between the level of expertee of the submitting node and the likelihood of the panel giving a diagnosis, we designated all of the submitting nodes as either established (E) or developing (D) in the limited number of cases with laboratory feedback (Table 4). The odds ratio calculated in this way shows that if the case was submitted from an established node, the DYSCERNE diagnosis was 7.714 times more likely to be positive rather than if the case was submitted from a developing node.
This work was funded as a research study and, of course, if the DDS were to be employed in clinical practice then costs would be incurred. On average, cases where a consensus opinion was reached were reviewed by five reviewers, and based on practice in our own centre we would estimate that 10–15 min of reviewer time was spent on each case. While collating results, the diagnosis suggested at the top was the one in which most experts agreed, and if three or more experts agreed on a single diagnosis this was considered as a strong evidence for the diagnosis. Further reviewers were senior clinicians paid at consultant level. Costs would vary depending on the typical salary for the country involved but are estimated at 16 Euros per case per reviewer for reviewer time, given the typical review panel of five experts. Added to this would be the costs for hosting of the website (7 Euros per case if utilised to full capacity) and for the clinical coordinator collating reports (40 Euros per case if salaried). An estimated cost per case might therefore be 127 Euros. Even if this is an underestimate of the time taken, it compares very favourably to an average genetic test of 500 Euros for a single gene or 1500 Euros for a ‘panel' test or exome using NextGeneration sequencing. Of course clinical diagnoses would need to be confirmed, but targeted testing would probably be cheaper than organising a whole battery of tests with no specific diagnosis in mind.11 In addition, some suggested clinical diagnoses would not be detected or would be difficult to detect on routine genetic testing, for example, teratogenic syndromes or some mosaic disorders.
The marked differences in the provision of genetic services between countries have obvious consequences for access to diagnosis.12 In some countries, diagnostic genetic testing is partly or wholly provided from commercial, private settings.13 The DDS is currently available to a number of professionals and to their patients in many countries with staffing shortages in clinical genetics or where access to more modern genetic diagnostic technologies is not available or is limited by a significant economic burden on the family. Thus, it is particularly relevant for low/middle income or developing countries. The DDS system was particularly helpful for professionals working in isolation or in developing countries. To access the system, a professional needs to be granted a site licence, however, the number of which remains limited at this point in time.
The DECR provided at the end of the evaluation of each case is a document sent to the submitting node electronically, with immediate benefits for the patient and the family. The DDS has an impact on the management of the patient, with advice about clinically relevant genetic or other investigations, imaging studies, recommendations for further specialised opinions, screening and, in some cases, treatment. Most importantly, in the cases of consensus clinical diagnosis, a recurrence risk was given that aided genetic counselling of the individual or the family. In some cases where a specific diagnosis could not be offered, the submitting clinician was at least directed to a group of disorders. A total of 23 different groups of disorders were differentially diagnosed in these cases. We think that assigning a condition to one of these groups is clinically relevant, as it might prove useful in the future for the families with a tentative diagnosis as laboratory diagnostic capabilities increase.
The contribution of the DDS to arriving at a diagnosis compares favourably to the types of genetic testing, such as chromosomal microarray analysis (aCGH). The diagnostic yield of aCGH was identified as 8.5% according to a recent study of >2000 postnatal cases.14 Of note, most cases accepted onto the DDS had negative or nonclinically relevant aCGH results. Though the use of aCGH as an initial screening test is becoming a standard clinical practice, our findings reinforce the fact that the DDS serves as a further tool in the diagnostic armamentarium for the specific group of dysmorphic patients in which standard laboratory investigations have given normal results, as it may lead to the suggestion of a clinical diagnosis that was previously not considered and thus allow the targeting of further specific diagnostic testing. However, we accept that clinical diagnoses are not always confirmed on testing and that any clinical diagnosis suggested in an individual case also needs to be considered in the context of molecular findings to arrive at the correct diagnosis on which management and counselling decisions are based.
We believe that platforms such as the DDS will have a place even in the era of NextGeneration Sequencing.15 This technology is still not widely available and there are several congenital dysmorphic conditions that are caused by environmental, multifactorial or epigenetic causes not diagnosed by this method. As it has always been the case in health services, a clinical insight often directs targeted testing and might save the cost of a whole-genome sequencing technique.11 Moreover, as laboratory diagnosis of rare dysmorphic syndromes improves, the attention of the clinical geneticist will shift to the clinical management of these patients that can be facilitated by systems such as the DDS. This type of approach could be a future model for regional genetic services as has already been tested in central Italy.16 It might be of value, particularly, where an urgent opinion is needed to facilitate the management of a newborn patient or to determine recurrence risk in a pregnant member of the family.
There are significant limitations to be considered regarding wider implementation of the DDS. DYSCERNE is a clinical genetic service that provides expert clinical opinions. Follow-up of the suggested diagnoses, decisions regarding genetic testing and the management of the patient/family are left to the judgement of the submitting clinician. This current study was not specifically designed to explore the results of the suggested genetic tests, as the decisions to undertake such tests and their availability were out of our control. In an effort to seek more objective feedback, we approached 40 randomly selected submitting clinicians to ask them what actions they took upon receiving the DYSCERNE diagnosis, and this provided some limited feedback on the genetic-testing results.
