Abstract
With the introduction of array comparative genomic hybridization (aCGH) techniques in the diagnostic setting of patients with developmental delay and congenital malformations, many new microdeletion syndromes have been recognized. One of these recently recognized microdeletion syndromes is the 16p11.2 deletion syndrome, associated with variable clinical outcomes including developmental delay, autism spectrum disorder, epilepsy, and obesity, but also apparently normal phenotype. We report on a 16-year-old patient with developmental delay, exhibiting retinis pigmentosa with progressive visual failure from the age of 9 years, ataxia, and peripheral neuropathy. Chromosomal microarray analysis identified a 1.7-Mb 16p11.2 deletion encompassing the 593-kb common deletion (∼29.5 to ∼30.1 Mb; Hg18) and the 220-kb distal deletion (∼28.74 to ∼28.95 Mb; Hg18) that partially included the CLN3 gene. As the patient's clinical findings were different from usual 16p11.2 microdeletion phenotypes and showed some features reminiscent of juvenile neuronal ceroid-lipofuscinosis (JNCL, Batten disease, OMIM 204200), we suspected and confirmed a mutation of the remaining CLN3 allele. This case further illustrates that unmasking of hemizygous recessive mutations by chromosomal deletion represents one explanation for the phenotypic variability observed in chromosomal deletion disorders.
Keywords: 16p11.2 deletion syndrome, juvenile neuronal ceroid-lipofuscinosis, hemizygous CLN3 mutation
Introduction
Common 16p11.2 microdeletion is defined as the presence of a 593-kb deletion at map position ∼29.5 to ∼30.1 Mb (Hg18), between two segmental duplications, suggesting NAHR as the causative mechanism.1 Adjacent and more distal to this 16p11.2 deletion, there is a less frequently reported 220-kb 16p11.2 deletion (∼28.74 to ∼28.95 Mb;Hg18) that was referred to as the ‘atypical 16p11.2 deletion'2 or ‘distal 16p11.2 deletion'.3 Larger 16p11.2 deletions were also reported, including the distal deletion but not the common 16p11.2 deletion.4, 5, 6 Finally, few cases encompassing both the distal and the common deletions were described in the literature.4, 7, 8, 9
Recurrent 16p11.2 deletions are associated with variable clinical features, including delayed language development, learning difficulties/intellectual disability, social impairments with or without autism spectrum disorder and minor dysmorphic facial features without a consistent pattern. They can also be associated with a normal phenotype.
Here, we present a patient who harbors an uncommon 16p11.2 microdeletion with some features reminiscent of juvenile neuronal ceroid-lipofuscinosis (JNCL) or Batten disease (OMIM 204200), a progressive neurodegenerative disorder, caused by recessive mutations in the CLN3 gene located on chromosome 16p11.2 and characterized by progressive visual failure from the early school years and deterioration in the early teenage years together with the onset of seizures, behavioral difficulties, until dementia. To the best of our knowledge, this is the first 16p11.2-deletion patient in whom unmasking of autosomal recessive gene mutation by a hemizygous deletion is demonstrated.
Materials and methods
Case history
The patient was the second child of non-consanguineous and healthy parents, with no relevant family history. The pregnancy and delivery were uneventful. His birthweight was 2830 g (−2 SD), length was 49 cm (−0.5 SD) and occipital-frontal circumference was 34 cm (−0.5 SD). He was hypotonic, was able to hold his head at 4 months, sat alone at 13 months, and was able to walk after 2 years. Speech development was also delayed. At the age of 9 years, acute visual impairment appeared with retinis pigmentosa and progressive visual loss. He presented recent ataxia and peripheral neuropathy.
On examination at the age of 16 years, his weight was 64.4 kg (+1 SD), his size was 1.68 m (median), and his head circumference was 55 cm (median). The patient had gynecoid obesity with purple stretch marks on the pelvic bones. No dysmorphic features was described. He reported sleeping troubles with very early wake-up. There was a bulimic symptomatology and he had behavioral difficulties with aggressive crisis in case of frustration. He suffered from severe fatigability and chronic constipation.
