Summary
The response of individual animals to mating signals depends on the sexual identity of the individual and the genetics of the mating targets, which represent the mating social context (social environment). However, how social signals are sensed and integrated during mating decisions remains a mystery. One of the models for understanding mating behaviors in molecular and cellular terms is the male courtship ritual in the fruit fly (Drosophila melanogaster). We have recently shown that a subset of gustatory receptor neurons (GRNs) that are enriched in the male appendages and express the ion channel ppk23 play a major role in the initiation and maintenance of male courtship via the perception of cuticular contact pheromones, and are likely to represent the main chemosensory pathway that influences mating decisions by males. Here we show that genetic feminization of ppk23-expressing GRNs in male flies resulted in a significant increase in male–male sexual attraction without an apparent impact on sexual attraction to females. Furthermore, we show that this increase in male–male sexual attraction is sensory specific, which can be modulated by variable social contexts. Finally, we show that feminization of ppk23-expressing sensory neurons lead to major transcriptional shifts, which may explain the altered interpretation of the social environment by feminized males. Together, these data indicate that the sexual cellular identity of pheromone sensing GRNs plays a major role in how individual flies interpret their social environment in the context of mating decisions.
Keywords: Fruit fly, Courtship, ppk23, Poxn, transformer, DEG/ENaC
Introduction
Sexually reproducing animals often show sexually dimorphic behaviors. One of the best-characterized models for understanding the role of genetics and neural circuits in controlling sex-specific behaviors is the fruit fly Drosophila melanogaster (Anand et al., 2001; Demir and Dickson, 2005; Manoli et al., 2005; Rideout et al., 2010; Ryner et al., 1996; Siwicki and Kravitz, 2009; Villella et al., 1997; Villella and Hall, 2008). Several studies have indicated that sex-specific innate mating behaviors are determined by a dedicated neuronal circuit that comprises neurons in the central and peripheral systems, and of which development and function are determined by the sex-specific splicing of the transcription factors fruitless (fru) and doublesex (dsx) (Manoli et al., 2005; Rideout et al., 2010; Stockinger et al., 2005).
Cuticular hydrocarbons (CHCs) serve as contact sex pheromones in flies and other insects (Ferveur, 2005; Kent et al., 2008; Krupp et al., 2008; Kuo et al., 2012; Yew et al., 2009). These data suggest that the gustatory system is likely to play an important role in the detection of sex-specific stimuli. This is supported by findings that several members of the gustatory receptor family play a role in the detection of pheromonal signals (Bray and Amrein, 2003; Koganezawa et al., 2010; Miyamoto and Amrein, 2008; Moon et al., 2009; Wang and Anderson, 2010; Wang et al., 2011; Watanabe et al., 2011). In addition, we and others have recently shown that a subset of sexually dimorphic GRNs in the male and female forelegs express both fru and the ion channel ppk23, and are likely the primary contact pheromone sensory neurons in the adult fly (Lu et al., 2012; Thistle et al., 2012; Toda et al., 2012). Because ppk23 seems to be exclusively expressed in fru-positive gustatory sensory neurons in the male appendages but not in any fru-positive central neurons (Lu et al., 2012), studies of the effects of these neurons on male courtship behavior represent an excellent opportunity to study the relative contribution of the gustatory system to courtship decisions, independent of the brain.
Although stereotypic, both the perception and production of pheromones is highly plastic across sex, species, and physical and social environmental conditions (Billeter et al., 2012; Everaerts et al., 2010; Ferveur, 2005; Kent et al., 2008; Krupp et al., 2008). Here we show that feminization of ppk23/fru-specific GRNs in the male appendages is sufficient to mimic the effects of mutations in the fru locus on male sexual behaviors, independent of the role of fruM in the brain. Our data suggest a simple behavioral model in which ppk23-expressing GRNs represent a focal integration point of social environmental cues and the genetic factors that determine cellular sexual identity, which together influence mating decisions of males.
