1. DISEASE CHARACTERISTICS
1.1 Name of the disease (synonyms)
Vici syndrome (VICIS) (Immunodeficiency with cleft lip/palate, cataract, hypopigmentation, and absent corpus callosum.)
1.2 OMIM# of the disease
242840
1.3 Name of the analyzed genes or DNA/chromosome segments
EPG5 (ectopic P-granules autophagy protein 5);
Alternative gene names: KIAA1632; HEEW1.
1.4 OMIM# of the gene(s)
615068
1.5 Mutational spectrum
Mutations in EPG5 are principally null mutations, most commonly comprising premature truncations, small insertions and deletions, and variants affecting the canonical splice sites.1 Missense mutations are less common but have been identified in a small number of families. To date, large deletions and duplications have not been tested for; however, given that the majority of cases have point mutations in keeping with expected inheritance patterns, this mutation class is not predicted to represent a frequent causative mechanism; to date, only a single case has been shown to harbour one pathogenic allele in the absence of any other potential causal variants (unpublished observation). Among the 24 cases of EPG5-related Vici syndrome screened in our laboratory to date, 38 variants have been detected that have been classed as neutral polymorphisms; identification of heterozygous alleles enables elimination of heterozygous deletions at these loci, but on an individual patient basis, significant proportions of the analyzed region remain uninformative.
Most EPG5 mutations are private and only one recurrent mutation has been published to date.1 EPG5 mutations are typically inherited from unaffected carrier parents; a single (unpublished) case has been identified with an EPG5 mutation showing de novo occurrence in the proband.
A small proportion of patients with diagnostic features of Vici syndrome do not have EPG5 mutations detectable on Sanger sequencing,1 suggesting either genetic heterogeneity or the presence of uncommon EPG5 mutations such as deep intronic mutations or large intragenic deletions/duplications.
1.6 Analytical methods
All 44 coding exons of EPG5 are analyzed by unidirectional Sanger sequencing with sensitivity to detect point mutations within analyzed regions approaching 100%.2 Multiplex ligation-dependent probe amplification (MLPA) may be required in future to detect submicroscopic deletions or duplications, although those are unlikely to have a major causative role (see 1.5). Similarly, reverse transcriptase PCR analysis of mRNA extracted from cultured fibroblasts may be required to screen for deep intronic EPG5 mutations causing aberrant RNA splicing, although again this is an unlikely mechanism on the basis of findings to date. Sequence variants are described following HGVS nomenclature guidelines (http://www.hgvs.org/) relative to the NCBI reference sequence NM_020964.2.
1.7 Analytical validation
Primers for EPG5 sequencing were designed to exclude common SNPs using the SNPcheck tool (http://www.ngrl.org.uk/Manchester/projects/informatics/snpcheck). Mutations identified on the initial screen should be confirmed bidirectionally using an independent biological sample from the index case or an affected relative.
1.8 Estimated frequency of the disease
(Incidence at birth (‘birth prevalence') or population prevalence. If known to be variable between ethnic groups, please report):
The birth prevalence of Vici syndrome is currently unknown but predicted to be low, with only 20 cases published to date.1, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 However, a proportion of cases are probably either undiagnosed or unreported, suggesting that this figure provides an underestimate of the actual frequency. Vici syndrome has been found in equal frequencies in the different ethnic groups studied. Only one recurrent EPG5 mutation has been identified to date, in the compound heterozygous state in an Italian and in the homozygous state in an unrelated Maltese patient without known parental consanguinity,1, 11 suggesting a possible founder effect.
1.9 Diagnostic setting

Comment:
Mutation analysis is mainly used for confirmation of a clinical diagnosis (on the basis of the presence of at least four of the five main diagnostic features, agenesis of the corpus callosum, cataracts, cardiomyopathy, skin hypopigmentation and immunodeficiency) and for accurate genetic counseling.
Preimplantation genetic diagnosis (PGD) may be offered to affected families with confirmed pathogenic mutations depending on the regulatory environment and facilities in their country.
