Abstract
The incidence of preterm birth is on the rise. The outcome of premature birth can vary widely, spanning completely normal development to severe neurological deficits, with a majority of children showing mild to moderate cognitive delay and increased incidence of neuropsychiatric conditions such as anxiety, attention deficit hyperactivity and autism spectrum disorders. Several animal models have been proposed to study the consequences of prematurity, one of the most promising being chronic perinatal hypoxia in mouse, which recapitulates the cognitive impairment, the partial recovery of function over time and the improvement after environmental enrichment. A major consequence of chronic perinatal hypoxia in animal models is delayed maturation of astrocytes, oligodendocytes and neurons, particularly inhibitory parvalbumin interneurons in the cerebral cortex. While delayed maturation can be seen as adaptive in that it allows prolonged neurogenesis and synaptic plasticity, it also leads to decreased myelination, aberrant neuron growth and likely impaired inhibitory regulation of electrical activity. Remarkably, interventions that increase cell maturation, such as environmental enrichment, reverse some of these cellular and behavioral deficits.
Introduction
Approximately 1-2% of live births in the United States are to very low birth weight infants (VLBW), defined as weighing under 1kg at birth. This has devastating consequences on neurological development, including striking decreases in cortical volume, white matter abnormalities and ventriculomegaly 1-4. Moreover, the incidence of live preterm births continues to rise steadily in recent decades 5. Functionally, VLBW children show increased incidence of developmental delays, motor disabilities and psychiatric illnesses such as anxiety disorders and autism spectrum disorders 1, 3, 4, 6-14. Importantly, many reports have shown that a substantial proportion of premature children recover over time. In a longitudinal study 15, more than 50% of VLBW manifested no significant neuropsychological differences as compared to term-born controls by the time they reach adulthood; among these, as many as 17% surpass term control for vocabulary skills, whereas about 40% maintain mild to severe cognitive impairment 15. With respect to the pathophysiology of abnormal neurological development in VLBW children, a percentage of VLBW children suffer from intraventricular hemorrhage or acute brain infarcts in early postnatal life, and manifest severe neurological deficits collectively referred to as “cerebral palsy”. However, the more frequent generalized neurological and behavioral disturbances described above are seen in the absence of brain infarcts; these are thought to be due to chronic hypoxic injury suffered by VLBW children as a result of immature lung development. Indeed, using a mouse model of chronic hypoxia, induced in rodent pups in the first week after birth (an age roughly corresponding to the third trimester of pregnancy in humans) investigators have been able to recapitulate the cortical volume loss, white matter abnormalities, ventriculomegaly and behavioral disturbances seen in the VLBW infant 16-22. Beyond the rodent, researchers have employed a range of animal models to study prematurity from baboon, sheep, to piglet and rabbit, with differing limitations and success, depending on the pathology being studied (for reviews see 17, 23, 24), however, the capacity for transgenic manipulations in the mouse makes it an attractive model to study the basic pathology of prematurity as well as options for treatment.
Recovery from hypoxic rearing in a mouse model of prematurity
As in the human condition observed in clinic, we have documented substantial recovery of many of the disturbances induced by hypoxic-rearing in our mouse model of perinatal hypoxia 19, 25. Nevertheless, some abnormalities remain; for example, cognitive deficits and increased anxiety behavior persist until adulthood, and our research has focused on elucidating the factors involved in mediating these differential recovery trajectories. We believe that chronic exposure to low levels of oxygen leads to a delay in brain maturation. This, on the one hand, can be conceptualized as an adaptive process that likely contributes to recovery from hypoxic injury, but on the other hand, a maturation delay may also be detrimental to developmental processes that occur during specific “critical” periods or in time-sensitive sequential events that are critical to subsequent developmental stages (Figure 1). Certainly, the persistence of behavioral deficits suggests that brain abnormalities related to these behaviors must exist. Abnormalities can exist on one or many levels of structure/function that are not necessarily mutually exclusive; from the integrity of cell numbers and synaptic connectivity, to large-scale structural integration of cells including intercellular architectural organization that condition neuronal network activity. This review attempts to examine perturbations at each of these levels of analysis and delineates the known underlying factors, synthesizing these data into a working hypothesis.
