SYNOPSIS
Intraventricular hemorrhage (IVH) is a major neurological complication of prematurity. Pathogenesis of intraventricular hemorrhage is attributed to intrinsic fragility of germinal matrix vasculature and to the fluctuation in the cerebral blood flow. Germinal matrix exhibits rapid angiogenesis orchestrating formation of immature vessels. The angiogenic vessels lack pericytes, display immature basal lamina low in fibronectin, and have astrocyte end-feet coverage deficient in glial fibrillary acidic protein. These factors contribute to the fragility of the germinal matrix vasculature. Fluctuation in the cerebral blood flow results from a wide range of respiratory and hemodynamic instability associated with the preterm infants. Prenatal glucocorticoid exposure remains the most effective means of preventing IVH. Therapies targeted to enhance the stability of the germinal matrix vasculature and minimize fluctuation in the cerebral blood flow might lead to more effective strategies in preventing IVH.
Keywords: germinal matrix hemorrhage, intraventricular hemorrhage, astrocytes, pericytes, angiogenesis, glucocorticoids, premature infants, indomethacin
Introduction
In the United States, about 12,000 premature infants develop intraventricular hemorrhage (IVH) every year. The incidence of moderate-to-severe IVH has remained almost stationary during the last two decades.1, 2 IVH is a major problem in premature infants, as a large number of them develop neurologic sequelae.3 Approximately 50–75% of preterm survivors with IVH develop cerebral palsy, mental retardation, and/or hydrocephalus.3, 4 Approximately, a quarter of non-disabled survivors develop psychiatric disorders and problems with executive function.5–7 According to the U.S. Census Bureau and the NICHD Neonatal Research Network, over 3600 new cases of mental retardation each year are children who were born premature and suffered IVH.8, 9 Hence, IVH and its resultant neurologic and psychiatric sequelae continue to be a major public health concern worldwide.
IVH typically initiates in the periventricular germinal matrix.10 This brain region is known to developmental neurobiologists as the ganglionic eminence (Fig. 1A). The germinal matrix consists of neuronal and glial precursor cells (Fig. 1B, C) and is most prominent on the head of caudate nucleus. The subependymal germinal matrix is highly vascular and is selectively vulnerable to hemorrhage. After 24 gestational weeks (gw), thickness of the germinal matrix decreases, and it almost disappears by 36–37 gw. When hemorrhage in the germinal matrix is substantial, the underlying ependyma breaks and germinal matrix hemorrhage progresses to IVH, as blood fills the lateral cerebral ventricle.
Figure 1. Morphology of germinal matrix.
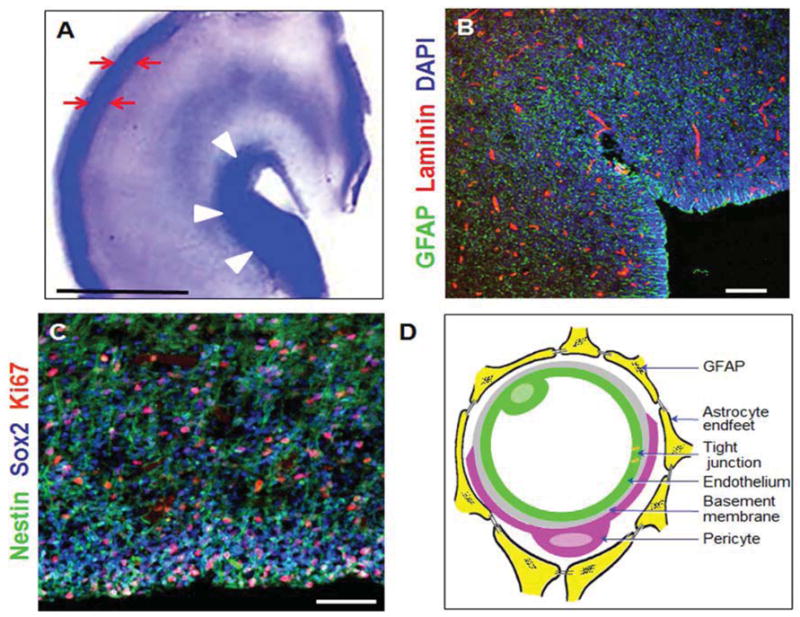
A) Representative cresyl violet staining of coronal section of the right-sided cerebral hemisphere of a 23 week preterm infant. Note cortical plate (arrows) and germinal matrix (arrow heads). Germinal matrix (violet staining) surrounds the whole ventricle, but is most conspicuous at the head of caudate nucleus. V, ventricle. Scale bar, 0.5 cm. B) Representative immunofluorescence of cryosection from germinal matrix of a 23 week premature infant labeled with DAPI (blue), GFAP (green), and laminin (vascular marker, red). Note germinal matrix is highly vascular (vascular endothelium in red) and enriched with GFAP (+) glial cells (green). C) Coronal brain section was double labeled with nestin (progenitor cells, green), Sox2 (radial glia, blue), and Ki67 (red, proliferation marker). Note nestin and Sox2 positive cells are abundant in the germinal matrix. Scale bar; 100 (B) and 50 μm (C). D) Schematic drawing of the blood brain barrier in cross section showing endothelium, endothelial tight junction, basal lamina, pericyte, and astrocyte endfeet.
PATHOGENESIS OF INTRAVENTRICULAR HEMORRHAGE
Pathogenesis of IVH is multifactorial, complex, and heterogeneous. An inherent fragility of the germinal matrix vasculature sets the ground for hemorrhage and fluctuation in the cerebral blood flow induces the rupture of vasculature (Box 1). If there are associated platelet or coagulation disorders, the homeostasis mechanisms are impaired which might accentuate the hemorrhage. Vaginal delivery, low Apgar score, severe respiratory distress syndrome, pneumothorax, hypoxia, hypercapnia, seizures, patent ductus arteriosus, infection, and others seem to increase primarily the fluctuations in the cerebral blood flow and thus, represent important risk factors to the development of IVH.
Box 1. Pathogenesis of germinal matrix vasculature.
Fragility of germinal matrix vasculature
Fluctuation in the cerebral blood flow
Platelet and coagulation disorder
What renders the germinal matrix vasculature fragile?
Blood vessels in the brain are unique as they form a blood-brain barrier (BBB). The BBB is a complex dynamic interface between blood and the brain, and consists of endothelial tight junctions, basal lamina, pericytes, and astrocyte end-feet in inside-out fashion (Fig 1D).11, 12 Logically, deficiency in any of the components of the BBB can weaken the vasculature and increase the propensity to hemorrhage. We have evaluated each of these components in the human germinal matrix and have unraveled a number of dissimilarities in the cellular and molecular components of this germinal matrix vasculature compared to the embryonic white matter and the cortical plate (Box 2).
Box 2. Fragility of germinal matrix vasculature.
Paucity of pericytes
Reduced fibronectin in the basal lamina
Reduced GFAP expression in the astrocyte endfeet
High vascular density and rapid angiogenesis in the germinal matrix
The germinal matrix exhibits rapid angiogenesis in contrast to other brain regions.13 This rapid endothelial proliferation contributes to the high vascular density of the germinal matrix. Both the vascular density and the cross-sectional area of the vasculature are higher in the human germinal matrix compared to the cortical plate (cerebral cortex) and embryonic white matter from 17–35 gw.14 In addition, the abundance of vessels and cross sectional area of vasculature increases with advancing gestational age in the second and third trimester of pregnancy.14 Intriguingly, vessels in the germinal matrix are circular in coronal sections whereas blood vessels in cerebral cortex and white matter are flat and elongated. The circular shape of the vessels suggests vasculature immaturity, which is consistent with the rapid ongoing angiogenesis in the germinal matrix. The high vascularity and rapid endothelial turnover is unique to the germinal matrix and can be attributed to the high metabolic demand of this brain region, which has a preponderance of proliferating, maturing, and migrating neuronal and glial precursor cells.
Paucity of pericytes in the germinal matrix vasculature
Pericytes are perivascular cells of the capillaries, venules, and arterioles (Fig. 2).15 They are enclosed in the basal lamina and wrap around the endothelial cells. They have complex and critical role to play in regulating angiogenesis, providing structural support to the vasculature, maintaining the BBB, and controlling neurovascular unit—endothelium, astrocytes, and neurons.16–18 Pericytes are the key players in the various stages of angiogenesis, including initiation, sprout extension, migration, and maturation of blood vessels.19 In response to angiogenic stimuli, they degrade basal lamina and migrate out of the microvasculature and upon completion of angiogenesis, they resume their position to strengthen vessels by synthesizing extracellular matrix and inducing endothelial maturation. Pericyte recruitment is regulated primarily by four ligand-receptor systems; the ligands include transforming growth factor β (TGFβ), platelet derived growth factor-B (PDGF-B), angiopoietin, and sphinogsine-1-phosphate.20 Deficiency of any of these ligands in transgenic animals results in failure of pericyte recruitment and consequent dilation of the vessels with increased propensity to hemorrhage.21
Figure 2.