Audiometric examination, metabolic exploration, and brain MRI did not reveal any anomaly. Retinal examination showed a pale optic disc with a narrowing of the vessels and bone spicular pigmentations in the peripheral retina. Vacuolated leukocytes were discovered on blood cell count without other hematological abnormalities (Table 1).
Table 1. Clinical features of our patient compared with previously reported cases of 16p11.2 deletions and patients with classic JNCL.
| Large 16p11.2 deletion | Common proximal 16p11.2 deletion | Atypical 16p11.2 deletion | Classic JNCL (1.02 kb deletion on CLN3 gene) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Clinical spectrum | Ballif et al., 20074 | Bochukova et al., 20107 | Bochukova et al., 20107 | Hanson et al., 20108 | Sampson et al., 20109 | Sampson et al., 20109 | Ghebranious et al., 20071 Bijlsma et al. 20092 | Bachmann-Gagescu et al., 20106 Baarge-Schaapveld et al., 2011 | Haltia, 200315 | Our patient |
| Progression of vision loss until blindness | na | na | na | Patient too young to evaluate manifestations reliably | na | na | − | − | + (Onset ∼2–5 years) | + (Onset 9 years) |
| Deterioration in cognitive skills, speech and mobility | na | na | na | na | na | − | − | + | + | |
| Seizures | na | na | na | + | na | + (Onset 1 year) | + | + (Onset 9–18 years) | − | |
| Premature death | na | na | na | na | na | − | − | + | na | |
| Behavioral problems, extrapyramidal signs, and sleep disturbance | na | na | na | + | + | + | + | + (Onset >10 years) | + | |
| Attention-deficit disorder | na | na | na | + | na | + | + | + | ||
| Autism spectrum disorder | na | na | na | na | na | + | + | − | − | |
| Developmental delay (learning difficulties/intellectual disability, delayed language development) | + | + | + | + | na | + | + | − | + | |
| Unusual facial morphology | na | na | na | + | na | − | + | − | − | |
| Obesity | + | + | + | − | − | + | + | − | + | |
| Neonatal hypotonia | na | na | na | na | na | − | + | − | + | |
| Congenital anomalies of the kidney and urinary tract | na | na | na | + | na | − | + | − | − | |
| Other major finding | na | na | na | +a | +b | − | − | − | − | |
Abbreviation: na, data not available.
Severe kyphosis.
Hirschprung disease and congenital joint contractures.
Classical and molecular cytogenetics
Chromosome analysis was performed on GTG-banded and RHG-banded metaphases prepared from cultured peripheral blood according to the standard protocols. Fluorescence in situ hybridization (FISH) was performed to confirm the genomic-copy-number aberration detected by array comparative genomic hybridization (aCGH). A commercially available probe, RP11-301D18 (29 776 143–29 961 746 – Hg18), located at 16p11.2, was obtained from BlueFISH, Amplitech (Compiegne, France) and was hybridized according to instructions provided by the manufacturer. At least 15 metaphase spreads and 100 interphase nuclei were analyzed under a fluorescence microscope (Leica Microsystems, Wetzlar, Germany).
Microarray-based comparative genomic hybridization (aCGH) was performed on genomic DNA isolated from peripheral blood lymphocytes (Kit Nucleospin Blood L, Macherey-Nagel, Eurl, Hoerdt, France) using an Agilent 180K oligonucleotide microarray system, according to the manufacturer's protocol (Agilent 180 K Whole Human genome Oligo Microarray kit, Agilent Technologies, Santa Clara, CA, USA). The array was scanned and analyzed with Feature Extraction 10.5 software (Agilent Technologies). Control DNA consisted of a sex-matched pool of genomic DNA (Promega, Madison, WI, USA). Genomic-copy-number aberrations were identified using the ADM-II algorithm of DNA analytics 4.0.76 (Agilent Technologies).