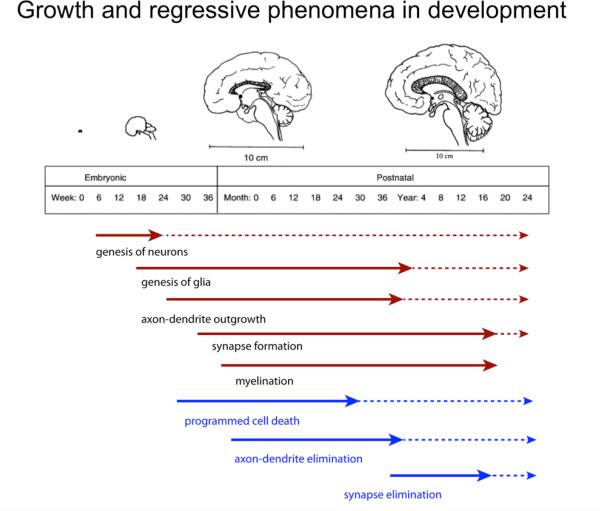
Figure 1.
Schematic outline of the major developmental processed occurring at embryonic and postnatal stages of human brain development. Growth processes are in red; regressive processes in blue. Broken arrows indicate continuation of a process at reduced levels or in restricted brain areas.
Cortical neurogenesis in perinatal injury
The decreases in cortical volume seen in VLBW children, and in response to postnatal chronic hypoxic injury in our animal model of prematurity, correspond to decreases in total number of neurons- particularly excitatory neurons, as they comprise of 90% of the neurons found in the cerebral cortex. Cortical volume following hypoxic injury in mice is decreased by approximately 24% (as compared to normoxic controls) at the end of chronic hypoxia exposure, which lasts from postnatal day 3 (P3) until P1116. The decreased number of neurons may be due to decreased neurogenesis or to increased cell death. Importantly, this phenomenon is not limited to our rodent model of chronic hypoxia, but decreased neurogenesis has also been observed in prematurely delivered rabbits. Remarkably, this decrease in cortical volume and corresponding neurogenesis is partially compensated by reactive processes that begin as soon as the animals are placed in normoxic environment. The recovery of cortical volume that occurs in the weeks following chronic hypoxia corresponds to a replenishment of the total number of excitatory neurons to those numbers seen in normoxic controls by the time mice reach early adulthood (6 weeks of age); this recovery occurs in part through an increase in cortical neurogenesis 16. It seems that hypoxic rearing promotes an increased “stem-cell like” capability of cortical astrocytes. It could also be that party damaged neurons “recover” their phenotype including marker immunoreactivity, a process that is favored by trophic factors such as Fibroblast Growth Factor (FGF) and Brain Derived Growth Factor (BDNF) signaling. Indeed, mice with genetic deletion of FGF receptor show much worse outcome and are unable to recover total neuron number after hypoxia 18.
Astroglia comprise of stereotypic star shaped protoplasmic astrocytes and astroglial stem cells, which exhibit a more radial like morphology. As with all neural stem cells, this subset of astroglia can self-renew and give rise to all three neural lineages: astrocytes, neurons and oligodendrocytes (microglia are thought to originate from the yolk sac). In the adult brain, stem cell activity of astroglia is restricted to the “neurogenic” regions of the brain: the dentate gyrus (DG) of the hippocampus and the subventricular zone (SVZ). However, during the first week postnatally, parenchymal astroglia retain stem cells properties also within the neocortex, but this ability decreases in the second week and is completely abolished by P15 26, 27. Interestingly however, we have shown in mice that hypoxic-rearing extends (or re-initiates) the period during which cortical astrocytes can give rise to new neurons beyond the first postnatal week, presumably contributing to the replenishment of excitatory neurons that we have observed during the recovery from hypoxic injury 27. Thus, the increased “stem-cell like” capability of cortical astrocytes in hypoxic animal could be seen as a delay in the normal maturation of astrocytes, from relatively simple cellular elements with wider potential to very specialized cells with much more complex machinery. The existence of a prolonged period of cortical astroglial “stemness” is not limited to the cortex, but a similar phenomenon has been demonstrated in other regions such as the subventricular zone (SVZ) and hippocampus 25, 27. Beyond the rodent brain, a recent study of post-mortem tissue from prematurely born infants showed increased proliferation of cortical cells expressing the transcription factor Sox2 28. Because Sox2 is typically expressed in the stem cell-like subpopulation of astroglia, this suggests that cortical astroglial cells of premature human infants may also show increased stem-cell like properties, however whether this reflects a prolonged period of “stemness” as in the rodent model, remains to be examined (Table 1).
Table 1.