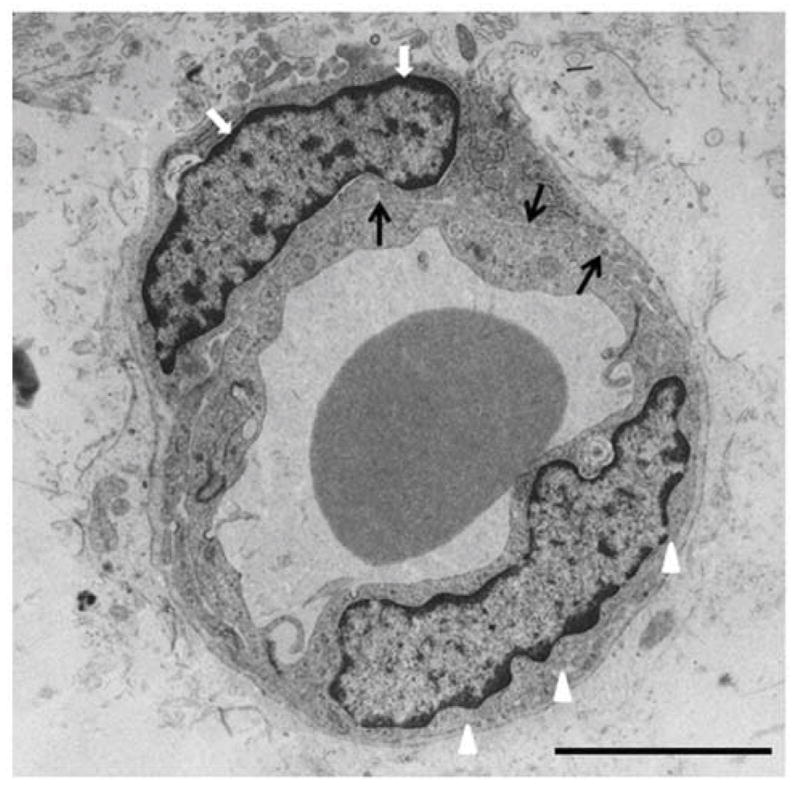
Electron micrograph showing endothelium (arrow heads) and pericyte (white arrows) separated by basal laminia (black arrows) in the white matter of 3 day old preterm rabbit pup (E29). Note pericyte wraps around the endothelium and is outer to the basal lamina. Scale bar, 4 μm.
Quantification of pericyte coverage and density in the brain of human preterm infants and fetuses has been performed in immuno-labeled sections and electron micrographs. These studies have revealed that the density of perivascular cells and their peri-endothelial coverage in the germinal matrix are reduced compared to the white matter and cerebral cortex on both ultrastructural and histochemical evaluation.22 Importantly, TGFβ expression is reduced in the germinal matrix microvasculature, which might be contributing to the paucity of pericytes.22 Indeed, low TGFβ concentration promotes endothelial proliferation and conversely, high TGFβ levels enhance capillary stabilization by facilitating pericyte recruitment. To conclude, rapid angiogenesis in the presence of low TGFβ results in abundance of angiogenic vessels deficient in pericytes, which leads to fragility of the germinal matrix microvasculature.
Deficient fibronectin in the basal lamina
Basal lamina is a key component of the BBB that surrounds pericytes and separates pericytes from astrocyte end-feet and endothelium.12, 23 Its formation and maintenance is assured by the endothelium, astrocytes, and pericytes. This contributes to the structural integrity of vasculature by virtue of its anchoring function. Basal lamina is composed of laminin, collagen, fibronectin, heparan sulfate proteoglycan, and perlecan.12, 24, 25 Knock out experiments have revealed the necessity of these molecules in the formation of blood vessels and maintenance of the vascular stability.26–28 Quantification of the constituents of basal lamina in human fetuses and preterm infants (postmortem) has revealed that fibronectin levels are significantly reduced in the germinal matrix vasculature compared to the cortex and white matter (Fig. 3A).29 However, other components of the basement membrane, including laminin (α1, α4, and α5), and α1 (IV) collagen, α5 (IV) collagen, and perlecan, are similarly expressed in the three brain regions. Given that polymerization of fibronectin into extracellular matrix controls stability of the vasculature and that fibronectin null mice exhibit cerebral hemorrhage, deficient fibronectin in the germinal matrix is likely to contribute to the fragility of germinal matrix vasculature.30, 31 Since TGFβ upregulates fibronectin and other components of the extracellular matrix, diminished TGFβ in the germinal matrix is consistent with low levels of fibronectin in the germinal matrix. Thus, upregulating TGFβ might elevate fibronectin levels in the basal lamina and strengthen the germinal matrix vasculature. However, TGFβ is a ubiquitously expressed growth factor; and there is no available strategy to increase the expression selectively of this molecule in the germinal matrix vasculature.
Figure 3. Germinal matrix vasculature is deficient in fibronectin and lack GFAP+ astrocyte endfeet coverage.
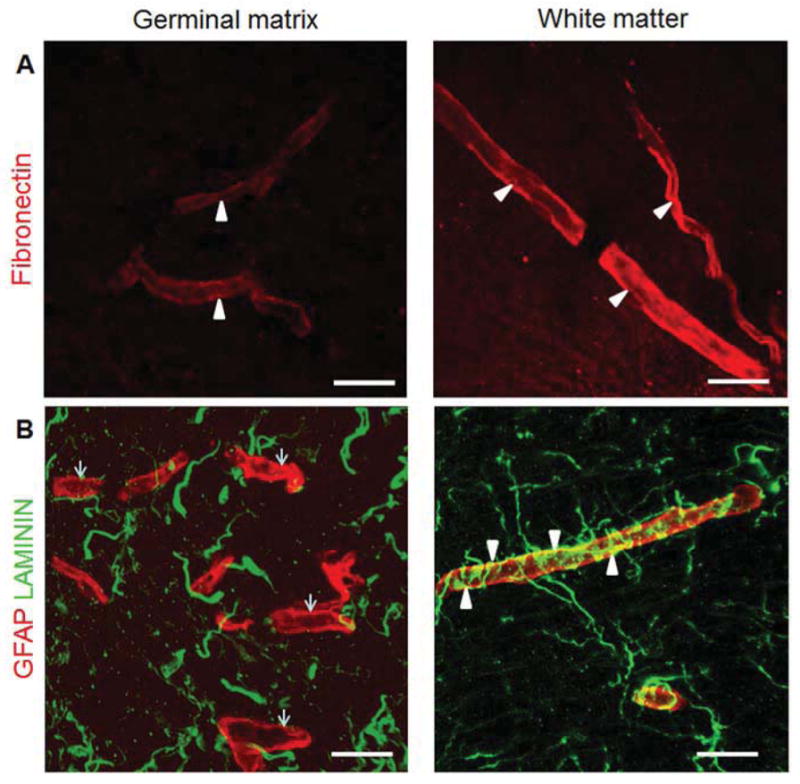
A) Representative immunofluorescence of cryosection from germinal matrix and white matter of a 24 week premature human infant labeled with fibronectin (red) specific antibody. Fibronectin is strongly expressed in the white matter whereas it is weakly expressed in the germinal matrix (arrowheads). Scale bar; 20μm. B) Cryosection from germinal matrix and white matter of a 24 week premature infant was double-labeled with CD34 (endothelium, red) and GFAP (astrocyte, green) specific antibody. GFAP positive astrocyte endfeet are intimately associated with the outer endothelial surface in the white matter (arrowheads). However, GFAP positive astrocytes are not covering endothelium in the germinal matrix (arrows).
Consistent with these studies, another report has shown that there is no significant difference in the expression of Collagen I, II, and IV between the germinal matrix and other brain regions of premature human infants.32 However, in beagle puppies, immunoreactivity of laminin and collagen V in the germinal matrix is greater at postnatal d4 compared to d1, and indomethacin treatment further increases the intensity of immunosignals for laminin and collagen V in the germinal matrix.33, 34 This suggests that deficiencies of these two molecules in germinal matrix vasculature of beagle puppies might contribute to the vascular weakness of this brain region. Together, the constituents of the basal lamina add to the stability of the vasculature; and a deficiency of fibronectin level in the basal lamina of the human germinal matrix might contribute to the vascular fragility of this brain region.
Reduced GFAP expression in the astrocyte end-feet
Astrocytes extend processes from the soma in all directions that cover the blood vessels. These astrocytic processes are known as endfeet, which unsheathe 99% of the BBB endothelium in adult brains. 35 The astrocytes contribute to the development of the BBB and regulate its function. Specifically, they provide structural integrity and control permeability of the BBB.12 Moreover, they are essential for function of neurons and the neurovascular unit. Astrocyte end-feet contain intermediate filaments, which form the cytoskeleton of the astrocytes. GFAP is a key component of these intermediate filaments. Studies in autopsy materials from fetuses and premature infants have shown that perivascular coverage by GFAP+ astrocyte end-feet is less in the germinal matrix compared to the cerebral cortex and the embryonic white matter (Fig. 3B).36 Since GFAP provides structural integrity and mechanical strength to the astrocyte end-feet,37–39 fragility of the germinal matrix vasculature can also be ascribed to reduced GFAP expression in the astrocyte end-feet.