Molecular genetics
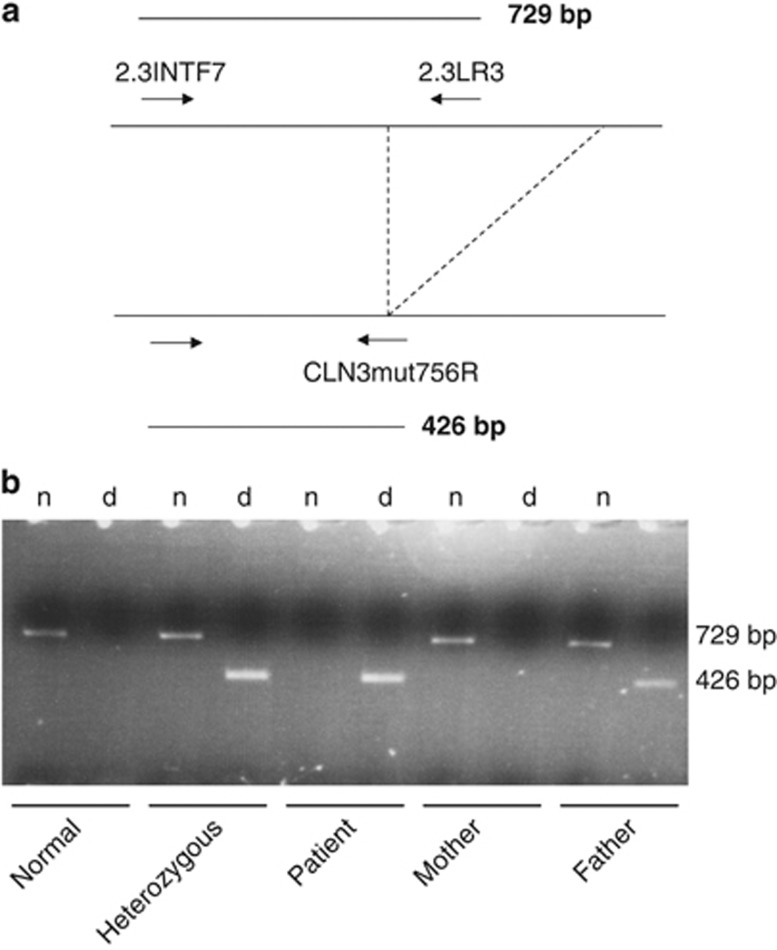
Allele-specific PCR for detection of the major 1.02-kb deletion in the CLN3 gene was performed according to Taschner et al.10 by using a primer spanning the deletion (2.3INTF7) in combination with one primer within the deletion (2.3LR3) and another primer spanning the deletion junction (CLN3mut756R) (Figure 2a). Briefly, 50 ng of genomic DNA was amplified in a total volume of 25 μl at a final concentration of 50 mmol/l KC1, 1.5 mmol/l MgCl2, 200 pmol/l dNTP, 0.004 U per 4 l of SuperTaq (HT Biotechnology Ltd, Cambridge, UK) in the presence of 5 pmol of primers 2.3LR3 (5′-GGGGGAGGACAAGCACTG-3′) and 2.3INTF7 (5′-CATTCTGTCACCCTTAGAAGCC-3′), and 4 pmol of primer CLN3mut756R (5′-GGACTTGAAGGACGGAGTCT-3′). Primers were designed using the genomic CLN3 sequence (Genbank Accession no. X99832).11 Denaturation was for 3 min at 94 °C, followed by 35 cycles of amplification with denaturation for 1 min at 94 °C, annealing for 2 min at 56 °C, and extension for 1 min at 72 °C, with a final extension for 10 min. Twenty microliter of PCR samples were analyzed on a 2% agarose gel.
