Abstract
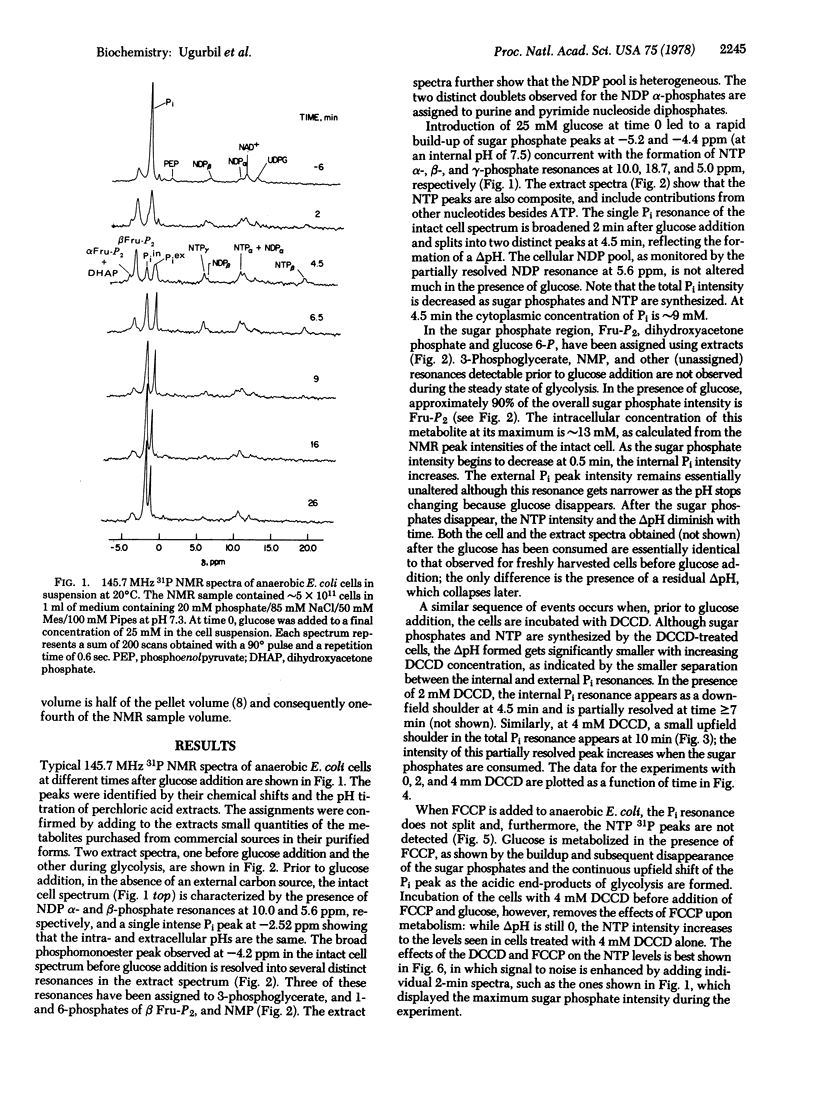
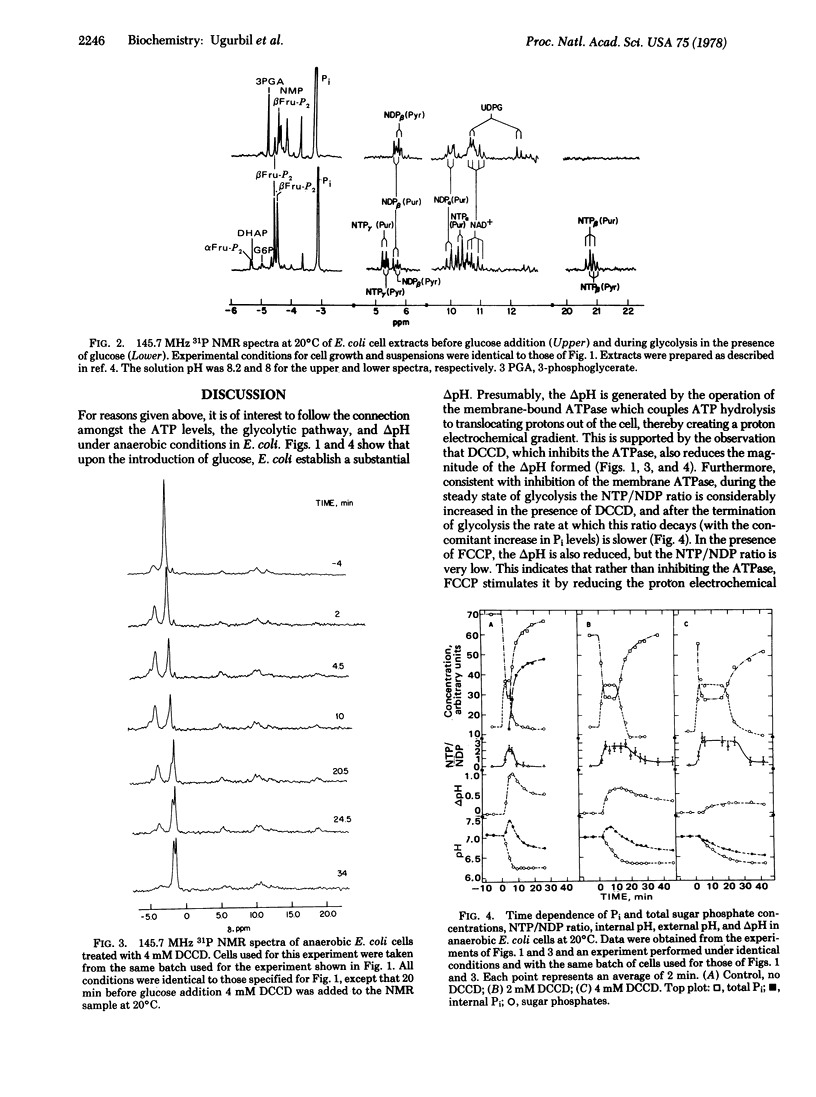
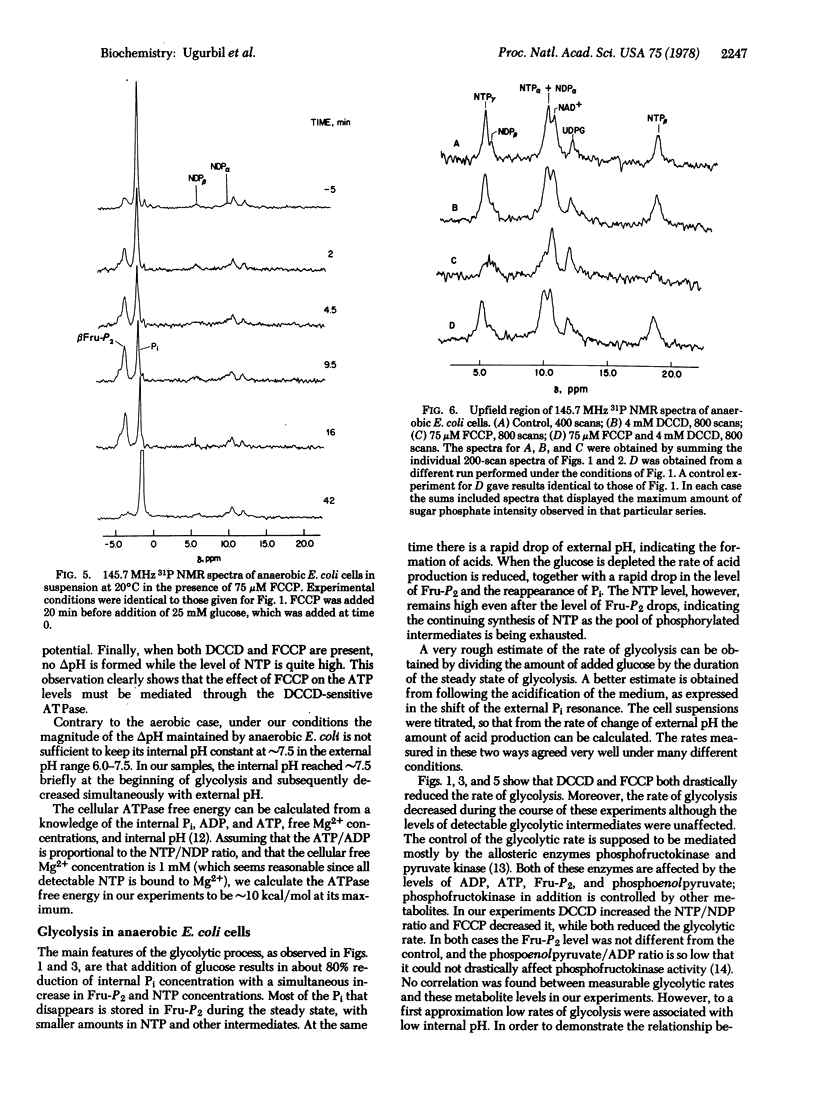
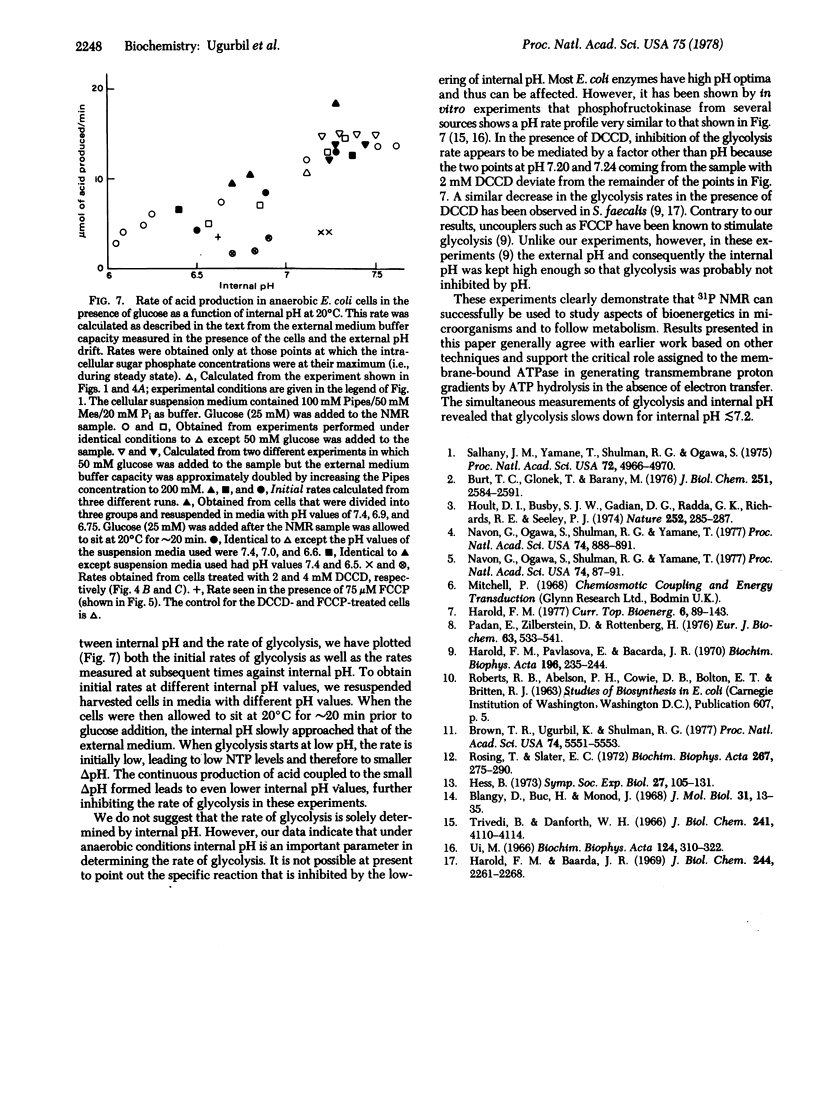
31P nuclear magnetic resonance spectra of glycolyzing, anaerobic Escherichia coli cells and their perchloric acid extracts were obtained at 145.7 MHz. Time-dependent intracellular concentrations of nucleoside di- and triphosphates, Pi, and sugar phosphates were measured during glycolysis with 2-min resolution, while intracellular and extra-cellular pH values were monitored simultaneously. Upon glucose addition, anaerobic E. coli cells rapidly produce acids and develop a transmembrane pH gradient (delta pH). Glycolysis rates were calculated from the changes in the external pH. It was found that glycolysis rates are strongly dependent on internal pH, sharply decreasing when the pH drops below approximately 7.2. The ATPase inhibitor, dicyclohexylcarbodiimide (DCCD), prevented NTP hydrolysis and inhibited delta pH formation. The uncoupler, carbonyl cyanide p-triflouromethoxyphenyl hydrazone (FCCP), drastically reduced both the delta pH and the NTP level. When the cells were previously treated with