Overall mechanism of germinal matrix vascular fragility
Germinal matrix exhibits rapid angiogenesis, in contrast to the other brain regions. The accelerated endothelial proliferation of this brain region is triggered by high levels of vascular endothelial growth factor (VEGF) and angiopoeitin-2, and reduced expression of TGF-β (Fig. 4).13 Since hypoxia is a key stimulant of these growth factors,40 we have evaluated hypoxia inducible factor-1α and have performed hypoxyprobe test. We have noted that hypoxia inducible factor-1α levels are elevated in the human germinal matrix compared to the cerebral cortex and white matter (unpublished data). In addition, hypoxyprobe [1-(2-hydroxy-3-piperidinyl)propyl 2-nitroimidazole hydrochloride] test shows lower oxygen gradient in the germinal matrix compared to the adjacent white matter.10 It seems that oxygen demand and utilization of this brain region is high as a result of its high metabolic activity. Indeed, germinal matrix harbors neuronal and glial precursor cells, which are in various phases of proliferation, migration, and maturation. Angiogenic vessels in the germinal matrix are naked endothelial tubes exhibiting paucity of pericytes and immature basal lamina low in fibronectin. In addition, GFAP is weakly expressed in the astrocyte endfeet. Together, a scarcity of pericytes, low fibronectin levels in the basement membrane, and reduced GFAP expression in the astrocyte endfeet contributes to the weakness of the germinal matrix BBB and to the vulnerability to hemorrhage. Importantly, IVH develops in the first three days of postnatal life and premature infants are relatively immune to hemorrhage after this period irrespective of the gestational age. This reduced propensity to hemorrhage after 3 days in preterm infants might be because of an increase in blood and tissue oxygen concentration after birth suppressing VEGF, angiopoetin-2 levels and the angiogenesis. A shutdown in angiogenesis shortly after birth would mature the angiogenic vessels making them resistant to rupture despite fluctuation in the CBF.
Figure 4. Mechanisms underlying fragility of germinal matrix vasculature.
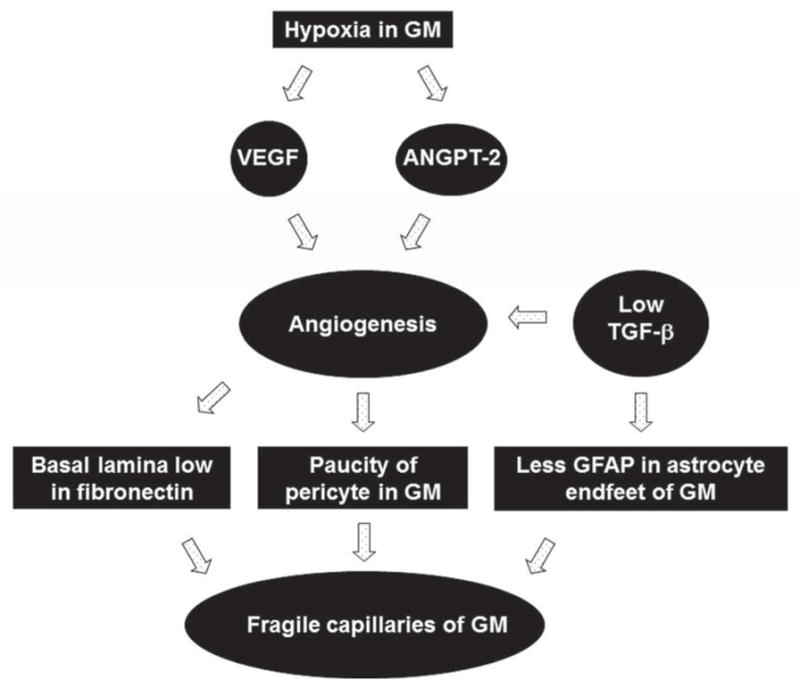
Hypoxic germinal matrix triggers upregulation of VEGF and angiopoieitn-2 expression. These growth factors induce angiogenesis. The angiogenic vessels of the germinal matrix exhibit paucity of pericytes and deficiency of fibronectin in immature basal lamina. Additionally, astrocyte endfeet in the germinal matrix vasculature display reduced expression of GFAP. These factors contribute to the fragility of the germinal matrix.
Disturbance in Cerebral Blood Flow
Fluctuation in the CBF
In premature infants with respiratory distress syndrome, two patterns of CBF velocity have been demarcated: one, stable pattern with equal peak and trough of systolic and diastolic flow velocity and other, fluctuating waveform with continuous alteration in systolic and diastolic flow velocity.41, 42 Fluctuating cerebral blood flow velocity during the first day of life strongly correlates with the occurrence of IVH and more importantly, elimination of this fluctuation in the CBF by intravenous pancuronium infusion markedly reduces the incidence of IVH.41–43 Since these studies were done in mid 1980s, it is possible that fluctuation in the CBF was related to the use of intermittent mandatory ventilation, giving rise to asynchrony between infant and ventilator breath, which can be eradicated by the use of paralytic agents. However, most of the neonatal units currently use synchronized ventilator modes, which minimizes infants “fighting” the ventilator and thus reduces fluctuations in the CBF velocity.44 Moreover, routine use of neuromuscular blocking agents in ventilated infants is not recommended because of uncertain long-term neurologic adverse effects of these agents. Other factors that contribute to the fluctuation of CBF velocity include patent ductus arteriosus, hypercarbia, hypotension, severe respiratory distress syndrome, and rapid infusion of sodium bicarbonate (Table 1).43, 45–47
Table 1.
Neonatal risk factors in the pathogenesis of IVH.
| Major Pathogenic mechanism | Putative mechanisms* | Risk factors | Preventive measures | |
|---|---|---|---|---|
|
| ||||
| 1. | Disturbance in CBF | 1. Fluctuation in CBF |
|
|
|
|
|||
|
|
|||
| 2. High cerebral venous pressure |
|
|
||
|
||||
| 3. Abnormal blood pressure |
|
|
||
| 4. Pressure passive circulation | Extreme prematurity and low birth weight (<1000g) Clinically unstable resulting from respiratory compromise, sepsis or other reasons |
|
||
|
| ||||
| 2 | inherent fragility of germinal matrix vasculature | Might be worsened by an inflammatory injury to the blood brain barrier | Hypoxic ischemic insult Sepsis | Prenatal GCs stabilizes the microvasculature by increasing:
|
|
| ||||
| 3. | Platelet and coagulation disturbances | Hemostatic failure | Thrombocytopenia Disseminated intravascular coagulopathy | Replacement of blood products |
Correlation of mechanisms with the risk factors and preventive measures is based on available evidence and author’s speculations.
Impaired autoregulation in premature infants
Cerebral autoregulation is the capability of the blood vessels in the brain to retain a constant CBF in spite of fluctuations in the blood pressure. The pressure passivity--impaired autoregulation—of CBF is associated with lower gestational age and birth weight, and is commonly noted in sick, ventilated, and hemodynamically unstable premature infants. Cerebral autoregulation has been evaluated in preterm infants using a number of methods, including Doppler, xenon clearance, near infra-red spectroscopy (NIRS), and spatially resolved spectroscopy (SRS).48,49,50, 51 Although these techniques involve continuous and repeated monitoring, the results of these studies have not been consistent. It was reported that NIRS identifies infants with impairment in cerebral autoregulation and that this is associated with high likelihood of severe IVH.52 Subsequent investigators realized continuous monitoring of CBF over extended period, because cerebral blood pressure passivity is not an “all-or-none phenomena”, but it can occur over varied time intervals.49 In VLBW infants, continuous monitoring of cerebral perfusion by NIRS and mean arterial blood pressure has shown that CBF is pressure passive for an average of 20% of the time; and there is strong correlation between pressure passive state and hypotension with lower birth weight and gestational age.49 Additionally, there is no association between impaired autoregulation and the occurrence of IVH.49 Another subsequent report, in which CBF was measured by SRS, showed strong association between diminished autoregulation and subsequent mortality; however, impaired autoregulation was not associated with the development of IVH.50 In a recent study on continuous monitoring of VLBW using NIRS, it has been shown that the correlation of regional cerebral oxygen saturation and mean blood pressure indicates pressure passivity of the CBF and these metrics may predict the occurrence of IVH.53 Hence, the role of impaired autoregulation in the development of IVH needs further evaluation.
Perinatal risk factors for IVH, fluctuation in the CBF, and germinal matrix fragility
A number of risk factors are associated with IVH, which directly or indirectly increase either the fluctuation in the CBF or the fragility of the germinal matrix microvasculature (Table 1). Hypotension is common in premature infants; however, there is conflicting data on the association between IVH and hypotension.49, 54, 55,56, 57 A correlation between hypotension and CBF seems to be complex and hypotension may not indicate reduced or disturbed CBF.58 A rise in central venous pressure (CVP) might contribute to the onset of IVH. Indeed, CVP is elevated in pneumothorax and in infants on mechanical ventilation using high mean airway pressure. Moreover, germinal matrix hemorrhage has been demonstrated to be mainly of venous origin in a study on autopsy materials from premature human infants.59 Prolonged positive pressure ventilation is also known to increase BBB permeability.60 A rapid infusion of sodium bicarbonate might contribute to the development of IVH. It is possible that a rapid infusion of a large dose of sodium bicarbonate will increase serum osmolality and arterial CO2 resulting in vasodilation and rupture of the microvasculature in the germinal matrix. However, there is disagreement on a causative role of bicarbonate in the development of IVH.61–63 Other risk factors for IVH include early onset sepsis, maternal chorioamnionitis, development of respiratory distress syndrome, recurrent tracheal suctioning, prolonged labor, hypoxia, hypercarbia and others (Table 1). It appears that most of these conditions contribute to the occurrence of IVH by disturbing the CBF. However, sepsis and hypoxia-ischemia might cause molecular and morphological changes in the microvasculature which may weaken the vessels of the germinal matrix. Coagulopathy does not seem to play a key role in pathogenesis of IVH, but can modify the risk and severity of IVH. A Cochrane systematic review showed that vitamin K administration did not affect the incidence of IVH.64 Fresh frozen plasma administration has also been tried without success. 65 However, several studies have reported that thrombocytopenia is a risk factor for IVH.66–68
Genetic factors and IVH
Mutations in the type IV procollagen gene, COL4A1, are associated with IVH in dizygotic preterm twins.69 Since inflammatory mediators and coagulation factors might have a role in the development of IVH, polymorphisms of pro-inflammatory cytokines and mutations in the coagulation factors have been evaluated as candidate genes that modify the severity and risk of IVH. Mutations in factor V Leiden, prothrombin G20210A, and IL1β have been implicated in the development of grade I and II IVH.70–72 Polymorphisms of IL-6 and TNFα are proposed as genetic modifiers of IVH risk.73, 74 In addition, polymorphism of methylene tetrahydrofolate reductase (MTHFR) gene has been reported in infants with IVH. C677T polymorphism in MTHFR enzyme is associated with high plasma homocysteine levels, which is a known risk factor for vascular disease.75 Together, mutations in coagulation, thrombophilia, and inflammation related genes might contribute to the development of IVH.