Results
Feminization of ppk23-expressing GRNs induces male–male courtship without altering the innate sexual preference for females
In previous work we have shown that the ion channel ppk23 and the gustatory neurons that express it play an essential role in the initiation and maintenance of normal male courtship behavior (Lu et al., 2012), by demonstrating that both mutations in ppk23 and blocking the activity of ppk23-expressing GRNs led to a defective male–female courtship behavior. On the other hand, we did not observe any effects of these manipulations on male–male courtship (Lu et al., 2012). We interpreted these data as suggesting that ppk23-expressing GRNs were mediating the behavioral response of males to aphrodisiac CHCs, which was further confirmed by the reduced behavioral response of ppk23 mutant males to the excitatory pheromone 7,11-heptacosadiene (7,11 HD) (Lu et al., 2012; Thistle et al., 2012). However, a calcium imaging study suggested that at least some ppk23-expressing GRNs can also respond to the inhibitory pheromone 7-tricosene (7-T) (Thistle et al., 2012). Together, these data suggested that ppk23-expressing GRNs represent a heterogeneous population of gustatory-like sensory neurons that are tuned to various classes of contact pheromones.
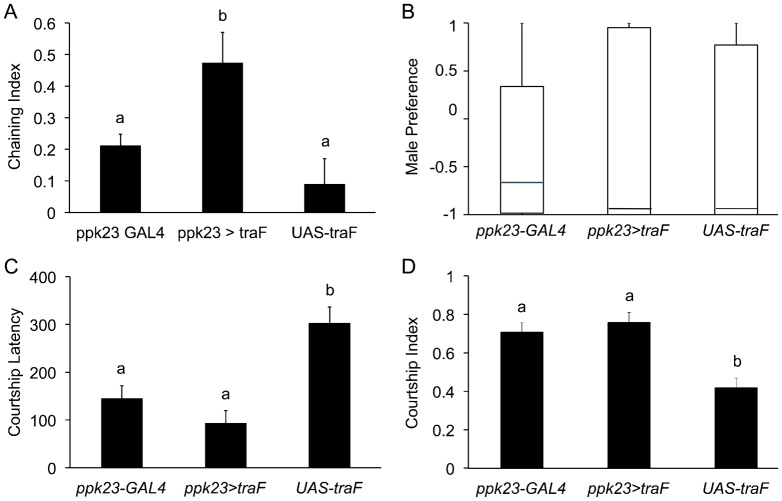
ppk23-expressing GRNs in the forelegs are sexually dimorphic, and express post-mitotically the sex-determination transcription factor fruitless (fru) (Lu et al., 2012; Thistle et al., 2012; Toda et al., 2012). Sex-determination factors such as fru and dsx are spliced into male or female-specific transcripts by the sex-specific splicing factor transformer (tra). Previous studies showed that overexpression of the female-specific transcript of tra (traF) is sufficient to induce female-like differentiation in male tissues, including the nervous system (Ferveur et al., 1997; Ferveur et al., 1995). Consequently, we hypothesized that feminization of ppk23-expressing GRNs with ectopic expression of traF in otherwise intact males will disrupt their normal function and will lead to similar mating phenotypes we observed in ppk23 mutant males. To our surprise, males with feminized ppk23-expressing GRNs showed robust male–male courtship behaviors measured by male chaining behavior (ANOVA, n = 6–8 groups, p<0.01**) (Fig. 1A). However, ppk23-feminized males retained their overall sexual preference for courting females when given a choice between wild-type male and female targets (Kruskal–Wallis rank sum test, p = 0.39) (Fig. 1B), and showed an overall normal courtship behavior towards wild-type females measured by courtship latency and index (Fig. 1C,D). These observations were in stark contrast to the inhibition of male courtship that we previously observed when ppk23-expressing cells were blocked by the ectopic expression of the tetanus toxin in these cells (Lu et al., 2012).
Fig. 1. Males with feminized ppk23-expressing GRNs show increased male–male courtship behavior.
