Abstract
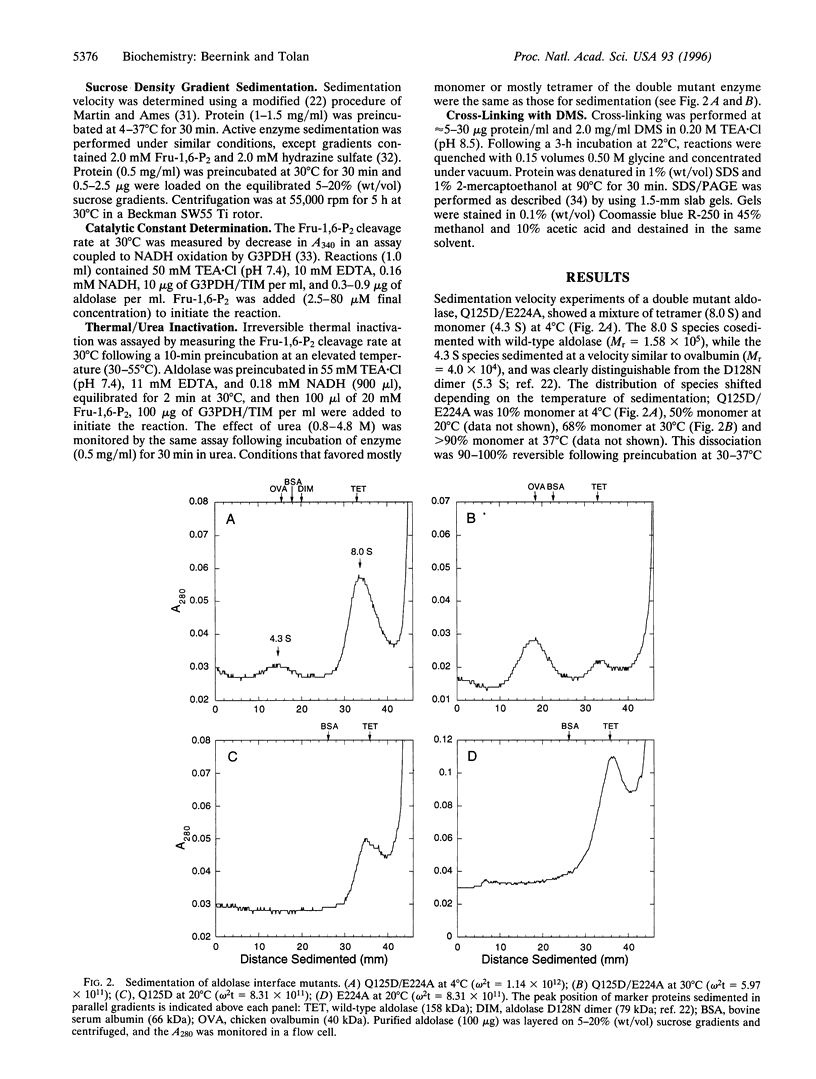
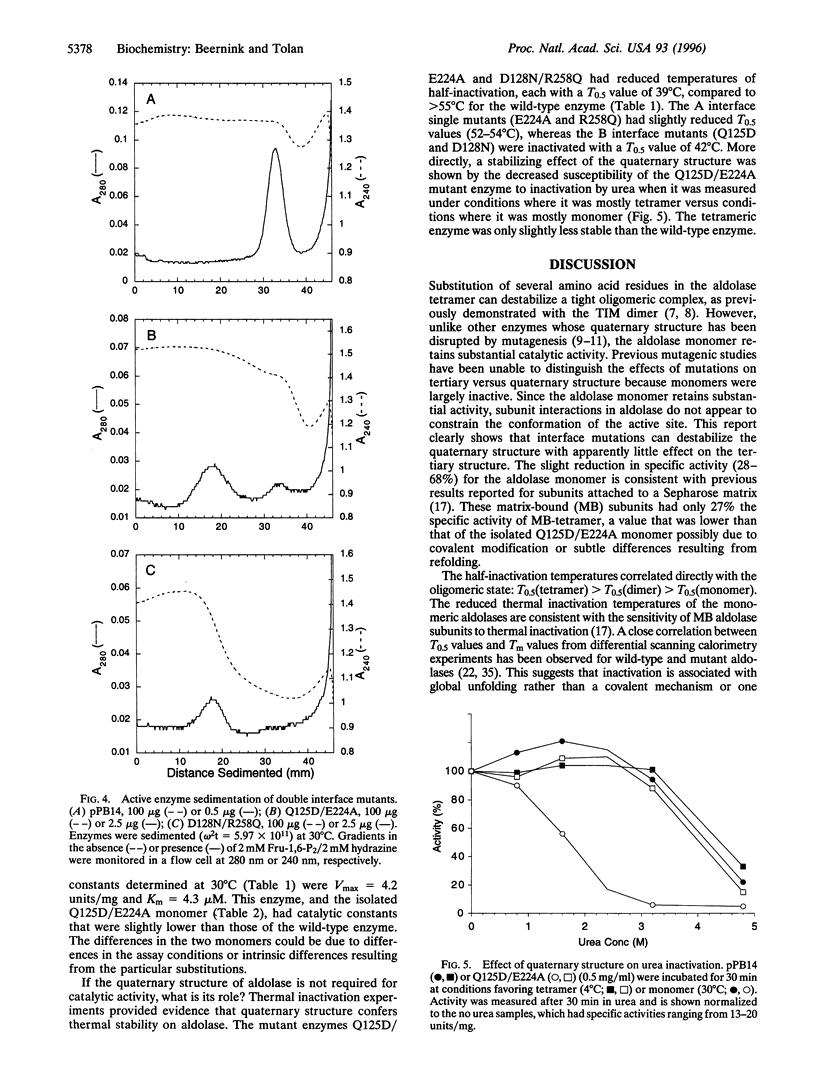
The fructose-1,6-bisphosphate aldolase (EC 4.1.2.13) homotetramer has been destabilized by site-directed mutagenesis at the two different subunit interfaces. A double mutant aldolase, Q125D/E224A, sediments as two distinct species, characteristic of a slow equilibrium, with velocities expected for the monomer and tetramer. The aldolase monomer is shown to be catalytically active following isolation from sucrose density gradients. The isolated aldolase monomer had 72% of the specific activity of the wild-type enzyme and a slightly lower Michaelis constant, clearly indicating that the quaternary structure is not required for catalysis. Cross-linking of the isolated monomer confirmed that it does not rapidly reequilibrate with the tetramer following isolation. There was a substantial difference between the tetramer and monomer in their inactivation by urea. The stability toward both urea and thermal inactivation of these oligomeric variants suggests a role for the quaternary structure in maintaining the stability of aldolase, which may be an important role of quaternary structure in many proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beernink P. T., Tolan D. R. Construction of a high-copy "ATG vector" for expression in Escherichia coli. Protein Expr Purif. 1992 Aug;3(4):332–336. doi: 10.1016/1046-5928(92)90009-l. [DOI] [PubMed] [Google Scholar]
- Beernink P. T., Tolan D. R. Subunit interface mutants of rabbit muscle aldolase form active dimers. Protein Sci. 1994 Sep;3(9):1383–1391. doi: 10.1002/pro.5560030904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchert T. V., Abagyan R., Jaenicke R., Wierenga R. K. Design, creation, and characterization of a stable, monomeric triosephosphate isomerase. Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1515–1518. doi: 10.1073/pnas.91.4.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchert T. V., Abagyan R., Kishan K. V., Zeelen J. P., Wierenga R. K. The crystal structure of an engineered monomeric triosephosphate isomerase, monoTIM: the correct modelling of an eight-residue loop. Structure. 1993 Nov 15;1(3):205–213. doi: 10.1016/0969-2126(93)90021-8. [DOI] [PubMed] [Google Scholar]
- Borchert T. V., Pratt K., Zeelen J. P., Callens M., Noble M. E., Opperdoes F. R., Michels P. A., Wierenga R. K. Overexpression of trypanosomal triosephosphate isomerase in Escherichia coli and characterisation of a dimer-interface mutant. Eur J Biochem. 1993 Feb 1;211(3):703–710. doi: 10.1111/j.1432-1033.1993.tb17599.x. [DOI] [PubMed] [Google Scholar]