PREVENTION OF INTRAVENTRICULAR HEMORRHAGE
Rationale of preventive therapies
Since IVH is primarily attributed to increased vascular fragility and disturbance in CBF, our strategies are directed to strengthening the germinal matrix vasculature and to stabilizing the CBF. Germinal matrix has a subset of vessels that are angiogenic, immature, and lack pericytes; and they thrive because of high levels of VEGF and angiopoietin2.13, 22 These blood vessels exhibit high fragility and propensity to bleed. It appears that the immature neovasculature are pruned within a few days of premature delivery, thus stabilizing the germinal matrix microvasculature. This is because oxygen concentration increases above intrauterine level shortly after birth, which possibly downregulates the VEGF levels in the germinal matrix. Indeed, preterm infants become relatively immune to the development of IVH after postnatal day 3. Prenatal glucocorticoids (GCs) or antenatal celecoxib also downregulates VEGF levels, which leads to apoptosis of naked endothelial cells, lacking pericyte coverage.13, 76 Hence, prenatal use of angiogenic inhibitors—GCs or celecoxib--trims the nascent vessels, but not the functional vessels protected by pericytes, thereby orchestrating a vascular network consisting of stable blood vessels.22, 76,77, 78 Fluctuations in the CBF is related to routine procedures performed in neonatal units, such as suctioning, handling, placing intravenous lines, and common problem associated with prematurity, including respiratory distress syndrome, patent ductus arteriosus, apneic episodes, seizures, hypoxemia, hypercarbia and others (Table 1). Hence, fluctuations in the CBF can be minimized by reducing the stimulation to the infant and appropriately managing the common complications of prematurity. Overall preventive approach is listed in Table 2.
Table 2.
Prevention of Intraventricular hemorrhage
| A. Prenatal interventions: |
|
| B. Care during infant delivery: | Optimize obstetric care and prevent prolonged labor |
| C. Postnatal interventions: |
|
Prenatal Pharmacological treatments to prevent IVH
Glucocorticoids (GCs)
In the United States, the preterm birth rate is about 12.5% and approximately 75% women in premature labor with gestational age of less than 34 weeks are treated with either betamethasone or dexamethasone.79 A number of studies have confirmed that prenatal GC reduces both severity and incidence of IVH.80, 81 The beneficial effect of prenatal GC is attributed to stabilization of the microvasculature of the germinal matrix and alleviation of disturbance in the CBF. Prenatal GC, as discussed above, suppresses angiogenesis in the germinal matrix microvasculature and thus trims the nascent and fragile vasculature, which are vulnerable to hemorrhage. Thus, GC exposure stabilizes the BBB of the germinal matrix; and infants treated with prenatal GC exhibit greater pericyte coverage, higher fibronectin levels and more GFAP in the astrocyte end-feet of the blood vessels of the germinal matrix compared to untreated infants.22, 29, 76 Moreover, it reduces the incidence and severity of respiratory distress syndrome, which might minimize fluctuation in the CBF. Postnatal betamethasone (0.1 mg/kg) also reduces cerebral flow velocity possibly by exerting a vasoconstrictor effect on cerebral vessels in preterm infants.82 Similarly, prenatal betamethasone has been shown to reduce cerebral blood flow by increasing cerebrovascular resistance in fetal sheep model.83
The optimal effects of prenatal GC have been observed after a complete course of 2 doses of betamethasone or 4 doses of dexamethasone when administered within a week of delivery of the premature newborn.84 However, benefits have also been noted with incomplete courses of GCs.85 Comparison of the two GCs—betamethasone and dexamethasone--has not conclusively shown superiority of one over the other and clinicians should choose whatever is available.85 Betamethasone exposed infants exhibit less severe respiratory distress syndrome, but more IVH, compared to prenatal dexamethasone treated infants.84, 86 There has been concern that prenatal dexamethasone might increase the incidence of periventricular leukomalacia.87 However, a subsequent study on a larger population clearly showed that there is no difference in the incidence of cystic PVL between dexamethasone and betamethasone exposed infants.88 This study also noted that both GCs are equally efficacious in preventing severe IVH, however there is a trend toward better efficacy for dexamethasone compared to betamethasone.88 Importantly, prenatal betamethasone is associated with a reduced risk for neonatal death compared with dexamethasone.88 Together, there is no recommendation for the use of one GC over the other, despite multiple clinical trials undertaken. Another key issue related to the use of prenatal steroid is single versus repeated course. Unfortunately, there is no agreement among the experts on the advantage of single vs. multiple course of GCs.85 There are concerns that multiple course of prenatal GC might have adverse effects on brain and other organ systems.
Phenobarbital and Magnesium sulfate
As etiopathogenesis of IVH was more mysterious in 80s than now, a number of agents without a concrete rationale were tried to prevent IVH. Phenobarbital and vitamin K are important to mention here, as these medications attracted the attention of investigators. Initial studies showed some protective effect of phenobarbital, however, subsequent clinical trials failed to confirm the protective effect of phenobarbital in preventing IVH.89–93 Maternal treatment of vitamin K or magnesium sulfate to prevent IVH did not demonstrate any benefit either.64, 94–96
Postnatal pharmacological treatment to prevent IVH
Indomethacin
Indomethacin, commonly used in premature neonates to close patent ductus arteriosus, has been shown to prevent IVH in several clinical trials. Indomethacin, a non-selective cyclo-oxygenase (COX) inhibitor, suppresses both COX1 and COX2 isoforms to reduce prostaglandin synthesis. It attenuates cerebral vascular hyperemic responses induced by hypoxia, hypercapnia, hypertension, and asphyxia.97, 98 It reduces alterations in the BBB permeability after cerebral ischemia and promotes maturation of basement membrane by increasing the expression of laminin and collagen V.33, 34, 99 The maturation of basal lamina upon indomethacin treatment can be attributed to COX2 inhibition, which suppresses angiogenesis and matures the germinal matrix vasculature.13
In a number of clinical trials, indomethacin treatment has shown short-term benefit of reducing the incidence of IVH.100,101, 102 Secondary analyses of a multicenter study based on gender have shown that indomethacin treatment reduces the rate of IVH in male infants, but not in female infants.103 However, another study on a larger population of preterm infants showed just a weak differential effect of indomethacin by sex.104 Since indomethacin reduces the occurrence of IVH, it was anticipated that this treatment would improve the neurodevelopment outcome of the infants. However, indomethacin treatment failed to reduce the rate of cerebral palsy, deafness and blindness on long term follow up.105, 106 A meta-analyses of 19 clinical trials also did not show any improvement in the long term outcome of indomethacin treated infants. 107, 108 Together, indomethacin prophylaxis has immediate benefits of reduction in symptomatic patent ductus arteriosus, and severe IVH; however, this does not impact long term neurodevelopmental outcomes. Hence, indomethacin is not recommended for routine prophylaxis against IVH. However, indomethacin is still being used in some neonatal units depending on clinical circumstances and personal preferences.
Ibuprofen is another non-selective COX inhibitor that has shown promise in closing patent ductus arteriosus. This compound does not reduce CBF, in contrast to indomethacin. More importantly, ibuprofen does not prevent IVH in premature infants.109, 110
Other clinical trials of unproven benefit
Postnatal phenobarbital has been used in a number of clinical trials to prevent IVH in 1980s, based on the premise that it might reduce abrupt changes in the CBF during tracheal suctioning, handling, and motor activity. 111–114, 115 However, phenobarbital did not reduce the incidence of IVH significantly. Another agent that drew the attention of investigators in 1980s was etamsylate. This compound reduces prostaglandin synthesis and promotes platelet adhesiveness. In addition, etamsylate induces polymerization of hyaluronic acid in the vascular basement membrane which might promote homeostasis and minimize bleeding. However, large clinical trials showed that etamsylate neither reduced the incidence of IVH nor enhanced the neurodevelopmental outcome of premature infants.116, 117 Pathogenesis of IVH has always puzzled the investigators and thus, a role of free radicals in the etiology of IVH cannot be totally excluded. Hence, vitamin E—a potent antioxidant--has been tried to prevent IVH in preterm infants without appreciable benefits.118, 119 To address the need to minimize asynchrony between infant and ventilator breath, pancuronium has been tried in 1980s and was found to reduce fluctuation in CBF and also the incidence of IVH.42 A relatively recent meta-analysis identified 6 clinical trials in which the use of neuromuscular blocking agent during mechanical ventilation was compared to no paralysis in newborn infants.120 It was concluded that neuromuscular paralyses with pancuronium reduced the rate of IVH. However, owing to complications associated with neuromuscular paralysis, routine use of pancuronium in extremely premature infants has not been recommended. The role of activated recombinant factor VII (rVIIa) in promoting coagulation is promising and it has been hypothesized that early (prophylactic) administration of rVIIa to extremely premature infants would reduce the incidence of IVH. However, IVH is not primarily a coagulation disorder and this hypothesis does not seem to be logical.