Overview of cortical abnormalities in a rodent model of prematurity and VLBW infants
| Cortical Unit | Rodent Hypoxia Model | Humans- VLBW |
|---|---|---|
| Excitatory Neurons | -Reduced number early after injury -Recovery by adulthood16, 18 |
Unknown |
| Inhibitory Neurons | -Persistent “immature” protein expression profile -Decreased cortical GABA content35 |
-Some evidence for delayed expression of proteins associated with “developmental switch” -Decreased cortical GABA signaling 34, 55 |
| Astroglia | -Retention of stem-cell like, early postnatal developmental properties -Reduction in glutamate transport27, 56 |
Unknown |
| Oligodendrocytes | -Increased “immature protein expression profile” -Functional abnormalities in white matter myelination20 |
-Structural and Functional abnormalities in white matter 2, 37, 57, 58 |
| Connectivity: Synapses, Networks | Unknown | Disrupted or aberrant connectivity38-40 |
Inhibitory neuron maturation in perinatal injury
In rodents, cortical GABAergic neurons initially depolarize surrounding cells in early postnatal development, however, this decreases steadily until the end of the second postnatal week, when cortical GABAergic neurons make a “switch” to an inhibitory, hyperpolarizing, phenotype 29, 30. While the details of the necessary and sufficient factors mediating this functional switch of GABAergic cells remain still largely unknown, it is thought to be dependent on local excitatory activity and involves the regulation of expression levels of the chloride transporters KCC2 and NKCC1. Both transporters colocalize with GABAA receptors, a ligand-gated ion channel which allows for chloride ion movement across the cell membrane. KCC2 moves chloride ions outside the cell, whereas NKCC1 acts in the opposite direction, increasing chloride intracellular levels. During early stages of embryogenesis, low levels of KCC2 and high levels of NKCC1 contribute to maintain high intracellular concentrations of chloride. At this time, GABAA receptor activation results in a massive outflow of chloride ions and depolarization of the cell. This mechanism of chloride homeostasis is reversed during postnatal development, as KCC2 upregulation and NKCC1 downregulation reverse the chloride gradient across the membrane, so that GABAA receptor activation results in chloride ion influx and hyperpolarization (for a review see 31-33). This “developmental switch” in GABAergic receptor function from hypo- to hyperpolarizing occurs in the first postnatal week in the rodent brain and during the last trimester of development of the human fetus. As in the rodent, in humans this coincides with a difference in expression levels of KCC2 and NKCC1, starting at about 20 weeks of gestation and reaching peak levels at 35 weeks. The timing of the maturation of the GABAergic system occurs at precisely the period when VLBW premature infants would be born. Indeed, studies have shown significant perturbation of this developmental process in the premature infant, with decreases in GABA, KCC2 and NKCC1 within the cortex34. It has been suggested that these decreases in cortical GABA content and possible alterations of GABAergic function may play a causal role in the increased incidence of several disorders observed within the preterm infant population including epilepsy, schizophrenia and autism 34.
Using our mouse model of postnatal chronic hypoxia, we examined whether there were parallel changes in cortical inhibitory neurons. Indeed, we have shown that, compared to normoxic controls, there is a significant decrease in parvalbumin (PV)+ GABAergic neurons and GABA content within the cortex of hypoxic-reared mice, and that this is attributable to long-lasting changes in their phenotype and, perhaps, functionality, although the functional question remains to be addressed 35.
Coincident with the functional maturation of cortical GABAergic neurons is the upregulation of calcium-binding proteins, including parvalbumin (PV), calretinin (CR) and calbindin (CB). PV+ cortical interneurons make up approximately 50% of the total cortical GABAergic population and are characterized by their fast-spiking capabilities. Distinct from the PV population are neurons that express the growth hormone inhibiting peptide somatostatin (SST). Like PV, SST neurons are born embryonically and migrate from the medial ganglionic eminence to the cortex, where they mature and upregulate expression of their phenotypic protein markers, PV and SST, respectively. Raising mice under hypoxic conditions significantly decreases the number of cortical and hippocampal neurons that express PV and SST proteins, but not those that express CR. Given that levels of GABA, PV and SST are all decreased in the cortex of hypoxic reared mice, it stands to reason that there may be simply be less interneurons as compared to normoxic controls; similar to what we have observed in cortical excitatory neurons. However, we have not seen group differences in neuronal cell death. Furthermore, using GAD1-GFP transgenic mice, we found no differences in number of neurons marked by green fluorescent protein (GFP) between hypoxic and normoxic-reared mice. Because GFP in these mice is a pan interneuron marker, allowing for the visualization of all interneurons, regardless of developmental stage, the data suggest that hypoxia did not change the number of interneurons per se, but rather the expression of phenotypic proteins associated with maturation of these cells.