DCCD, FCCP collapsed the delta pH while the NTP levels remained high. It is concluded that ATP produced by glycolysis is hydrolyzed by the membrane ATPase to generate a delta pH and that FCCP stimulates ATP hydrolysis by ATPase and collapses the proton gradient.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blangy D., Buc H., Monod J. Kinetics of the allosteric interactions of phosphofructokinase from Escherichia coli. J Mol Biol. 1968 Jan 14;31(1):13–35. doi: 10.1016/0022-2836(68)90051-x. [DOI] [PubMed] [Google Scholar]
- Brown T. R., Ugurbil K., Shulman R. G. 31P nuclear magnetic resonance measurements of ATPase kinetics in aerobic Escherichia coli cells. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5551–5553. doi: 10.1073/pnas.74.12.5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt C. T., Glonek T., Bárány M. Analysis of phosphate metabolites, the intracellular pH, and the state of adenosine triphosphate in intact muscle by phosphorus nuclear magnetic resonance. J Biol Chem. 1976 May 10;251(9):2584–2591. [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R., Baron C., Abrams A. Inhibition of membrane-bound adenosine triphosphatase and of cation transport in Streptococcus faecalis by N,N'-dicyclohexylcarbodiimide. J Biol Chem. 1969 May 10;244(9):2261–2268. [PubMed] [Google Scholar]