Optimizing care of fetuses and premature newborns
Prenatal interventions
Inter-hospital transport of extremely premature infants is associated with increased incidence and severity of IVH. This correlation has remained constant in recent years.121 Hence, pregnant mothers in preterm labor should be transported to a tertiary care center specializing in high-risk delivery. Prolonged labor might increase the risk of IVH and should be managed appropriately. Data on the incidence of IVH in Cesarean section vs. vaginal delivery is inconsistent122, 123 and thus, infants are delivered based on the decisions made by obstetricians.
Postnatal interventions
There is no specific recommendation about neonatal resuscitation of premature infants to prevent IVH. However, restoration of normal oxygenation and ventilation immediately at birth is important as hypoxemia and hypercarbia can cause fluctuation in the CBF which might contribute to IVH. While preventing metabolic acidosis and accomplishing normal perfusion is important, a rapid sodium bicarbonate infusion might add to the risk of IVH. After infants have been transferred to the neonatal unit, gentle and synchronized ventilation, prompt closure of ductus arteriosus, and maintenance of normal O2 and CO2 levels in the arterial blood are important preventive measures. Preventing pneumothorax, apneic episodes, seizures, as well as minimizing tracheal suctioning and handling will prevent disturbances in the CBF. Reducing stimulation and gentle caretaking decreases the incidence of IVH.124 Effect of surfactant treatment on the development of IVH is unclear. Use of high frequency ventilators does not increase the risk of IVH125 and therefore, an individualized approach in selection of appropriate ventilator should be pursued.
Together, the incidence of IVH has remained unchanged over the last couple of decades, despite advances in care of newborns. Use of prenatal GCs remains the most effective strategy in the prevention of IVH.
SUMMARY
IVH is a major complication of prematurity. IVH usually initiates in the periventricular germinal matrix and progresses to IVH upon the rupture of the underlying ependyma. The pathogenesis of IVH is ascribed to the intrinsic fragility of germinal matrix vasculature and to fluctuations in the CBF. The germinal matrix exhibits accelerated angiogenesis, which orchestrates formation of nascent vessels that lack pericytes, display immature basal lamina low in fibronectin, and have astrocyte endfeet coverage deficient in GFAP. These morphological and molecular factors contribute to the fragility of the germinal matrix vasculature. Importantly, pressure passive circulation is frequent in the premature infants and CBF fluctuates with the changes in internal milieu (O2, CO2, pH, osmolarity), CVP, blood pressure, and cardiac output secondary to inadequate ventilation, pneumothorax, severe lung disease, hemodynamic instability, sepsis, dehydration, patent ductus arteriosus, frequent suctioning, increased handling, and other factors. Recent studies have suggested roles of genes encoding coagulation factors, inflammatory cytokines, and collagen in the pathogenesis of IVH. Prenatal GCs are the most effective in preventing IVH and are the standard care. There is no long term advantage of using postnatal indomethacin. There is a need of improved therapy to prevent IVH and its neurodevelopmental sequelae. Therapies designed to enhance the stability of the germinal matrix vasculature and reduce fluctuation of CBF might lead to more effective strategies in preventing IVH.
KEY POINTS.
Pathogenesis of intraventricular hemorrhage (IVH) is ascribed to the intrinsic weakness of germinal matrix vasculature and to the fluctuation in the cerebral blood flow.
The germinal matrix displays accelerated angiogenesis that orchestrates formation of nascent vessels that lack pericytes, display immature basal lamina low in fibronectin, and have astrocyte end-feet coverage deficient in glial fibrillary acidic protein (GFAP). These morphological and molecular factors contribute to the fragility of the germinal matrix vasculature.
The fluctuations in the cerebral blood flow is attributed to the cardiorespiratory and hemodynamic instability frequently associated with extremely premature infants, including hypotension, hypoxia, pneumothorax, patent ductus arteriosus, and others.
Prenatal glucocorticoids have emerged as the most effective intervention to prevent IVH. Therapies designed to enhance the stability of the germinal matrix vasculature and reduce fluctuation of CBF could lead to strategies that are more effective in preventing IVH.
Acknowledgments
Source of funding: NIH/NINDS grant RO1 NS071263 (PB)
Authors thank Drs. Laura Ment and Barabara Stonstreet for the critical review of the manuscript and Joanne Abrahams for the assistance with images.
Footnotes
Conflict of interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fanaroff AA, Stoll BJ, Wright LL, et al. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol. 2007;196(2):147, e141–148. doi: 10.1016/j.ajog.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 2.Jain NJ, Kruse LK, Demissie K, et al. Impact of mode of delivery on neonatal complications: trends between 1997 and 2005. J Matern Fetal Neonatal Med. 2009;22(6):491–500. doi: 10.1080/14767050902769982. [DOI] [PubMed] [Google Scholar]
- 3.Sherlock RL, Anderson PJ, Doyle LW, et al. Neurodevelopmental sequelae of intraventricular haemorrhage at 8 years of age in a regional cohort of ELBW/very preterm infants. Early Hum Dev. 2005;81(11):909–916. doi: 10.1016/j.earlhumdev.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Luu TM, Ment LR, Schneider KC, et al. Lasting effects of preterm birth and neonatal brain hemorrhage at 12 years of age. Pediatrics. 2009;123(3):1037–1044. doi: 10.1542/peds.2008-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Indredavik MS, Vik T, Evensen KA, et al. Perinatal risk and psychiatric outcome in adolescents born preterm with very low birth weight or term small for gestational age. J Dev Behav Pediatr. 2010;31(4):286–294. doi: 10.1097/DBP.0b013e3181d7b1d3. [DOI] [PubMed] [Google Scholar]
- 6.Nosarti C, Giouroukou E, Micali N, et al. Impaired executive functioning in young adults born very preterm. J Int Neuropsychol Soc. 2007;13(4):571–581. doi: 10.1017/S1355617707070725. [DOI] [PubMed] [Google Scholar]
- 7.Whitaker AH, Feldman JF, Lorenz JM, et al. Neonatal head ultrasound abnormalities in preterm infants and adolescent psychiatric disorders. Arch Gen Psychiatry. 2011;68(7):742–752. doi: 10.1001/archgenpsychiatry.2011.62. [DOI] [PubMed] [Google Scholar]
- 8.Rushing S, Ment LR. Preterm birth: a cost benefit analysis. Semin Perinatol. 2004;28(6):444–450. doi: 10.1053/j.semperi.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 9.McCrea HJ, Ment LR. The diagnosis, management, and postnatal prevention of intraventricular hemorrhage in the preterm neonate. Clin Perinatol. 2008;35(4):777–792. vii. doi: 10.1016/j.clp.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ballabh P. Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatr Res. 2010;67(1):1–8. doi: 10.1203/PDR.0b013e3181c1b176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16(1):1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 12.Persidsky Y, Ramirez SH, Haorah J, et al. Blood-brain barrier: structural components and function under physiologic and pathologic conditions. J Neuroimmune Pharmacol. 2006;1(3):223–236. doi: 10.1007/s11481-006-9025-3. [DOI] [PubMed] [Google Scholar]
- 13.Ballabh P, Xu H, Hu F, et al. Angiogenic inhibition reduces germinal matrix hemorrhage. Nat Med. 2007;13(4):477–485. doi: 10.1038/nm1558. [DOI] [PubMed] [Google Scholar]
- 14.Ballabh P, Braun A, Nedergaard M. Anatomic analysis of blood vessels in germinal matrix, cerebral cortex, and white matter in developing infants. Pediatr Res. 2004;56(1):117–124. doi: 10.1203/01.PDR.0000130472.30874.FF. [DOI] [PubMed] [Google Scholar]
- 15.Sa-Pereira I, Brites D, Brito MA. Neurovascular unit: a focus on pericytes. Mol Neurobiol. 2012;45(2):327–347. doi: 10.1007/s12035-012-8244-2. [DOI] [PubMed] [Google Scholar]