Connectivity and cortical network integration in perinatal injury
Despite the aforementioned remarkable recovery in cortical volume and excitatory neuron numbers, mice reared in hypoxic conditions continue to show behavioral disturbances on cognitive and emotional measures well into adulthood and long after excitatory neurons have been replenished to normal numbers. Therefore neurobiological perturbations must exist that account for these abnormalities. For example, the mere presence of new excitatory neurons identified though immunohistochemistry does not speak to the functionality of this newly generated cortical population. Electrophysiological recordings of these cells would be necessary to understand their capacity to function within their network. Critical relationships between cortical areas and the targets of descending connections may form aberrantly because some neurons are born after hypoxic-rearing and therefore considerably later than those born in a normoxic mouse. Moreover, since hypoxia occurs during the critical period when synaptogenesis and synaptic pruning occur in development, it is likely that neuronal connections are perturbed more generally. Disturbed connectivity in premature infants is suggested by abnormal trajectories of white matter growth and by functional connectivity studies. Although alterations in neuronal arborization and synaptic density have not yet been examined in human tissue, decreased arborization and synaptic density have been shown in a sheep model of fetal ischemia 36-41. In hypoxic mice, connectivity has not been assessed anatomically, but a diffusion tensor imaging (DTI) study showed perturbations of long-range connectivity 19. Finally, beyond the physical connections themselves, the functionality of these connections can also contribute to decreased performance; for example, delayed myelination may decrease effective and coordinated neuronal communication with their targets. Again, perturbations in myelination and connectivity have been described in VLBW infants such that abnormalities in language brain centers and cortical and white matter connectivity are perturbed when examined by fMRI and DTI 36, 38, 39, 42-44. Importantly, in spite of the evidence for abnormal connectivity in the VLBW infant, to date, studies have not examined whether this is also true at a microstructural level in rodent models of prematurity. Electrophysiological and structural connectivity experiments from imaging to tracing studies will be necessary to explore both connections and their functionality on a level of cell-cell interaction and within larger networks, both regional and beyond.
Abnormal development of cortical excitatory/inhibitory networks in prematurity
Development of cortical connections relies heavily on interactions between excitatory and inhibitory neurons. In mice, excitatory neurons are generated starting from embryonic day 11 and in humans, excitatory neurogenesis in the cerebral cortex has been reported to start around the 7th week of gestation, and is completed by the 15th -18th week of gestation (Figure 1). Although recent reports have suggested that neurogenesis arising from the subventricular zone (SVZ) may actually continue until 28 gestational weeks 28, it is not clear whether this is cortical neurogenesis or whether these cells may migrate to the olfactory bulb. In mouse, inhibitory neurons arise from specific regions of the ventral telencephalon, namely the medial and the caudal ganglionic eminence (MGE and CGE). In humans, however, a sizable proportion of inhibitory cells may arise, like the excitatory neurons, from dorsal telencephalon 45. After being generated, interneurons migrate to the cortical primordium, where they integrate with radially-migrating excitatory neurons to form local networks, although little is known about how these two events are synchronized and reciprocally regulated.
Interneurons may be grouped into different classes according to their developmental origins, morphological features, expression of specific neuropeptides and calcium-binding proteins, mode of interaction with excitatory neurons, localization with respect to cortical layers, and electrophysiological properties. In this view, excitatory and inhibitory neurons, which are profoundly different with regard to their developmental origin and timing of differentiation, are functionally coupled together to form processing units, named cortical columns. Thus, the birth, migration and integration of cortical pyramidal neurons and interneurons occur between E11-E18 in mouse and the first 5 months of gestation in humans (Figure 1) (for a review, see 46). However, neurons do continue to be generated through postnatal life in restricted niches, such as the hippocampal DG and in the SVZ, where newly generated neurons then migrate along the rostral migratory stream to the olfactory bulb. Given their vulnerable timing, these processes are likely to be particularly disrupted in the premature infant.
Following neurogenesis, and during the third trimester of gestation in humans and in the first 10 days after birth in rodents, maturational processes of these newly born cells preside. As such, cortical neurons elaborate their axons and dendrites, establish synapses and later on, prune back these synapses, presumably to form coherent, specialized networks of activity (Figure 1). There are unique, area-specific patterns of connectivity, morphology of neurons and electrophysiological properties. During this time, glial cells –both astrocytes and oligodendrocytes--are being generated and mature their morphology and functional properties, upregulating proteins associated with a mature glial phenotype such as glial fibrillary acidic protein and glutamine synthetase in astrocytes and myelin basic protein in oligodendrocytes, while downregulating vimentin, Sox2 and NG2 (for reviews see 47-49. As such, glia also lose their capacity for stem-cell like activity and “retire” to their mature functional profiles including regulating neurotransmitter turnover and providing trophic support to surrounding neurons.