- Brenner-Holzach O., Leuthardt F. Hybridisation of aldolase from Drosophila, blocked at the active site, with native C aldolase from calf brain. Eur J Biochem. 1972 Dec 18;31(3):423–426. doi: 10.1111/j.1432-1033.1972.tb02548.x. [DOI] [PubMed] [Google Scholar]
- Casal J. I., Ahern T. J., Davenport R. C., Petsko G. A., Klibanov A. M. Subunit interface of triosephosphate isomerase: site-directed mutagenesis and characterization of the altered enzyme. Biochemistry. 1987 Mar 10;26(5):1258–1264. doi: 10.1021/bi00379a009. [DOI] [PubMed] [Google Scholar]
- Chan W. W. Matrix-bound protein subunits. Biochem Biophys Res Commun. 1970 Dec 9;41(5):1198–1204. doi: 10.1016/0006-291x(70)90213-5. [DOI] [PubMed] [Google Scholar]
- Chan W. W., Mawer H. M. Studies on protein subunits. II. Preparation and properties of active subunits of aldolase bound to a matrix. Arch Biochem Biophys. 1972 Mar;149(1):136–145. doi: 10.1016/0003-9861(72)90307-4. [DOI] [PubMed] [Google Scholar]
- Chan W. W., Mort J. S., Chong D. K., Macdonald P. D. Studies on protein subunits. 3. Kinetic evidence for the presence of active subunits during the renaturation of muscle aldolase. J Biol Chem. 1973 Apr 25;248(8):2778–2784. [PubMed] [Google Scholar]
- DISCHE Z., LANDSBERG E. A colorimetric procedure for the determination of triose phosphate and fructose-1,6-diphosphate in presence of other sugars. Biochim Biophys Acta. 1960 Mar 25;39:144–147. doi: 10.1016/0006-3002(60)90130-x. [DOI] [PubMed] [Google Scholar]
- Davies G. E., Stark G. R. Use of dimethyl suberimidate, a cross-linking reagent, in studying the subunit structure of oligomeric proteins. Proc Natl Acad Sci U S A. 1970 Jul;66(3):651–656. doi: 10.1073/pnas.66.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrig T., Muhoberac B. B., Brems D., Bosron W. F. Monomers of human beta 1 beta 1 alcohol dehydrogenase exhibit activity that differs from the dimer. J Biol Chem. 1993 Jun 5;268(16):11721–11726. [PubMed] [Google Scholar]
- Iglesias A. A., Andreo C. S. NADP-dependent malate dehydrogenase (decarboxylating) from sugar cane leaves. Kinetic properties of different oligomeric structures. Eur J Biochem. 1990 Sep 24;192(3):729–733. doi: 10.1111/j.1432-1033.1990.tb19283.x. [DOI] [PubMed] [Google Scholar]
- Lebherz H. G. Evidence for the lack of subunit exchange between aldolase tetramers in vivo. J Biol Chem. 1975 Sep 25;250(18):7388–7391. [PubMed] [Google Scholar]