Results
Classical and molecular cytogenetics
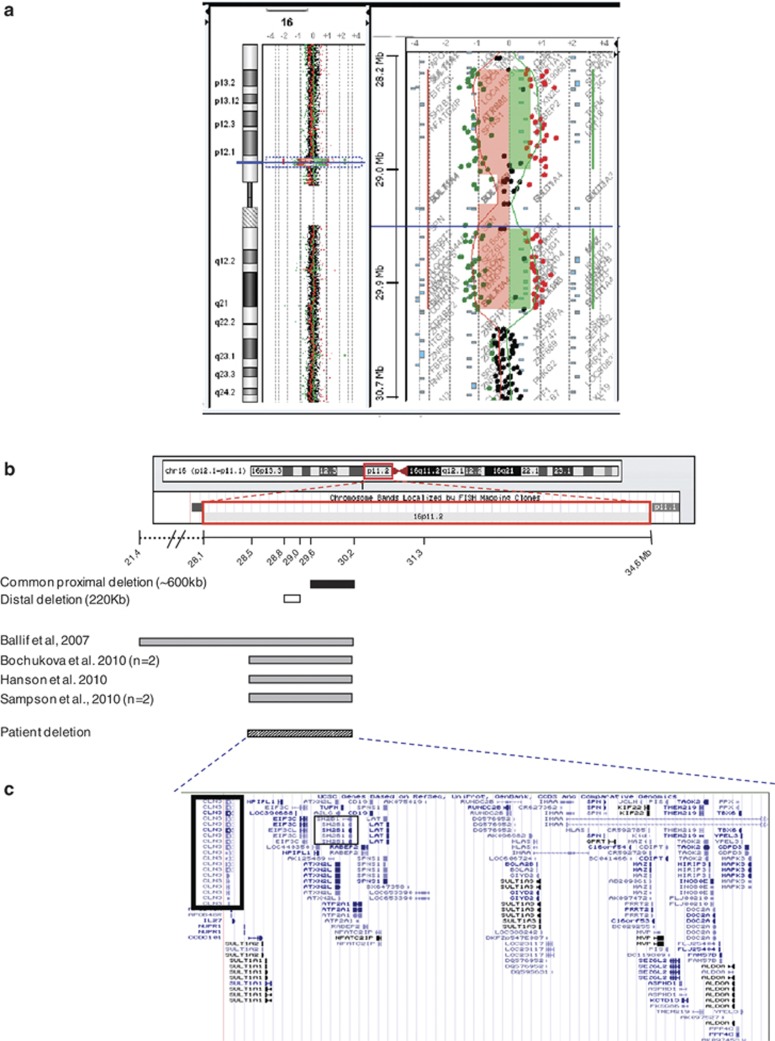
By using GTG and RHG bandings, the patient was initially judged to have a normal 46,XY karyotype at 550 band level. aCGH analysis revealed a ∼1.7-Mb interstitial deletion of chromosome 16p11.2 (28 399 426–30 098 210 – Hg18), stretching from oligonucleotide A_16_P40598212, the first-deleted oligonucleotide, to oligonucleotide A_16_P20443172, the last-deleted oligonucleotide (Figure 1a), and excluded the presence of any other genomic imbalance, except copy number variations (CNV) previously described in the normal population according to the Database of Genomic Variants (http://projects.tcag.ca/variation) (data not shown).
Figure 1.
(a) Oligo array-CGH of whole chromosome 16 with ideogram illustrating deletion and enlargement of the ∼1.7-Mb interstitial deletion of chromosome 16p11.2 (28, 491, 925–30, 190, 709), stretching from oligonucleotides A_16_P40598212 to A_16_P20443172 (hg18 – NCBI Build 36.1). (b) Schematic representation of the 16p11.2 chromosomal region. The figure shows the chromosomal 16 ideogram and the distance in Mb from p-telomere with location of the patient deletion (hatched box) and deletions previously found on reported cases (grey boxes). The black box indicates the common proximal deletion (∼ 600 kb). The white box indicates the distal deletion (∼220 kb). (c) The region of interest showing the positions of implicated genes consistent with the UCSC Genome Browser. CLN3 and SH2B1 genes are highlighted in black. The full colour version of this figure is available at European Journal of Human Genetics online.
Metaphase FISH analysis using RP11-301D18 probe demonstrated a hemizygous deletion of chromosome 16p11.2, which is consistent with aCGH results. Parental karyotype and FISH using the same probe showed no 16p11.2 deletion or other rearrangement involving this locus, indicating a de novo occurrence (data not shown).