Furthermore, inhibitory neurons require an extended period of maturation that in rodents extends into adolescence. During this extended period, neuronal connections and synapses, which are originally produced in excess, are pruned in an activity-dependent way, to lead to mature patterns of connectivity. Because the organization of the cerebral cortex into functional modules occurs within narrow critical periods of time, it could be argued that any impairment in generation and maturation of both excitatory and inhibitory neurons may have profound consequences on the functional outcome of a given brain region.
Hypoxic injury induces a delay in maturation
We observed that in hypoxic-reared mice 1) cortical astrocytes remain in an immature, stem-cell like state for an extended period of time, and 2) cortical inhibitory neurons show a more immature protein expression profile. These findings might suggest that the cortical milieu of hypoxic-reared mice is developmentally stunted. While this delay in maturation may well facilitate recovery, allowing for a prolonged period of cortical neurogenesis and synaptic plasticity, these new cells may be impaired in their ability to integrate into functional circuitry, leading to the formation of aberrant patterns of connectivity (Box 1).
Unfortunately, it is not yet clear whether this delay in maturation of interneurons during the recovery process is adaptive or maladaptive. Most likely, the prolonged plasticity endangered by the maturation delay is in part beneficial by allowing alternative patterns of connectivity to emerge to compensate for lost functions, and in part deleterious, because it may cause excessive “growth” and delay the establishment of mature patterns of connectivity. Indeed, manipulations that enhance maturational processes ameliorate recovery. One of such manipulations is environmental enrichment, which consists of cognitive and physical stimulation. In rodents, this is achieved through the introduction of running wheels and a changing, novel environment of toys and burrowing tubes. Environmental enrichment has been shown to enhance maturational processes, i.e., increasing the complexity of dendritic arbors of newly generated neurons 50-53. Environmental enrichment of hypoxic-reared mice induced a more mature protein expression profile in cortical interneurons (increased PV and SST expression) and reversed cognitive deficits such that enriched, hypoxic-reared mice were no different from normoxic-reared controls 21, 25. Excitingly, the use of environmental enrichment as a therapeutic intervention also seems valuable in the clinic. Longitudinal studies have shown that environmental factors such as two parent households and increased maternal education predict augmented recovery in prematurely born children 54. Nevertheless, enrichment of infants is time consuming, costly, and sometimes not entirely feasible in the premature infant. Therefore, the underlying factors involved in accelerating maturational processes need to be understood in order to develop novel, targeted therapeutic options that can be used to intervene early on in the developmental process.
Conclusions
Animal models of prematurity help us understand the pathophysiology of abnormal development in children suffering from prematurity and its complications, suggesting new treatments. Important insights derived from such models are that the brain reacts to perinatal insults by long-lasting adaptations which include increased neurogenesis as well as delayed neuronal and glial maturation. Delayed maturations of neurons and glial cells is thought to be responsible for most of the behavioral deficits.
Key Points.
Many human premature infants and animal models of perinatal hypoxic injury recover gross anatomical deficits, but microstructural and functional alterations persists
Astroglial cells are delayed in their maturation and retain stem cell properties, prolonging neurogenesis
A portion of PV and SST inhibitory neurons subtypes remain immature and likely function abnormally
There is likely aberrant connectivity and altered balance of excitation/inhibition in hypoxic-reared animals.
Box 1. Evidence suggesting that chronic hypoxia induces a delay in cortical maturation.
Cortical astroglial cells remain pluripotent, show self-renewal several days after hypoxic-rearing (P15) and express stem-cell markers; a phenotype typically restricted to the first postnatal week.
Oligodendrocyte progenitors increase their proliferation and show protein expression profiles that are also consistent with earlier stages of maturation following hypoxic-rearing.
Cortical interneurons fail to properly upregulate mature protein expression markers (such as parvalbumin and somatostatin) after hypoxic-rearing
Interventions that increase cell maturation, such as environmental enrichment, reverse the interneuron deficits.
Acknowledgments
This work was supported by NIH grants P01 NS062686, R01 MH067715 and R01 NS060750 (F.M.V); and NS is the recipient of a Canadian Institute of Health Fellowship.