- Harold F. M., Pavlasová E., Baarda J. R. A transmembrane pH gradient in Streptococcus faecalis: origin, and dissipation by proton conductors and N,N'-dicyclohexylcarbodimide. Biochim Biophys Acta. 1970;196(2):235–244. doi: 10.1016/0005-2736(70)90011-8. [DOI] [PubMed] [Google Scholar]
- Hess B. Organization of glycolysis: oscillatory and stationary control. Symp Soc Exp Biol. 1973;27:105–131. [PubMed] [Google Scholar]
- Hoult D. I., Busby S. J., Gadian D. G., Radda G. K., Richards R. E., Seeley P. J. Observation of tissue metabolites using 31P nuclear magnetic resonance. Nature. 1974 Nov 22;252(5481):285–287. doi: 10.1038/252285a0. [DOI] [PubMed] [Google Scholar]
- Navon G., Ogawa S., Shulman R. G., Yamane T. 31P nuclear magnetic resonance studies of Ehrlich ascites tumor cells. Proc Natl Acad Sci U S A. 1977 Jan;74(1):87–91. doi: 10.1073/pnas.74.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navon G., Ogawa S., Shulman R. G., Yamane T. High-resolution 31P nuclear magnetic resonance studies of metabolism in aerobic Escherichia coli cells. Proc Natl Acad Sci U S A. 1977 Mar;74(3):888–891. doi: 10.1073/pnas.74.3.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padan E., Zilberstein D., Rottenberg H. The proton electrochemical gradient in Escherichia coli cells. Eur J Biochem. 1976 Apr 1;63(2):533–541. doi: 10.1111/j.1432-1033.1976.tb10257.x. [DOI] [PubMed] [Google Scholar]
- Rosing J., Slater E. C. The value of G degrees for the hydrolysis of ATP. Biochim Biophys Acta. 1972 May 25;267(2):275–290. doi: 10.1016/0005-2728(72)90116-8. [DOI] [PubMed] [Google Scholar]
- Salhany J. M., Yamane T., Shulman R. G., Ogawa S. High resolution 31P nuclear magnetic resonance studies of intact yeast cells. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4966–4970. doi: 10.1073/pnas.72.12.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi B., Danforth W. H. Effect of pH on the kinetics of frog muscle phosphofructokinase. J Biol Chem. 1966 Sep 10;241(17):4110–4112. [PubMed] [Google Scholar]
- Ui M. A role of phosphofructokinase in pH-dependent regulation of glycolysis. Biochim Biophys Acta. 1966 Aug 24;124(2):310–322. doi: 10.1016/0304-4165(66)90194-2. [DOI] [PubMed] [Google Scholar]