(A) Male–male chaining index in feminized flies (ppk23>traF) and two parental controls (ppk23-GAL4, UAS-traF). Feminized males showed higher male chaining behavior relative to males from parental lines. (B) Feminized males preferred females to males in choice courtship assays. Boxplots show the distribution of choice behaviors (1, male; −1 female). (C,D) Feminized males show normal courtship behavior towards wild-type females. Feminization of ppk23-expressing GRNs had no effect on either latency or courtship index relative to parental ppk23-GAL4 males. UAS-traF parental males showed consistent longer latency (C) and reduced courtship index (D), which were likely due to unrelated factors present in the genetic background of this specific transgenic line. The different letters (a,b) in parts A, C, and D represent groups that are significantly different from each other based on ANOVA post hoc tests.
We originally identified ppk23 as a gustatory-enriched Degenerin/epithelial sodium channel (DEG/ENaC) by screening for genes that were not expressed in the Poxn mutant (Lu et al., 2012). Animals that carry mutations in Poxn lack all external gustatory sensilla (Awasaki and Kimura, 1997; Boll and Noll, 2002; Dambly-Chaudière et al., 1992; Nottebohm et al., 1992; Nottebohm et al., 1994; Vervoort et al., 1995). Poxn also retains its expression in all postmitotic GRNs and thus serves as an excellent marker for these neurons. As a result, we hypothesized that if the effects of feminizing ppk23-expressing GRNs are indeed due to gustatory functions, then feminizing the complete gustatory sensory system in males should lead to a phenotype that is similar to the one we observed in ppk23-feminized males. To completely feminize the gustatory system we expressed UAS-traF with a previously published Poxn-GAL4 line (Boll and Noll, 2002). As we expected, males with feminized GRNs showed a robust chaining behavior that was indistinguishable from males with the feminization of ppk23-expressing GRNs only (supplementary material Fig. S1A). However, in contrast to ppk23-feminized, Poxn-feminized males showed a clear preference to courting males over females (supplementary material Fig. S1B). Nevertheless, when offered a wild-type female as a mating target, Poxn-feminized males actively courted virgin females with the same tenacity as parental and sibling controls (supplementary material Fig. S1C,D). These data indicated that courtship decisions in males were also affected by ppk23-independent GRNs, and suggested that ectopic feminization of the gustatory sensory system was sufficient to induce a dramatic shift from heterosexual to homosexual behaviors in Drosophila males. In both ppk23-GAL4 and Poxn-GAL4 studies we used the parental lines as wild-type controls as has been described in previous studies that used the UAS-traF transgene (Fernández et al., 2010; Hoxha et al., 2013; Lazareva et al., 2007; Shirangi et al., 2013). Although our data suggest that the homozygous UAS-traF parental line shows some male chaining behavior, our analyses indicated that chaining is significantly higher when traF was expressed by either ppk23-GAL4 or Poxn-GAL4. Thus, we conclude that feminization of chemosensory neurons was sufficient to induce chaining behavior in males.
Feminization of ppk23-expressing cells does not increase the sexual attractiveness of manipulated males
Although we did not observe expression of ppk23 outside the chemosensory system, it is still possible that some of the observed effects on male–male courtship were due to qualitative or quantitative changes in the production of cuticular pheromone signals in feminized males via direct or indirect effects on the pheromone producing oenocytes (Billeter et al., 2009). To test this possibility we first examined the attractiveness of ppk23-feminized males as courtship targets for wild-type males. We expected that wild-type males would become more sexually attracted to feminized males than non-feminized males. However, our data indicated that the attractiveness of manipulated males did not differ from wild-type parental controls (supplementary material Fig. S2A,B). We also analyzed the CHC profiles of feminized and wild-type parental males by using gas chromatography (FID) and combined gas chromatography/mass spectrometry (GC/MS). As with behavior, we did not observe a significant effect of the ppk23-feminization on the overall CHC profile or any of the individual compounds (supplementary material Fig. S2C). These data indicate that the observed increase in male–male courtship in feminized males is due to changes in sensory functions rather than their pheromonal signature.