We acknowledge Laura Ment, Vittorio Gallo, Tamas Horvath, Michael Schwartz and Joseph Madri for useful discussions, and Allyson Vermaak for technical assistance. The authors declare no competing financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8:110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Volpe JJ. Cerebral white matter injury of the premature infant-more common than you think. Pediatrics. 2003;112:176–180. doi: 10.1542/peds.112.1.176. [DOI] [PubMed] [Google Scholar]
- 3.Vaccarino FM, Ment LR. Injury and repair in developing brain. Arch Dis Child Fetal Neonatal Ed. 2004;89:F190–192. doi: 10.1136/adc.2003.043661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371:261–269. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- 5.Martin As, Scahill L, Kratochvil CJ. Pediatric psychopharmacology : principles and practice. 2nd ed Oxford University Press; Oxford ; New York: 2011. p. xxvi.p. 810. [Google Scholar]
- 6.Volpe JJ. Cognitive deficits in premature infants. N Engl J Med. 1991;325:276–278. doi: 10.1056/NEJM199107253250409. [DOI] [PubMed] [Google Scholar]
- 7.Peterson BS, Vohr B, Staib LH, et al. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. Jama. 2000;284:1939–1947. doi: 10.1001/jama.284.15.1939. [comment]. [DOI] [PubMed] [Google Scholar]
- 8.Parker J, Mitchell A, Kalpakidou A, et al. Cerebellar growth and behavioural & neuropsychological outcome in preterm adolescents. Brain : a journal of neurology. 2008;131:1344–1351. doi: 10.1093/brain/awn062. [DOI] [PubMed] [Google Scholar]
- 9.Nosarti C, Giouroukou E, Micali N, et al. Impaired executive functioning in young adults born very preterm. Journal of the International Neuropsychological Society : JINS. 2007;13:571–581. doi: 10.1017/S1355617707070725. [DOI] [PubMed] [Google Scholar]
- 10.Moore GS, Kneitel AW, Walker CK, et al. Autism risk in small- and large-forgestational-age infants. American journal of obstetrics and gynecology. 2012;206:314, e311–319. doi: 10.1016/j.ajog.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luu TM, Ment LR, Schneider KC, et al. Lasting effects of preterm birth and neonatal brain hemorrhage at 12 years of age. Pediatrics. 2009;123:1037–1044. doi: 10.1542/peds.2008-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Limperopoulos C. Autism spectrum disorders in survivors of extreme prematurity. Clinics in perinatology. 2009;36:791–805. vi. doi: 10.1016/j.clp.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Anderson P, Doyle LW. Victorian Infant Collaborative Study G. Neurobehavioral outcomes of school-age children born extremely low birth weight or very preterm in the 1990s. JAMA. 2003. 289:3264–3272. doi: 10.1001/jama.289.24.3264. [DOI] [PubMed] [Google Scholar]
- 14.Allin M, Walshe M, Fern A, et al. Cognitive maturation in preterm and term born adolescents. Journal of neurology, neurosurgery, and psychiatry. 2008;79:381–386. doi: 10.1136/jnnp.2006.110858. [DOI] [PubMed] [Google Scholar]
- 15.Luu TM, Vohr BR, Allan W, et al. Evidence for catch-up in cognition and receptive vocabulary among adolescents born very preterm. Pediatrics. 2011;128:313–322. doi: 10.1542/peds.2010-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fagel DM, Ganat Y, Silbereis J, et al. Cortical neurogenesis enhanced by chronic perinatal hypoxia. Exp Neurol. 2006;199:77–91. doi: 10.1016/j.expneurol.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Scafidi J, Fagel DM, Ment LR, et al. Modeling premature brain injury and recovery. Int J Dev Neurosci. 2009;27:863–871. doi: 10.1016/j.ijdevneu.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fagel DM, Ganat Y, Cheng E, et al. Fgfr1 is required for cortical regeneration and repair after perinatal hypoxia. J Neurosci. 2009;29:1202–1211. doi: 10.1523/JNEUROSCI.4516-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chahboune H, Ment LR, Stewart WB, et al. Hypoxic Injury during Neonatal Development in Murine Brain: Correlation between In Vivo DTI Findings and Behavioral Assessment. Cereb Cortex. 2009 doi: 10.1093/cercor/bhp068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jablonska B, Scafidi J, Aguirre A, et al. Oligodendrocyte regeneration after neonatal hypoxia requires FoxO1-mediated p27Kip1 expression. J Neurosci. 2012;32:14775–14793. doi: 10.1523/JNEUROSCI.2060-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Douglas RM, Ryu J, Kanaan A, et al. Neuronal death during combined intermittent hypoxia/hypercapnia is due to mitochondrial dysfunction. American journal of physiology Cell physiology. 