2. TEST CHARACTERISTICS
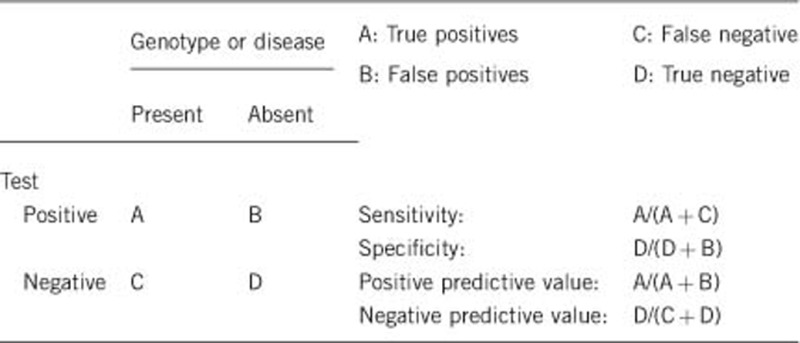
2.1 Analytical sensitivity
(proportion of positive tests if the genotype is present)
Sanger sequencing as the primary testing strategy will detect point mutations with close to 100% sensitivity.2 Overall sensitivity will be complete barring rare variants disrupting PCR primer binding and mutations undetectable by Sanger sequencing of coding regions, which are predicted to be in the minority. As an estimation, given the presence of a single patient with only one heterozygous EPG5 mutation identified from 24 cases screened to date in our laboratory, an analytical sensitivity of 98% (47/48 chromosomes) could be attributed. This figure does not incorporate the four patients in whom no EPG5 mutation has been identified, the rationale being that they are more likely to represent cases unlinked to the EPG5 locus, as discussed in 1.5.
2.2 Analytical specificity
(proportion of negative tests if the genotype is not present)
Sequence analysis: 100%.
2.3 Clinical sensitivity
(proportion of positive tests if the disease is present)
Clinical sensitivity in cases of Vici syndrome where all the main diagnostic features (agenesis of the corpus callosum, cataracts, cardiomyopathy, skeletal myopathy, skin hypopigmentation and immunodeficiency) are present is likely to be very high; absence of a positive result is likely to be related to the analytical sensitivity (see 2.1). Cases where not all diagnostic features are present are likely to result in a lower sensitivity. Locus heterogeneity or presence of mutations not detectable on routine Sanger sequencing has been suggested for 2/18 cases reported by Cullup et al.,1 in whom some but not all diagnostic features were present.
2.4 Clinical specificity
(proportion of negative tests if the disease is not present)
Considering early-onset and severity of Vici syndrome, clinical specificity is likely to be 100%.
2.5 Positive clinical predictive value
(life-time risk to develop the disease if the test is positive)
Vici syndrome is a severe condition, and most signs and symptoms are present at birth. The positive clinical predictive value (for example the likelihood of a fetus to develop features of Vici syndrome if found to carry recessive familial EPG5 mutations on prenatal testing) is likely to be 100%. Some features of Vici syndrome (for example, cataracts, cardiomyopathy and the associated immunodeficiency) are not always present at birth, but are expected to evolve over the first years of life with a likelihood approaching 100%.
2.6 Negative clinical predictive value
(probability not to develop the disease if the test is negative)
Index case in that family had been tested:
The negative clinical predictive value is likely to be 100% if the index case in the family had been tested and was found positive for EPG5 mutations.
Index case in that family had not been tested:
Unknown but probably high.
3. Clinical utility
3.1 (Differential) diagnostics: The tested person is clinically affected
(To be answered if in 1.9 ‘A' was marked)
3.1.1 Can a diagnosis be made other than through a genetic test?
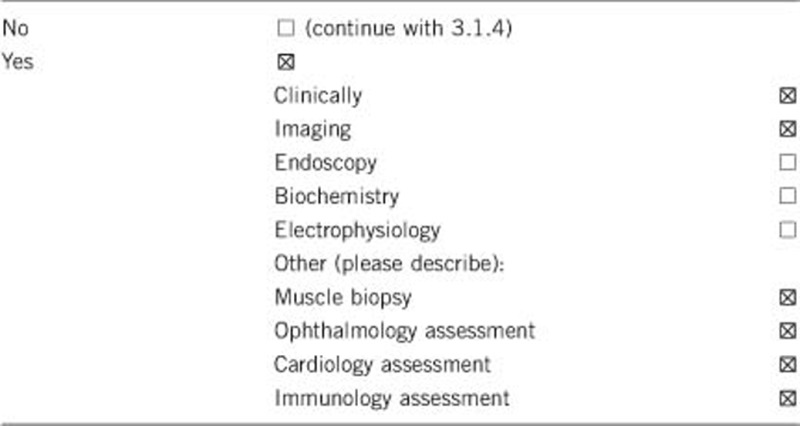
Comment:
The diagnosis of Vici syndrome is essentially a clinical diagnosis, based on the presence of at least four out of the five main diagnostic features: agenesis of the corpus callosum, cataracts, cardiomyopathy, skin hypopigmentation and immunodeficiency.1 In addition to a clinical assessment, brain magnetic resonance (MR) imaging, ophthalmology assessment (including slit lamp examination), cardiology assessment (including ECG and cardiac ultrasound) and tests to assess immune function are recommended to establish the presence of the main diagnostic features.