- Lebherz H. G. Stability of quaternary structure of mammalian and avian fructose diphosphate aldolases. Biochemistry. 1972 Jun 6;11(12):2243–2250. doi: 10.1021/bi00762a006. [DOI] [PubMed] [Google Scholar]
- Lusty C. J. Catalytically active monomer and dimer forms of rat liver carbamoyl-phosphate synthetase. Biochemistry. 1981 Jun 23;20(13):3665–3674. doi: 10.1021/bi00516a001. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Morris A. J., Tolan D. R. Site-directed mutagenesis identifies aspartate 33 as a previously unidentified critical residue in the catalytic mechanism of rabbit aldolase A. J Biol Chem. 1993 Jan 15;268(2):1095–1100. [PubMed] [Google Scholar]
- Nguyen T. T., Muench K. A., Bryant F. R. Inactivation of the recA protein by mutation of histidine 97 or lysine 248 at the subunit interface. J Biol Chem. 1993 Feb 15;268(5):3107–3113. [PubMed] [Google Scholar]
- Penhoet E. E., Rutter W. J. Catalytic and immunochemical properties of homomeric and heteromeric combinations of aldolase subunits. J Biol Chem. 1971 Jan 25;246(2):318–323. [PubMed] [Google Scholar]
- Porvari K. S., Herrala A. M., Kurkela R. M., Taavitsainen P. A., Lindqvist Y., Schneider G., Vihko P. T. Site-directed mutagenesis of prostatic acid phosphatase. Catalytically important aspartic acid 258, substrate specificity, and oligomerization. J Biol Chem. 1994 Sep 9;269(36):22642–22646. [PubMed] [Google Scholar]
- RACKER E. Enzymatic synthesis and breakdown of desoxyribose phosphate. J Biol Chem. 1952 May;196(1):347–365. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain M. S., Lebherz H. G. Hybridization between fructose diphosphate aldolase subunits derived from diverse biological systems: anomolous hybridization behavior of some aldolase subunit types. Arch Biochem Biophys. 1986 Jan;244(1):35–41. doi: 10.1016/0003-9861(86)90091-3. [DOI] [PubMed] [Google Scholar]
- Sygusch J., Beaudry D., Allaire M. Molecular architecture of rabbit skeletal muscle aldolase at 2.7-A resolution. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7846–7850. doi: 10.1073/pnas.84.22.7846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. W., Ott J., Eckstein F. The rapid generation of oligonucleotide-directed mutations at high frequency using phosphorothioate-modified DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8765–8785. doi: 10.1093/nar/13.24.8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traut T. W. Dissociation of enzyme oligomers: a mechanism for allosteric regulation. Crit Rev Biochem Mol Biol. 1994;29(2):125–163. doi: 10.3109/10409239409086799. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]