2010;298:C1594–1602. doi: 10.1152/ajpcell.00298.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai J, Tuong CM, Zhang Y, et al. Mouse intermittent hypoxia mimicking apnoea of prematurity: effects on myelinogenesis and axonal maturation. The Journal of pathology. 2012;226:495–508. doi: 10.1002/path.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Back SA, Riddle A, Dean J, et al. The instrumented fetal sheep as a model of cerebral white matter injury in the premature infant. Neurotherapeutics. 2012;9:359–370. doi: 10.1007/s13311-012-0108-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinney HC, Volpe JJ. Modeling the encephalopathy of prematurity in animals: the important role of translational research. Neurol Res Int. 2012;2012:295389. doi: 10.1155/2012/295389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salmaso N, Silbereis J, Komitova M, et al. Environmental Enrichment Increases the GFAP+ Stem Cell Pool and Reverses Hypoxia-Induced Cognitive Deficits in Juvenile Mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:8930–8939. doi: 10.1523/JNEUROSCI.1398-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laywell E, Rakic P, Kukekov VG, et al. Identification of a multipotent astrocytic stem cell in the immature and adult mouse brain. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:13883–13888. doi: 10.1073/pnas.250471697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bi B, Salmaso N, Komitova M, et al. Cortical glial fibrillary acidic protein-positive cells generate neurons after perinatal hypoxic injury. J Neurosci. 2011;31:9205–9221. doi: 10.1523/JNEUROSCI.0518-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malik S, Vinukonda G, Vose LR, et al. Neurogenesis continues in the third trimester of pregnancy and is suppressed by premature birth. J Neurosci. 2013;33:411–423. doi: 10.1523/JNEUROSCI.4445-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- 30.Owens DF, Kriegstein AR. Is there more to GABA than synaptic inhibition? Nat Rev Neurosci. 2002;3:715–727. doi: 10.1038/nrn919. [DOI] [PubMed] [Google Scholar]
- 31.Ben-Ari Y, Khalilov I, Kahle KT, et al. The GABA excitatory/inhibitory shift in brain maturation and neurological disorders. Neuroscientist. 2012;18:467–486. doi: 10.1177/1073858412438697. [DOI] [PubMed] [Google Scholar]
- 32.Fiumelli H, Woodin MA. Role of activity-dependent regulation of neuronal chloride homeostasis in development. Curr Opin Neurobiol. 2007;17:81–86. doi: 10.1016/j.conb.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 33.Kriegstein AR, Owens DF. GABA may act as a self-limiting trophic factor at developing synapses. Sci STKE. 2001:pe1. doi: 10.1126/stke.2001.95.pe1. 2001. [DOI] [PubMed] [Google Scholar]
- 34.Robinson S, Mikolaenko I, Thompson I, et al. Loss of cation-chloride cotransporter expression in preterm infants with white matter lesions: implications for the pathogenesis of epilepsy. J Neuropathol Exp Neurol. 2010;69:565–572. doi: 10.1097/NEN.0b013e3181dd25bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Komitova M, Xenos D, Salmaso N, et al. Hypoxia-induced developmental delays of inhibitory interneurons are reversed by environmental enrichment in the postnatal mouse forebrain. J Neurosci. 2013;33:13375–13387. doi: 10.1523/JNEUROSCI.5286-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Imai M, Watanabe H, Yasui K, et al. Functional connectivity of the cortex of term and preterm infants and infants with Down's syndrome. Neuroimage. 2013 doi: 10.1016/j.neuroimage.2013.04.080. [DOI] [PubMed] [Google Scholar]
- 37.Pavlova MA, Krageloh-Mann I. Limitations on the developing preterm brain: impact of periventricular white matter lesions on brain connectivity and cognition. Brain. 2013;136:998–1011. doi: 10.1093/brain/aws334. [DOI] [PubMed] [Google Scholar]