- 16.Balabanov R, Dore-Duffy P. Role of the CNS microvascular pericyte in the blood-brain barrier. J Neurosci Res. 1998;53(6):637–644. doi: 10.1002/(SICI)1097-4547(19980915)53:6<637::AID-JNR1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 17.Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9(6):685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 18.Armulik A, Genove G, Mae M, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468(7323):557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 19.Hirschi KK, D’Amore PA. Control of angiogenesis by the pericyte: molecular mechanisms and significance. EXS. 1997;79:419–428. doi: 10.1007/978-3-0348-9006-9_18. [DOI] [PubMed] [Google Scholar]
- 20.Gaengel K, Genove G, Armulik A, et al. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29(5):630–638. doi: 10.1161/ATVBAHA.107.161521. [DOI] [PubMed] [Google Scholar]
- 21.Lindahl P, Johansson BR, Leveen P, et al. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277(5323):242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 22.Braun A, Xu H, Hu F, et al. Paucity of pericytes in germinal matrix vasculature of premature infants. J Neurosci. 2007;27(44):12012–12024. doi: 10.1523/JNEUROSCI.3281-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stratman AN, Davis GE. Endothelial cell-pericyte interactions stimulate basement membrane matrix assembly: influence on vascular tube remodeling, maturation, and stabilization. Microsc Microanal. 2012;18(1):68–80. doi: 10.1017/S1431927611012402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tilling T, Engelbertz C, Decker S, et al. Expression and adhesive properties of basement membrane proteins in cerebral capillary endothelial cell cultures. Cell Tissue Res. 2002;310(1):19–29. doi: 10.1007/s00441-002-0604-1. [DOI] [PubMed] [Google Scholar]
- 25.Hallmann R, Horn N, Selg M, et al. Expression and function of laminins in the embryonic and mature vasculature. Physiol Rev. 2005;85(3):979–1000. doi: 10.1152/physrev.00014.2004. [DOI] [PubMed] [Google Scholar]
- 26.Forsberg E, Kjellen L. Heparan sulfate: lessons from knockout mice. J Clin Invest. 2001;108(2):175–180. doi: 10.1172/JCI13561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gould DB, Phalan FC, Breedveld GJ, et al. Mutations in Col4a1 cause perinatal cerebral hemorrhage and porencephaly. Science. 2005;308(5725):1167–1171. doi: 10.1126/science.1109418. [DOI] [PubMed] [Google Scholar]
- 28.George EL, Georges-Labouesse EN, Patel-King RS, et al. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119(4):1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- 29.Xu H, Hu F, Sado Y, et al. Maturational changes in laminin, fibronectin, collagen IV, and perlecan in germinal matrix, cortex, and white matter and effect of betamethasone. J Neurosci Res. 2008;86(7):1482–1500. doi: 10.1002/jnr.21618. [DOI] [PubMed] [Google Scholar]
- 30.Mao Y, Schwarzbauer JE. Fibronectin fibrillogenesis, a cell-mediated matrix assembly process. Matrix Biol. 2005;24(6):389–399. doi: 10.1016/j.matbio.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 31.Sottile J, Hocking DC, Langenbach KJ. Fibronectin polymerization stimulates cell growth by RGD-dependent and -independent mechanisms. J Cell Sci. 2000;113(Pt 23):4287–4299. doi: 10.1242/jcs.113.23.4287. [DOI] [PubMed] [Google Scholar]
- 32.Anstrom JA, Thore CR, Moody DM, et al. Morphometric assessment of collagen accumulation in germinal matrix vessels of premature human neonates. Neuropathol Appl Neurobiol. 2005;31(2):181–190. doi: 10.1111/j.1365-2990.2004.00626.x. [DOI] [PubMed] [Google Scholar]
- 33.Ment LR, Stewart WB, Ardito TA, et al. Beagle pup germinal matrix maturation studies. Stroke. 1991;22(3):390–395. doi: 10.1161/01.str.22.3.390. [DOI] [PubMed] [Google Scholar]
- 34.Ment LR, Stewart WB, Ardito TA, et al. Indomethacin promotes germinal matrix microvessel maturation in the newborn beagle pup. Stroke. 1992;23(8):1132–1137. doi: 10.1161/01.str.23.8.1132. [DOI] [PubMed] [Google Scholar]
- 35.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57(2):173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 36.El-Khoury N, Braun A, Hu F, et al. Astrocyte end-feet in germinal matrix, cerebral cortex, and white matter in developing infants. Pediatr Res. 2006;59(5):673–679. doi: 10.1203/01.pdr.0000214975.85311.9c. [DOI] [PubMed] [Google Scholar]
- 37.Liedtke W, Edelmann W, Bieri PL, et al. GFAP is necessary for the integrity of CNS white matter architecture and long-term maintenance of myelination. Neuron. 1996;17(4):607–615. doi: 10.1016/s0896-6273(00)80194-4. [DOI] [PubMed] [Google Scholar]
- 38.Trimmer PA, Reier PJ, Oh TH, et al. An ultrastructural and immunocytochemical study of astrocytic differentiation in vitro: changes in the composition and distribution of the cellular cytoskeleton. J Neuroimmunol. 1982;2(3–4):235–260. doi: 10.1016/0165-5728(82)90058-3. [DOI] [PubMed] [Google Scholar]
- 39.Ribotta MG, Menet V, Privat A. Glial scar and axonal regeneration in the CNS: lessons from GFAP and vimentin transgenic mice. Acta Neurochir Suppl. 2004;89:87–92. doi: 10.1007/978-3-7091-0603-7_12. [DOI] [PubMed] [Google Scholar]
- 40.Mu D, Jiang X, Sheldon RA, et al. Regulation of hypoxia-inducible factor 1alpha and induction of vascular endothelial growth factor in a rat neonatal stroke model. Neurobiol Dis. 2003;14(3):524–534. doi: 10.1016/j.nbd.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 41.Perlman JM, McMenamin JB, Volpe JJ. Fluctuating cerebral blood-flow velocity in respiratory-distress syndrome. Relation to the development of intraventricular hemorrhage. N Engl J Med. 1983;309(4):204–209. doi: 10.1056/NEJM198307283090402. [DOI] [PubMed] [Google Scholar]
- 42.Perlman JM, Goodman S, Kreusser KL, et al. Reduction in intraventricular hemorrhage by elimination of fluctuating cerebral blood-flow velocity in preterm infants with respiratory distress syndrome. N Engl J Med. 1985;312(21):1353–1357. doi: 10.1056/NEJM198505233122104. [DOI] [PubMed] [Google Scholar]
- 43.Van Bel F, Van de Bor M, Stijnen T, et al. Aetiological role of cerebral blood-flow alterations in development and extension of peri-intraventricular haemorrhage. Developmental medicine and child neurology. 1987;29(5):601–614. doi: 10.1111/j.1469-8749.1987.tb08502.x. [DOI] [PubMed] [Google Scholar]
- 44.Rennie JM, South M, Morley CJ. Cerebral blood flow velocity variability in infants receiving assisted ventilation. Arch Dis Child. 1987;62(12):1247–1251. doi: 10.1136/adc.62.12.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mullaart RA, Hopman JC, De Haan AF, et al. Cerebral blood flow fluctuation in low-risk preterm newborns. Early Hum Dev. 1992;30(1):41–48. doi: 10.1016/0378-3782(92)90085-u. [DOI] [PubMed] [Google Scholar]
- 46.Mullaart RA, Hopman JC, Rotteveel JJ, et al. Cerebral blood flow fluctuation in neonatal respiratory distress and periventricular haemorrhage. Early Hum Dev. 1994;37(3):179–185. doi: 10.1016/0378-3782(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 47.Coughtrey H, Rennie JM, Evans DH. Variability in cerebral blood flow velocity: observations over one minute in preterm babies. Early Hum Dev. 1997;47(1):63–70. doi: 10.1016/s0378-3782(96)01769-0. [DOI] [PubMed] [Google Scholar]
- 48.du Plessis AJ. Cerebrovascular injury in premature infants: current understanding and challenges for future prevention. Clin Perinatol. 2008;35(4):609–641. v. doi: 10.1016/j.clp.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 49.Soul JS, Hammer PE, Tsuji M, et al. Fluctuating pressure-passivity is common in the cerebral circulation of sick premature infants. Pediatr Res. 2007;61(4):467–473. doi: 10.1203/pdr.0b013e31803237f6. [DOI] [PubMed] [Google Scholar]
- 50.Wong FY, Leung TS, Austin T, et al. Impaired autoregulation in preterm infants identified by using spatially resolved spectroscopy. Pediatrics. 2008;121(3):e604–611. doi: 10.1542/peds.2007-1487. [DOI] [PubMed] [Google Scholar]
- 51.Caicedo A, De Smet D, Naulaers G, et al. Cerebral tissue oxygenation and regional oxygen saturation can be used to study cerebral autoregulation in prematurely born infants. Pediatr Res. 2011;69(6):548–553. doi: 10.1203/PDR.0b013e3182176d85. [DOI] [PubMed] [Google Scholar]
- 52.Tsuji M, Saul JP, du Plessis A, et al. Cerebral intravascular oxygenation correlates with mean arterial pressure in critically ill premature infants. Pediatrics. 2000;106(4):625–632. doi: 10.1542/peds.106.4.625. [DOI] [PubMed] [Google Scholar]
- 53.Alderliesten T, Lemmers PM, Smarius JJ, et al. Cerebral oxygenation, extraction, and autoregulation in very preterm infants who develop peri-intraventricular hemorrhage. J Pediatr. 2013;162(4):698–704. e692. doi: 10.1016/j.jpeds.2012.09.038. [DOI] [PubMed] [Google Scholar]