In cases where the genetic diagnosis of Vici syndrome has not been established, additional laboratory investigations (for example, transferrin isolelectric focussing), genetic testing and a muscle biopsy will often be performed to exclude multisystem disorders with similar features such as primary glycosylation defects, ciliopathies or mitochondrial disorders. Where performed, supportive features on muscle biopsy include light microscopy abnormalities comprising increased fiber size variability, increased internal nuclei and vacuolization,6 and ultrastructural changes on electron microscopy such as numerous vacuoles3 and abnormalities of mitochondrial morphology and localization.
3.1.2 Describe the burden of alternative diagnostic methods to the patient
The burden of assessments and investigations required to establish the clinical diagnosis of Vici syndrome are on the whole acceptable, but brain MR imaging will often require general anesthesia, particularly in small children.
A muscle biopsy is an invasive procedure that requires local or general anesthesia and carries a small risk of bleeding, infection and scarring.
3.1.3 How is the cost effectiveness of alternative diagnostic methods to be judged?
Unknown. However muscle biopsy in particular is a costly procedure, making diagnostic approaches relying on this procedure potentially less cost-effective than those relying on primary genetic testing. EPG5 is a relatively large gene, but new technologies such as next generation sequencing may reduce costs and turnaround times.
3.1.4 Will disease management be influenced by the result of a genetic test?

3.2 Predictive Setting: The tested person is clinically unaffected but carries an increased risk based on family history
(To be answered if in 1.9 ‘B' was marked)
Not applicable
3.2.1 Will the result of a genetic test influence lifestyle and prevention?
If the test result is positive (please describe)
Not applicable
If the test result is negative (please describe)
Not applicable
3.2.2 Which options in view of lifestyle and prevention does a person at-risk have if no genetic test has been done (please describe)?
Not applicable
3.3 Genetic risk assessment in family members of a diseased person
(To be answered if in 1.9 ‘C' was marked)
3.3.1 Does the result of a genetic test resolve the genetic situation in that family?
Yes, in all cases where recessive EPG5 mutations of proven pathogenicity have been identified. As outlined above, exclusion of EPG5 mutations should lead to a search for other disease-causing genes associated with a similar phenotype or to the consideration of an alternative diagnosis.
3.3.2 Can a genetic test in the index patient save genetic or other tests in family members?
A positive genetic test can save other clinical tests in similarly affected relatives. A positive genetic test enables genetic confirmation of the clinical diagnosis in similarly affected relatives and identification of the heterozygous carrier state in their unaffected parents, with a view to prenatal diagnosis.
3.3.3 Does a positive genetic test result in the index patient enable a predictive test in a family member?
Yes, identification of the mutation in the proband enables carrier testing in at risk relatives and confirmation of the diagnosis in other affected family members. In cases where not all features of Vici syndrome are present at the point of genetic diagnosis, identification of mutations in the proband may also help to predict development of symptoms (for example, a cardiomyopathy) commonly associated with Vici syndrome.
3.4 Prenatal diagnosis
(To be answered if in 1.9 ‘D' was marked)
3.4.1 Does a positive genetic test result in the index patient enable a prenatal diagnosis?
Yes, prenatal and preimplantation diagnosis can be performed in the family, if requested and in accordance with regulation and facilities in specific countries.
4. If applicable, further consequences of testing
Please assume that the result of a genetic test has no immediate medical consequences. Is there any evidence that a genetic test is nevertheless useful for the patient or his/her relatives? (Please describe)
The result of the genetic test is currently principally of use for the resolution of a clinical diagnosis in the patient and similarly affected relatives. In addition, a molecular genetic diagnosis will enable carrier parents of the index case to make informed reproductive decisions and allow future family planning. Molecular genetic confirmation of the clinical diagnosis will reduce the number of additional investigations (such as a muscle biopsy), which are invasive and expensive.