- 38.Ball G, Boardman JP, Aljabar P, et al. The influence of preterm birth on the developing thalamocortical connectome. Cortex. 2013;49:1711–1721. doi: 10.1016/j.cortex.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 39.Lubsen J, Vohr B, Myers E, et al. Microstructural and functional connectivity in the developing preterm brain. Semin Perinatol. 2011;35:34–43. doi: 10.1053/j.semperi.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mullen KM, Vohr BR, Katz KH, et al. Preterm birth results in alterations in neural connectivity at age 16 years. Neuroimage. 2011;54:2563–2570. doi: 10.1016/j.neuroimage.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dean JM, McClendon E, Hansen K, et al. Prenatal cerebral ischemia disrupts MRI-defined cortical microstructure through disturbances in neuronal arborization. Sci Transl Med. 2013;5:168ra167. doi: 10.1126/scitranslmed.3004669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smyser CD, Inder TE, Shimony JS, et al. Longitudinal analysis of neural network development in preterm infants. Cereb Cortex. 2010;20:2852–2862. doi: 10.1093/cercor/bhq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Myers EH, Hampson M, Vohr B, et al. Functional connectivity to a right hemisphere language center in prematurely born adolescents. NeuroImage. 2010;51:1445–1452. doi: 10.1016/j.neuroimage.2010.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Constable RT, Ment LR, Vohr BR, et al. Prematurely born children demonstrate white matter microstructural differences at 12 years of age, relative to term control subjects: an investigation of group and gender effects. Pediatrics. 2008;121:306–316. doi: 10.1542/peds.2007-0414. [DOI] [PubMed] [Google Scholar]
- 45.Letinic K, Zoncu R, Rakic P. Origin of GABAergic neurons in the human neocortex. Nature. 2002;417:645–649. doi: 10.1038/nature00779. [DOI] [PubMed] [Google Scholar]
- 46.Stevens HE, Smith KM, Rash BG, et al. Neural stem cell regulation, fibroblast growth factors, and the developmental origins of neuropsychiatric disorders. Front Neurosci. 2010;4 doi: 10.3389/fnins.2010.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Molofsky AV, Krencik R, Ullian EM, et al. Astrocytes and disease: a neurodevelopmental perspective. Genes Dev. 2012;26:891–907. doi: 10.1101/gad.188326.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nishiyama A, Komitova M, Suzuki R, et al. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci. 2009;10:9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- 49.Nguyen L, Borgs L, Vandenbosch R, et al. The Yin and Yang of cell cycle progression and differentiation in the oligodendroglial lineage. Ment Retard Dev Disabil Res Rev. 2006;12:85–96. doi: 10.1002/mrdd.20103. [DOI] [PubMed] [Google Scholar]
- 50.Liu N, He S, Yu X. Early natural stimulation through environmental enrichment accelerates neuronal development in the mouse dentate gyrus. PLoS One. 2012;7:e30803. doi: 10.1371/journal.pone.0030803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baroncelli L, Braschi C, Spolidoro M, et al. Nurturing brain plasticity: impact of environmental enrichment. Cell Death Differ. 2010;17:1092–1103. doi: 10.1038/cdd.2009.193. [DOI] [PubMed] [Google Scholar]
- 52.Redila VA, Christie BR. Exercise-induced changes in dendritic structure and complexity in the adult hippocampal dentate gyrus. Neuroscience. 2006;137:1299–1307. doi: 10.1016/j.neuroscience.2005.10.050. [DOI] [PubMed] [Google Scholar]
- 53.Eadie BD, Redila VA, Christie BR. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. J Comp Neurol. 2005;486:39–47. doi: 10.1002/cne.20493. [DOI] [PubMed] [Google Scholar]
- 54.Ment LR, Vohr B, Allan W, et al. Change in cognitive function over time in very low-birth-weight infants. Jama. 2003;289:705–711. doi: 10.1001/jama.289.6.705. [DOI] [PubMed] [Google Scholar]
- 55.Robinson S, Li Q, Dechant A, et al. Neonatal loss of gamma-aminobutyric acid pathway expression after human perinatal brain injury. J Neurosurg. 2006;104:396–408. doi: 10.3171/ped.2006.104.6.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raymond M, Li P, Mangin JM, et al. Chronic perinatal hypoxia reduces glutamate-aspartate transporter function in astrocytes through the Janus kinase/signal transducer and activator of transcription pathway. J Neurosci. 2011;31:17864–17871. doi: 10.1523/JNEUROSCI.3179-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nosarti C, Giouroukou E, Healy E, et al. Grey and white matter distribution in very preterm adolescents mediates neurodevelopmental outcome. Brain : a journal of neurology. 2008;131:205–217. doi: 10.1093/brain/awm282. [DOI] [PubMed] [Google Scholar]
- 58.Back SA. Perinatal white matter injury: the changing spectrum of pathology and emerging insights into pathogenetic mechanisms. Ment Retard Dev Disabil Res Rev. 2006;12:129–140. doi: 10.1002/mrdd.20107. [DOI] [PubMed] [Google Scholar]