Molecular genetics
Mutation analysis of the CLN3 gene in the patient revealed on the remaining allele a 1.02-kb deletion (c.461_677del, NM_001042432.1), previously reported as the major causative deletion in JNCL. The father of the patient was found to be a heterozygous carrier of this mutation. No mutation in the CLN3 gene was detected in the mother (Figure 2b), suggesting that submicroscopic deletion of 16p11.2 occurred de novo on the maternal allele.
Figure 2.
(a) Direct detection of the major deletion in the CLN3 gene by allele-specific PCR. (b) The patient has the 426-bp major deletion fragment. The mother of the patient has the 726-bp wild-type fragment. The father of the patient has both. n: amplification of the wild-type allele. d: amplification of the deleted allele.
Discussion
Here, we reported on a 16-year-old patient with developmental delay, behavioral difficulties, and obesity consistent with 16p11.2 deletion. He also exhibited neurodegenerative features from the age of 9 years. The interstitial deletion of chromosome 16p11.2 (28 491 925–30 190 709 – Hg18) revealed by aCGH encompassed the 220-kb deletion and extended through the common 593-kb 16p11.2-deleted region (Figure 1b). Five patients with this 1.7-Mb deletion have been described in the literature (Figure 1b).7, 8, 9 All patients had developmental delay except for one patient who was too young to evaluate this reliably.8 In addition to the developmental delay, one patient was hyperactive and two had behavioral problems (Table 1). The deleted region includes the SH2B1 gene, known to be involved in leptin and insulin signaling, which is consistent with hyperphagia and gynecoid obesity of our patient. Indeed, several authors provided evidence that CNV of SH2B1 may significantly contribute to human obesity, as deletion carriers exhibited hyperphagia and severe insulin resistance.6, 7 Of interest, besides the patient reported here, three out of the five patients mentioned in Figure 1b for whom we have information about the body mass index, had obesity in addition to their developmental delay. Neurodegenerative features have not been described in 16p11.2 patients with or without 16p11.2 common deletion. As aCGH excluded the presence of any other pathogenic genomic imbalance, we suspected that, in addition to causing clinical features of 16p11.2-deletion syndrome, the deletion identified on chromosome 16 unmasked a hemizygous gene mutation, leading to the complex phenotype of our patient.
The deletion contained 58 protein-coding genes, including the CLN3 gene (Figure 1c). The CLN3 gene encodes a 438-amino-acid glycosylated membrane protein almost certainly located in the lysosome and in additional locations in the neurons.12 The function of CLN3 remains unknown but proposed functions include lysosomal acidification, sequestration of lysosomal enzymes, degradation of proteins and small molecule transport, organelle fusion, and apoptosis.13, 14 Mutations in CLN3 cause JNCL, the most common of a group of inherited, autosomal recessive, progressive, and ultimately fatal neurodegenerative disorders that share the clinical features of progressive loss of vision, myoclonic seizures, loss of cognitive function, pyramidal and extrapyramidal motor dysfunctions, and the pathologic features of progressive neuronal loss, with an accumulation of lipofuscin-like autofluorescent storage material in the cytoplasm of neurons and other cells (Table 1).15 The presence of fingerprint patterns in the lysosomes of different tissues and the finding of vacuolated lymphocytes are considered as pathognomonic for JNCL.
Vacuolated lymphocytes on peripheral blood film testing were present in our patient, which strongly strengthens our hypothesis that the distinct neurological features of the patient could be derived from a hemizygous CLN3-gene mutation associated with the submicroscopic 16p11.2 deletion, even if the patient did not present all JNCL features, especially myoclonic seizures (Table 1). Mutation analysis of the CLN3 gene in the patient by allele-specific PCR revealed a 1.02-kb deletion on the remaining allele (NG_008654.1: g.10373_11338del). This common mutation is a c.461_677del (NM_001042432.1) and deletes 217 bp of coding sequence, including exons 7 and 8 (Figure 2b). Forty-one mutations and five polymorphisms have been described in CLN3 (http://www.ucl.ac.uk/ncl/cln3.shtml – accessed on 19 November 2007). Approximately 85% of JNCL patients are homozygous for this 1.02-kb deletion, resulting in a frameshift and a premature stop. The remaining patients are mostly compound heterozygotes for the 1.02-kb deletion and for one of the many other mutations described so far.16 In some patients, the second mutation has not yet been identified,12 suggesting the interest of using aCGH to detect 16p11.2 microdeletion on the remaining allele.