Acknowledgments
This work was supported by EuroGentest2 (Unit 2: ‘Genetic testing as part of health care'), a Coordination Action under FP7 (Grant Agreement Number 261469) and the European Society of Human Genetics. TC and HJ were supported by a grant from the Guy's and St. Thomas' Charitable Foundation (Grant number 070404). MG and ALK are supported by the Leducq Foundation, the MRC and the BHF.
The authors declare no conflict of interest.
References
- Cullup T, Kho AL, Dionisi-Vici C, et al. Recessive mutations in EPG5 cause Vici syndrome, a multisystem disorder with defective autophagy. Nat Genet. 2013;45:83–87. doi: 10.1038/ng.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellard S, Shields B, Tysoe C, et al. Semi-automated unidirectional sequence analysis for mutation detection in a clinical diagnostic setting. Genet Test Mol Biomarkers. 2009;13:381–386. doi: 10.1089/gtmb.2008.0096. [DOI] [PubMed] [Google Scholar]
- Al-Owain M, Al-Hashem A, Al-Muhaizea M, et al. Vici syndrome associated with unilateral lung hypoplasia and myopathy. AmJ Med Genet Part A. 2010;152A:1849–1853. doi: 10.1002/ajmg.a.33421. [DOI] [PubMed] [Google Scholar]
- Chiyonobu T, Yoshihara T, Fukushima Y, et al. Sister and brother with Vici syndrome: agenesis of the corpus callosum, albinism, and recurrent infections. AmJ Med Genet Part A. 2002;109:61–66. doi: 10.1002/ajmg.10298. [DOI] [PubMed] [Google Scholar]
- del Campo M, Hall BD, Aeby A, et al. Albinism and agenesis of the corpus callosum with profound developmental delay: Vici syndrome, evidence for autosomal recessive inheritance. AmJ Med Genet Part A. 1999;85:479–485. doi: 10.1002/(sici)1096-8628(19990827)85:5<479::aid-ajmg9>3.3.co;2-4. [DOI] [PubMed] [Google Scholar]
- McClelland V, Cullup T, Bodi I, et al. Vici syndrome associated with sensorineural hearing loss and evidence of neuromuscular involvement on muscle biopsy. AmJ Med Genet Part A. 2010;152A:741–747. doi: 10.1002/ajmg.a.33296. [DOI] [PubMed] [Google Scholar]
- Miyata R, Hayashi M, Sato H, et al. Sibling cases of Vici syndrome: sleep abnormalities and complications of renal tubular acidosis. AmJ Med Genet Part A. 2007;143:189–194. doi: 10.1002/ajmg.a.31584. [DOI] [PubMed] [Google Scholar]
- Ozkale M, Erol I, Gumus A, Ozkale Y, Alehan F. Vici syndrome associated with sensorineural hearing loss and laryngomalacia. PediatrNeurol. 2012;47:375–378. doi: 10.1016/j.pediatrneurol.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Rogers CR, Aufmuth B, Monesson S.Vici Syndrome: A Rare Autosomal Recessive Syndrome with Brain Anomalies, Cardiomyopathy, and Severe Intellectual Disability Case Reports in Genetics 20112011Article ID 421582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vici CD, Sabetta G, Gambarara M, et al. Agenesis of the corpus callosum, combined immunodeficiency, bilateral cataract, and hypopigmentation in two brothers. AmJ Med Genet Part A. 1988;29:1–8. doi: 10.1002/ajmg.1320290102. [DOI] [PubMed] [Google Scholar]
- Said E, Soler D, Sewry C. Vici syndrome—a rapidly progressive neurodegenerative disorder with hypopigmentation, immunodeficiency and myopathic changes on muscle biopsy. AmJ Med Genet Part A. 2012;158A:440–444. doi: 10.1002/ajmg.a.34273. [DOI] [PubMed] [Google Scholar]
- Finocchi A, Angelino G, Cantarutti N, et al. Immunodeficiency in Vici syndrome: a heterogeneous phenotype. AmJ Med Genet Part A. 2012;158A:434–439. doi: 10.1002/ajmg.a.34244. [DOI] [PubMed] [Google Scholar]