Sustainability is the main issue, as currently the coordinator and the expert reviewers participate free of charge. The case submission forms require careful completion by submitting clinicians, and gatekeeping is a time-consuming process. Only a finite number of cases can be reviewed at any point in time. In some case, particularly those with fewer dysmorphic features, there was no response or low response from reviewers. Despite these limitations, these results demonstrate that the DDS system is a digital clinical genetic service that can improve accessibility and delivery of high-quality diagnostic services and fulfil individual needs for diagnosis as identified by user groups.17
In the era of genomic medicine, the integration of the trained intuition of dysmorphology and NextGeneration sequencing would be very productive in research and clinical translation.
Acknowledgments
The DYSCERNE project was funded by grants from the European Commission Public Health Executive Agency, the European Society of Human Genetics and the Manchester Biomedical Research Centre.
Appendix
Members of the DYSCERNE expert panel:
Amiel J, Baraitser M, Brueton L, Brunner H, Chrzanowska K, Dallapiccola B, del Campo Casanelles M, Devriendt K, Donnai D, Fitzpatrick D, Gillessen-Kaesbach G, Houge G, Kerr B, Krajewska-Walasek M, Lacombe D, Meinecke P, Metcalfe K, Mortier G, Odent S, Philip N, Prescott T, Raas-Rothschild A, Rauch A, Rittinger O, Salonen R, Schrander-Stumpel C, Suri M, Temple K, Tolmie J, Van Der Burgt I, Verloes A, Wieczorek D and Zenker M.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on European Journal of Human Genetics website (http://www.nature.com/ejhg)
Contributor Information
the DYSCERNE expert panel:
J Amiel, M Baraitser, L Brueton, H Brunner, K Chrzanowska, B Dallapiccola, M del Campo Casanelles, K Devriendt, D Donnai, D Fitzpatrick, G Gillessen-Kaesbach, G Houge, B Kerr, M Krajewska-Walasek, D Lacombe, P Meinecke, K Metcalfe, G Mortier, S Odent, N Philip, T Prescott, A Raas-Rothschild, A Rauch, O Rittinger, R Salonen, C Schrander-Stumpel, M Suri, K Temple, J Tolmie, I Van Der Burgt, A Verloes, D Wieczorek, and M Zenker
Supplementary Material
References
- Mathews TJ, MacDorman MF. Infant mortality statistics from the 2003 period linked birth/infant death data set. Natl Vital Stat Rep. 2006;54:1–29. [PubMed] [Google Scholar]
- McCandless SE, Brunger JW, Cassidy SB.The burden of genetic disease on inpatient care in a children's hospital Am J Hum Genet 200474121–127.(Erratum in: Am J Hum Genet. 2004; 74: 788). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeschler JB, Shevell M, The American Academy of Pediatrics Committee on Genetics Clinical genetic evaluation of the child with mental retardation or developmental delays. Pediatrics. 2006;117:2304–2316. doi: 10.1542/peds.2006-1006. [DOI] [PubMed] [Google Scholar]
- DYSCERNE ® www.dyscerne.org Accessed on 13 November 2012..
- London Medical Databases. Winter–Baraitser Dysmorphology Database www.lmdatabases.com Accessed on 15 April 2013..
- Basel-Vanagaite L, Dallapiccola B, Ramirez-Solis R, et al. The E3 ubiquitin ligase UBE3B is mutated in an autosomal-recessive blepharophimosis-intellectual disability syndrome. Abstract: 15th Manchester Dysmorphology Conference. 2012.
- Johns Hopkins University and National Center for Biotechnology Information. Online Mendelian Inheritance in ManTM www.ncbi.nlm.nih.gov/omim Accessed on 15 April 2013.
- Orphanet ® www.orpha.net Accessed on 15 April 2013..
- POSSUM. Pictures of Standard Syndromes and Undiagnosed Malformations www.possum.net.au Accessed on 15 April 2013..
- Reardon W, Donnai D. Dysmorphology demystified. Arch Dis Child Fetal Neonatal Ed. 2007;92:F225–F229. doi: 10.1136/adc.2006.110619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Editorial: Can we all just get along Nat Genet 201244833. [DOI] [PubMed] [Google Scholar]
- Harris R, Reid M. Medical genetic services in 31 countries: an overview. Eur J Hum Genet. 1997;5 (Suppl 2:3–21. [PubMed] [Google Scholar]
- Zimmern RL, Khoury MJ. The impact of genomics on public health practice: the case for change. Public Health Genomics. 2012;15:118–124. doi: 10.1159/000334840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Shaw CA, Patel A, et al. Clinical implementation of chromosomal microarray analysis: summary of 2513 postnatal cases. PLoS One. 2007;2:e327. doi: 10.1371/journal.pone.0000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch A, Wieczorek D, Graf E, et al. Range of genetic mutations associated with severe non-syndromic sporadic intellectual disability: an exome sequencing study. Lancet. 2012;380:1674–1682. doi: 10.1016/S0140-6736(12)61480-9. [DOI] [PubMed] [Google Scholar]
- Dentici ML, Tarani L, Digilio MC, et al. RDDR: a dysmorphology diagnostic network for newborns in central Italy. J Matern Fetal Neonatal Med. 2012;25 (Suppl 4:121–123. doi: 10.3109/14767058.2012.714989. [DOI] [PubMed] [Google Scholar]
- Donnai D. Genetic services. Clin Genet. 2002;61:1–6. doi: 10.1034/j.1399-0004.2002.610101.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.