This case provides further evidence that phenotypic variability among patients with similar deletion syndromes may, in part, be due to hemizygous expression of a recessive mutation on the non-deleted homolog. Indeed, a review article by Coman and Gardner17 highlights that this mechanism has already been demonstrated by several authors.18, 19, 20, 21, 22, 23 However, to our knowledge, this is the first report of JNCL caused by an unmasked mutation of CLN3 in a 16p11.2-deletion syndrome. This report illustrates the importance of searching for CLN3 mutation when larger 16p11.2 deletion is associated with neurodegenerative features.
Acknowledgments
We thank the patient and his family for their kind cooperation and permission to publish this case. aCGH analysis was supported by grants from the DGOS (Direction Générale de l'Offre de Soins).
DATA ARCHIVING
The data were submitted to DECIPHER: Database of Chromosomal Imbalance and Phenotype in Humans using Ensembl Resources (http://decipher.sanger.ac.uk/).
The authors declare no conflict of interest.
References
- Ghebranious N, Giampietro PF, Wesbrook FP, Rezkalla SH. A novel microdeletion at 16p11.2 harbors candidate genes for aortic valve development, seizure disorder, and mild mental retardation. Am J Med Genet A. 2007;143A:1462–1471. doi: 10.1002/ajmg.a.31837. [DOI] [PubMed] [Google Scholar]
- Bijlsma EK, Gijsbers ACJ, Schuurs-Hoeijmakers JHM, et al. Extending the phenotype of recurrent rearrangements of 16p11.2: deletions in mentally retarded patients without autism and in normal individuals. Eur J Med Genet. 2009;52:77–87. doi: 10.1016/j.ejmg.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Barge-Schaapveld DQCM, Maas SM, Polstra A, Knegt LC, Hennekam RCM. The atypical 16p11.2 deletion: a not so atypical microdeletion syndrome. Am J Med Genet A. 2011;155A:1066–1072. doi: 10.1002/ajmg.a.33991. [DOI] [PubMed] [Google Scholar]
- Ballif BC, Hornor SA, Jenkins E, et al. Discovery of a previously unrecognized microdeletion syndrome of 16p11.2-p12.2. Nat Genet. 2007;39:1071–1073. doi: 10.1038/ng2107. [DOI] [PubMed] [Google Scholar]
- Battaglia A, Novelli A, Bernardini L, Igliozzi R, Parrini B. Further characterization of the new microdeletion syndrome of 16p11.2-p12.2. Am J Med Genet A. 2009;149A:1200–1204. doi: 10.1002/ajmg.a.32847. [DOI] [PubMed] [Google Scholar]
- Bachmann-Gagescu R, Mefford HC, Cowan C, et al. Recurrent 200-kb deletions of 16p11.2 that include the SH2B1 gene are associated with developmental delay and obesity. Genet Med. 2010;12:641–647. doi: 10.1097/GIM.0b013e3181ef4286. [DOI] [PubMed] [Google Scholar]
- Bochukova EG, Huang N, Keogh J, et al. Large, rare chromosomal deletions associated with severe early-onset obesity. Nature. 2010;463:666–670. doi: 10.1038/nature08689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson E, Nasir RH, Fong A, et al. Cognitive and behavioral characterization of 16p11.2 deletion syndrome. J Dev Behav Pediatr. 2010;31:649–657. doi: 10.1097/DBP.0b013e3181ea50ed. [DOI] [PubMed] [Google Scholar]
- Sampson MG, Coughlin CR, Kaplan P, et al. Evidence for a recurrent microdeletion at chromosome 16p11.2 associated with congenital anomalies of the kidney and urinary tract (CAKUT) and Hirschsprung disease. Am J Med Genet A. 2010;152A:2618–2622. doi: 10.1002/ajmg.a.33628. [DOI] [PubMed] [Google Scholar]