- 54.Weindling AM, Wilkinson AR, Cook J, et al. Perinatal events which precede periventricular haemorrhage and leukomalacia in the newborn. Br J Obstet Gynaecol. 1985;92(12):1218–1223. doi: 10.1111/j.1471-0528.1985.tb04865.x. [DOI] [PubMed] [Google Scholar]
- 55.Muller AM, Morales C, Briner J, et al. Loss of CO2 reactivity of cerebral blood flow is associated with severe brain damage in mechanically ventilated very low birth weight infants. Eur J Paediatr Neurol. 1997;1(5–6):157–163. [PubMed] [Google Scholar]
- 56.Watkins AM, West CR, Cooke RW. Blood pressure and cerebral haemorrhage and ischaemia in very low birthweight infants. Early Hum Dev. 1989;19(2):103–110. doi: 10.1016/0378-3782(89)90120-5. [DOI] [PubMed] [Google Scholar]
- 57.Miall-Allen VM, de Vries LS, Whitelaw AG. Mean arterial blood pressure and neonatal cerebral lesions. Arch Dis Child. 1987;62(10):1068–1069. doi: 10.1136/adc.62.10.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lightburn MH, Gauss CH, Williams DK, et al. Cerebral blood flow velocities in extremely low birth weight infants with hypotension and infants with normal blood pressure. J Pediatr. 2009;154(6):824–828. doi: 10.1016/j.jpeds.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ghazi-Birry HS, Brown WR, Moody DM, et al. Human germinal matrix: venous origin of hemorrhage and vascular characteristics. AJNR Am J Neuroradiol. 1997;18(2):219–229. [PMC free article] [PubMed] [Google Scholar]
- 60.Stonestreet BS, McKnight AJ, Sadowska G, et al. Effects of duration of positive-pressure ventilation on blood-brain barrier function in premature lambs. Journal of applied physiology. 2000;88(5):1672–1677. doi: 10.1152/jappl.2000.88.5.1672. [DOI] [PubMed] [Google Scholar]
- 61.Van de Bor M, Van Bel F, Lineman R, et al. Perinatal factors and periventricular-intraventricular hemorrhage in preterm infants. Am J Dis Child. 1986;140(11):1125–1130. doi: 10.1001/archpedi.1986.02140250051035. [DOI] [PubMed] [Google Scholar]
- 62.Wigglesworth JS, Keith IH, Girling DJ, et al. Hyaline membrane disease, alkali, and intraventricular haemorrhage. Arch Dis Child. 1976;51(10):755–762. doi: 10.1136/adc.51.10.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dykes FD, Lazzara A, Ahmann P, et al. Intraventricular hemorrhage: a prospective evaluation of etiopathogenesis. Pediatrics. 1980;66(1):42–49. [PubMed] [Google Scholar]
- 64.Crowther CA, Crosby DD, Henderson-Smart DJ. Vitamin K prior to preterm birth for preventing neonatal periventricular haemorrhage. Cochrane Database Syst Rev. 2010;(1):CD000229. doi: 10.1002/14651858.CD000229.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Beverley DW, Pitts-Tucker TJ, Congdon PJ, et al. Prevention of intraventricular haemorrhage by fresh frozen plasma. Arch Dis Child. 1985;60(8):710–713. doi: 10.1136/adc.60.8.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Van de Bor M, Briet E, Van Bel F, et al. Hemostasis and periventricular-intraventricular hemorrhage of the newborn. Am J Dis Child. 1986;140(11):1131–1134. doi: 10.1001/archpedi.1986.02140250057036. [DOI] [PubMed] [Google Scholar]
- 67.Lupton BA, Hill A, Whitfield MF, et al. Reduced platelet count as a risk factor for intraventricular hemorrhage. Am J Dis Child. 1988;142(11):1222–1224. doi: 10.1001/archpedi.1988.02150110100029. [DOI] [PubMed] [Google Scholar]
- 68.Shirahata A, Nakamura T, Shimono M, et al. Blood coagulation findings and the efficacy of factor XIII concentrate in premature infants with intracranial hemorrhages. Thromb Res. 1990;57(5):755–763. doi: 10.1016/0049-3848(90)90033-9. [DOI] [PubMed] [Google Scholar]
- 69.Bilguvar K, DiLuna ML, Bizzarro MJ, et al. COL4A1 mutation in preterm intraventricular hemorrhage. J Pediatr. 2009;155(5):743–745. doi: 10.1016/j.jpeds.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Debus O, Koch HG, Kurlemann G, et al. Factor V Leiden and genetic defects of thrombophilia in childhood porencephaly. Arch Dis Child Fetal Neonatal Ed. 1998;78(2):F121–124. doi: 10.1136/fn.78.2.f121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gopel W, Gortner L, Kohlmann T, et al. Low prevalence of large intraventricular haemorrhage in very low birthweight infants carrying the factor V Leiden or prothrombin G20210A mutation. Acta Paediatr. 2001;90(9):1021–1024. doi: 10.1080/080352501316978101. [DOI] [PubMed] [Google Scholar]
- 72.Ryckman KK, Feenstra B, Shaffer JR, et al. Replication of a genome-wide association study of birth weight in preterm neonates. J Pediatr. 2012;160(1):19–24. e14. doi: 10.1016/j.jpeds.2011.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gopel W, Hartel C, Ahrens P, et al. Interleukin-6-174-genotype, sepsis and cerebral injury in very low birth weight infants. Genes Immun. 2006;7(1):65–68. doi: 10.1038/sj.gene.6364264. [DOI] [PubMed] [Google Scholar]
- 74.Adcock K, Hedberg C, Loggins J, et al. The TNF-alpha -308, MCP-1 -2518 and TGF-beta1 +915 polymorphisms are not associated with the development of chronic lung disease in very low birth weight infants. Genes Immun. 2003;4(6):420–426. doi: 10.1038/sj.gene.6363986. [DOI] [PubMed] [Google Scholar]
- 75.Harteman JC, Groenendaal F, van Haastert IC, et al. Atypical timing and presentation of periventricular haemorrhagic infarction in preterm infants: the role of thrombophilia. Developmental medicine and child neurology. 2012;54(2):140–147. doi: 10.1111/j.1469-8749.2011.04135.x. [DOI] [PubMed] [Google Scholar]
- 76.Vinukonda G, Dummula K, Malik S, et al. Effect of prenatal glucocorticoids on cerebral vasculature of the developing brain. Stroke. 2010;41(8):1766–1773. doi: 10.1161/STROKEAHA.110.588400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 78.Winkler F, Kozin SV, Tong RT, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6(6):553–563. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 79.Meadow WL, Bell A, Sunstein CR. Statistics, not memories: what was the standard of care for administering antenatal steroids to women in preterm labor between 1985 and 2000? Obstet Gynecol. 2003;102(2):356–362. doi: 10.1016/s0029-7844(03)00510-6. [DOI] [PubMed] [Google Scholar]
- 80.Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;(3):CD004454. doi: 10.1002/14651858.CD004454.pub2. [DOI] [PubMed] [Google Scholar]
- 81.Shankaran S, Bauer CR, Bain R, et al. Relationship between antenatal steroid administration and grades III and IV intracranial hemorrhage in low birth weight infants. The NICHD Neonatal Research Network. Am J Obstet Gynecol. 1995;173(1):305–312. doi: 10.1016/0002-9378(95)90219-8. [DOI] [PubMed] [Google Scholar]
- 82.Cambonie G, Mesnage R, Milesi C, et al. Betamethasone impairs cerebral blood flow velocities in very premature infants with severe chronic lung disease. J Pediatr. 2008;152(2):270–275. doi: 10.1016/j.jpeds.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 83.Lohle M, Muller T, Wicher C, et al. Betamethasone effects on fetal sheep cerebral blood flow are not dependent on maturation of cerebrovascular system and pituitary-adrenal axis. The Journal of physiology. 2005;564(Pt 2):575–588. doi: 10.1113/jphysiol.2004.077537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brownfoot FC, Crowther CA, Middleton P. Different corticosteroids and regimens for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2008;(4):CD006764. doi: 10.1002/14651858.CD006764.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wapner R, Jobe AH. Controversy: antenatal steroids. Clin Perinatol. 2011;38(3):529–545. doi: 10.1016/j.clp.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Feldman DM, Carbone J, Belden L, et al. Betamethasone vs dexamethasone for the prevention of morbidity in very-low-birthweight neonates. Am J Obstet Gynecol. 2007;197(3):284, e281–284. doi: 10.1016/j.ajog.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 87.Baud O, Foix-L’Helias L, Kaminski M, et al. Antenatal glucocorticoid treatment and cystic periventricular leukomalacia in very premature infants. N Engl J Med. 1999;341(16):1190–1196. doi: 10.1056/NEJM199910143411604. [DOI] [PubMed] [Google Scholar]
- 88.Lee BH, Stoll BJ, McDonald SA, et al. Adverse neonatal outcomes associated with antenatal dexamethasone versus antenatal betamethasone. Pediatrics. 2006;117(5):1503–1510. doi: 10.1542/peds.2005-1749. [DOI] [PubMed] [Google Scholar]
- 89.Shankaran S, Cepeda EE, Ilagan N, et al. Pharmacokinetic basis for antenatal dosing of phenobarbital for the prevention of neonatal intracerebral hemorrhage. Dev Pharmacol Ther. 1986;9(3):171–177. doi: 10.1159/000457089. [DOI] [PubMed] [Google Scholar]
- 90.Shankaran S, Cepeda EE, Ilagan N, et al. Antenatal phenobarbital for the prevention of neonatal intracerebral hemorrhage. Am J Obstet Gynecol. 1986;154(1):53–57. doi: 10.1016/0002-9378(86)90392-3. [DOI] [PubMed] [Google Scholar]
- 91.Thorp JA, Ferrette-Smith D, Gaston LA, et al. Combined antenatal vitamin K and phenobarbital therapy for preventing intracranial hemorrhage in newborns less than 34 weeks’ gestation. Obstet Gynecol. 1995;86(1):1–8. doi: 10.1016/0029-7844(95)00091-5. [DOI] [PubMed] [Google Scholar]
- 92.Thorp JA, Parriott J, Ferrette-Smith D, et al. Antepartum vitamin K and phenobarbital for preventing intraventricular hemorrhage in the premature newborn: a randomized, double-blind, placebo-controlled trial. Obstet Gynecol. 1994;83(1):70–76. [PubMed] [Google Scholar]
- 93.Kaempf JW, Porreco R, Molina R, et al. Antenatal phenobarbital for the prevention of periventricular and intraventricular hemorrhage: a double-blind, randomized, placebo-controlled, multihospital trial. J Pediatr. 1990;117(6):933–938. doi: 10.1016/s0022-3476(05)80141-6. [DOI] [PubMed] [Google Scholar]
- 94.Mitani M, Matsuda Y, Shimada E, et al. Short- and long-term outcomes in babies born after antenatal magnesium treatment. J Obstet Gynaecol Res. 2011;37(11):1609–1614. doi: 10.1111/j.1447-0756.2011.01583.x. [DOI] [PubMed] [Google Scholar]
- 95.Basu SK, Chickajajur V, Lopez V, et al. Immediate clinical outcomes in preterm neonates receiving antenatal magnesium for neuroprotection. J Perinat Med. 2012;40(2):185–189. doi: 10.1515/JPM.2011.094. [DOI] [PubMed] [Google Scholar]
- 96.Perlman JM, Risser RC, Gee JB. Pregnancy-induced hypertension and reduced intraventricular hemorrhage in preterm infants. Pediatr Neurol. 1997;17(1):29–33. doi: 10.1016/s0887-8994(97)00073-8. [DOI] [PubMed] [Google Scholar]
- 97.Coyle MG, Oh W, Stonestreet BS. Effects of indomethacin on brain blood flow and cerebral metabolism in hypoxic newborn piglets. Am J Physiol. 1993;264(1 Pt 2):H141–149. doi: 10.1152/ajpheart.1993.264.1.H141. [DOI] [PubMed] [Google Scholar]
- 98.Coyle MG, Oh W, Petersson KH, et al. Effects of indomethacin on brain blood flow, cerebral metabolism, and sagittal sinus prostanoids after hypoxia. Am J Physiol. 1995;269(4 Pt 2):H1450–1459. doi: 10.1152/ajpheart.1995.269.4.H1450. [DOI] [PubMed] [Google Scholar]
- 99.Zuckerman SL, Mirro R, Armstead WM, et al. Indomethacin reduces ischemia-induced alteration of blood-brain barrier transport in piglets. Am J Physiol. 1994;266(6 Pt 2):H2198–2203. doi: 10.1152/ajpheart.1994.266.6.H2198. [DOI] [PubMed] [Google Scholar]
- 100.Schmidt B, Davis P, Moddemann D, et al. Long-term effects of indomethacin prophylaxis in extremely-low-birth-weight infants. N Engl J Med. 2001;344(26):1966–1972. doi: 10.1056/NEJM200106283442602. [DOI] [PubMed] [Google Scholar]
- 101.Ment LR, Oh W, Ehrenkranz RA, et al. Low-dose indomethacin and prevention of intraventricular hemorrhage: a multicenter randomized trial. Pediatrics. 1994;93(4):543–550. [PubMed] [Google Scholar]
- 102.Fowlie PW, Davis PG. Prophylactic indomethacin for preterm infants: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2003;88(6):F464–466. doi: 10.1136/fn.88.6.F464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ment LR, Vohr BR, Makuch RW, et al. Prevention of intraventricular hemorrhage by indomethacin in male preterm infants. J Pediatr. 2004;145(6):832–834. doi: 10.1016/j.jpeds.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 104.Ohlsson A, Roberts RS, Schmidt B, et al. Male/female differences in indomethacin effects in preterm infants. J Pediatr. 2005;147(6):860–862. doi: 10.1016/j.jpeds.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 105.Ment LR, Vohr B, Oh W, et al. Neurodevelopmental outcome at 36 months’ corrected age of preterm infants in the Multicenter Indomethacin Intraventricular Hemorrhage Prevention Trial. Pediatrics. 1996;98(4 Pt 1):714–718. [PubMed] [Google Scholar]
- 106.Ment LR, Vohr B, Allan W, et al. Outcome of children in the indomethacin intraventricular hemorrhage prevention trial. Pediatrics. 2000;105(3 Pt 1):485–491. doi: 10.1542/peds.105.3.485. [DOI] [PubMed] [Google Scholar]
- 107.Fowlie PW, Davis PG. Prophylactic intravenous indomethacin for preventing mortality and morbidity in preterm infants. Cochrane Database Syst Rev. 2002;(3):CD000174. doi: 10.1002/14651858.CD000174. [DOI] [PubMed] [Google Scholar]
- 108.Fowlie PW, Davis PG, McGuire W. Prophylactic intravenous indomethacin for preventing mortality and morbidity in preterm infants. Cochrane Database Syst Rev. 2010;(7):CD000174. doi: 10.1002/14651858.CD000174.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dani C, Bertini G, Pezzati M, et al. Prophylactic ibuprofen for the prevention of intraventricular hemorrhage among preterm infants: a multicenter, randomized study. Pediatrics. 2005;115(6):1529–1535. doi: 10.1542/peds.2004-1178. [DOI] [PubMed] [Google Scholar]
- 110.Van Overmeire B, Allegaert K, Casaer A, et al. Prophylactic ibuprofen in premature infants: a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2004;364(9449):1945–1949. doi: 10.1016/S0140-6736(04)17477-1. [DOI] [PubMed] [Google Scholar]
- 111.Bedard MP, Shankaran S, Slovis TL, et al. Effect of prophylactic phenobarbital on intraventricular hemorrhage in high-risk infants. Pediatrics. 1984;73(4):435–439. [PubMed] [Google Scholar]
- 112.Donn SM, Roloff DW, Goldstein GW. Prevention of intraventricular haemorrhage in preterm infants by phenobarbitone. A controlled trial. Lancet. 1981;2(8240):215–217. doi: 10.1016/s0140-6736(81)90470-0. [DOI] [PubMed] [Google Scholar]
- 113.Anwar M, Kadam S, Hiatt IM, et al. Phenobarbitone prophylaxis of intraventricular haemorrhage. Arch Dis Child. 1986;61(2):196–197. doi: 10.1136/adc.61.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Morgan ME, Massey RF, Cooke RW. Does phenobarbitone prevent periventricular hemorrhage in very low-birth-weight babies?: a controlled trial. Pediatrics. 1982;70(2):186–189. [PubMed] [Google Scholar]
- 115.Kuban KC, Leviton A, Krishnamoorthy KS, et al. Neonatal intracranial hemorrhage and phenobarbital. Pediatrics. 1986;77(4):443–450. [PubMed] [Google Scholar]
- 116.Cooke RW, Morgan ME. Prophylactic ethamsylate for periventricular haemorrhage. Arch Dis Child. 1984;59(1):82–83. doi: 10.1136/adc.59.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Benson JW, Drayton MR, Hayward C, et al. Multicentre trial of ethamsylate for prevention of periventricular haemorrhage in very low birthweight infants. Lancet. 1986;2(8519):1297–1300. doi: 10.1016/s0140-6736(86)91432-7. [DOI] [PubMed] [Google Scholar]
- 118.Fish WH, Cohen M, Franzek D, et al. Effect of intramuscular vitamin E on mortality and intracranial hemorrhage in neonates of 1000 grams or less. Pediatrics. 1990;85(4):578–584. [PubMed] [Google Scholar]
- 119.Brion LP, Bell EF, Raghuveer TS. Vitamin E supplementation for prevention of morbidity and mortality in preterm infants. Cochrane Database Syst Rev. 2003;(4):CD003665. doi: 10.1002/14651858.CD003665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cools F, Offringa M. Neuromuscular paralysis for newborn infants receiving mechanical ventilation. Cochrane Database Syst Rev. 2005;(2):CD002773. doi: 10.1002/14651858.CD002773.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mohamed MA, Aly H. Transport of premature infants is associated with increased risk for intraventricular haemorrhage. Arch Dis Child Fetal Neonatal Ed. 2010;95(6):F403–407. doi: 10.1136/adc.2010.183236. [DOI] [PubMed] [Google Scholar]
- 122.Anderson GD, Bada HS, Shaver DC, et al. The effect of cesarean section on intraventricular hemorrhage in the preterm infant. Am J Obstet Gynecol. 1992;166(4):1091–1099. doi: 10.1016/s0002-9378(11)90594-8. discussion 1099–1101. [DOI] [PubMed] [Google Scholar]
- 123.Wadhawan R, Vohr BR, Fanaroff AA, et al. Does labor influence neonatal and neurodevelopmental outcomes of extremely-low-birth-weight infants who are born by cesarean delivery? Am J Obstet Gynecol. 2003;189(2):501–506. doi: 10.1067/s0002-9378(03)00360-0. [DOI] [PubMed] [Google Scholar]
- 124.Szymonowicz W, Yu VY, Walker A, et al. Reduction in periventricular haemorrhage in preterm infants. Arch Dis Child. 1986;61(7):661–665. doi: 10.1136/adc.61.7.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Henderson-Smart DJ, Cools F, Bhuta T, et al. Elective high frequency oscillatory ventilation versus conventional ventilation for acute pulmonary dysfunction in preterm infants. Cochrane Database Syst Rev. 2007;(3):CD000104. doi: 10.1002/14651858.CD000104.pub2. [DOI] [PubMed] [Google Scholar]