- Taschner PE, De Vos N, Breuning MH. Rapid detection of the major deletion in the Batten disease gene CLN3 by allele specific PCR. J Med Genet. 1997;34:955–956. doi: 10.1136/jmg.34.11.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison HM, Hofmann SL, Becerra CHR, et al. Genomic structure and complete nucleotide sequence of the Batten disease gene CLN3. Genomics. 1997;40:346–350. doi: 10.1006/geno.1996.4576. [DOI] [PubMed] [Google Scholar]
- Mole SE, Williams RE, Goebel HH. Correlations between genotype, ultrastructural morphology and clinical phenotype in the neuronal ceroid lipofuscinoses. Neurogenetics. 2005;6:107–126. doi: 10.1007/s10048-005-0218-3. [DOI] [PubMed] [Google Scholar]
- Phillips SN, Benedict JW, Weimer JM, Pearce DA. CLN3, the protein associated with batten disease: structure, function and localization. J Neurosci Res. 2005;79:573–583. doi: 10.1002/jnr.20367. [DOI] [PubMed] [Google Scholar]
- Rakheja D, Narayan SB, Bennett MJ. The function of CLN3P, the Batten disease protein. Mol Genet Metab. 2008;93:269–274. doi: 10.1016/j.ymgme.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Haltia M. The neuronal ceroid-lipofuscinoses. J Neuropathol Exp Neurol. 2003;62:1–13. doi: 10.1093/jnen/62.1.1. [DOI] [PubMed] [Google Scholar]
- Lauronen L, Munroe PB, Jarvela I, et al. Delayed classic and protracted phenotypes of compound heterozygous juvenile neuronal ceroid lipofuscinosis. Neurology. 1999;52:360–365. doi: 10.1212/wnl.52.2.360. [DOI] [PubMed] [Google Scholar]
- Coman DJ, Gardner RJM. Deletions that reveal recessive genes. Eur J Hum Genet. 2007;15:1103–1104. doi: 10.1038/sj.ejhg.5201919. [DOI] [PubMed] [Google Scholar]
- Lee ST, Nicholls RD, Bundey S, et al. Mutations of the P gene in oculocutaneous albinism, ocular albinism, and Prader-Willi syndrome plus albinism. N Engl J Med. 1994;330:529–534. doi: 10.1056/NEJM199402243300803. [DOI] [PubMed] [Google Scholar]
- Liburd N, Ghosh M, Riazuddin S, et al. Novel mutations of MYO15A associated with profound deafness in consanguineous families and moderately severe hearing loss in a patient with Smith-Magenis syndrome. Hum Genet. 2001;109:535–541. doi: 10.1007/s004390100604. [DOI] [PubMed] [Google Scholar]
- Griffin AE, Cobb B, Anderson P, et al. Detection of hemizygosity in Hermansky-Pudlak syndrome by quantitative real-time PCR. Clin Genet. 2005;68:23–30. doi: 10.1111/j.1399-0004.2005.00461.x. [DOI] [PubMed] [Google Scholar]
- Flipsen-ten Berg K, van Hasselt PM, Eleveld MJ, et al. Unmasking of a hemizygous WFS1 gene mutation by a chromosome 4p deletion of 8.3 Mb in a patient with Wolf-Hirschhorn syndrome. Eur J Hum Genet. 2007;15:1132–1138. doi: 10.1038/sj.ejhg.5201899. [DOI] [PubMed] [Google Scholar]
- Shimada S, Miya K, Oda N, et al. An unmasked mutation of EIF2B2 due to submicroscopic deletion of 14q24.3 in a patient with vanishing white matter disease. Am J Med Genet A. 2012;158A:1771–1777. doi: 10.1002/ajmg.a.35431. [DOI] [PubMed] [Google Scholar]
- Blumkin L, Kivity S, Lev D, et al. A compound heterozygous missense mutation and a large deletion in the KCTD7 gene presenting as an opsoclonus-myoclonus ataxia-like syndrome. J Neurol. 2012;259:2590–2598. doi: 10.1007/s00415-012-6545-z. [DOI] [PubMed] [Google Scholar]