Feminization of GRNs does not alter gross axonal wiring patterns in the thoracic ganglion
ppk23-expressing GRNs are about two-fold more abundant in male relative to female forelegs, and show a sexual dimorphic axonal midline crossing in the thoracic ganglia of males but not females (Lu et al., 2012). It has been shown that the axonal midline crossing of GRNs in the male depends on the expression of the male forms of the two main sex-determination transcription factors fru and dsx (Mellert et al., 2010). Since the splicing of both fruM and dsxM depends on the sex-dependent splicing of tra (Robinett et al., 2010; Verhulst et al., 2010), we hypothesized that the ectopic expression of traF in ppk23- or Poxn-expressing GRNs in males may have resulted in the inhibition of axonal midline crossing, which subsequently led to aberrant male sexual behaviors. However, anatomical analyses of midline crossing in feminized ppk23 or Poxn males revealed no gross changes in axonal wiring patterns relative to wild-type controls (Independent sample t-tests; n = 5–6 per genotype) (Fig. 2A–E). We also did not observe any effects of feminization of overall number of ppk23-positive cells in males or females (supplementary material Fig. S3). We cannot explain why feminization by the ectopic expression of traF did not inhibit axonal midline crossing as was previously reported for direct manipulations of the fruM transcripts in Poxn neurons (Mellert et al., 2010). Nevertheless, our data suggest that the behavioral outcomes of chemosensory feminization are not directly related to the status of axonal midline crossing or to the relative abundance of ppk23-positive cells in forelegs.
Fig. 2. Feminization of ppk23-expressing chemosensory neurons does not affect their gross axonal projection patterns.
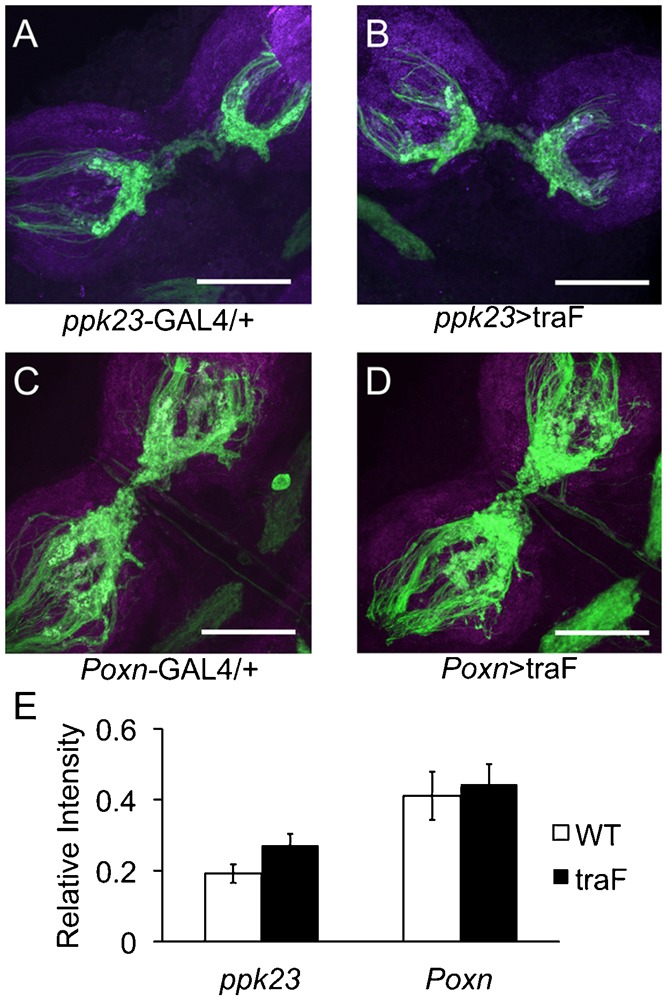
(A) Membrane-tethered GFP (UAS-mCD8::GFP) was expressed by ppk23-GAL4 (wild-type pattern). (B) UAS-mCD8::GFP was co-expressed with UAS-traF by ppk23-GAL4. (C) mCD8::GFP was expressed by the pan-gustatory Poxn-GAL4 line. (D) UAS-mCD8::GFP was co-expressed with UAS-traF by Poxn-GAL4. (E) Quantification of relative fluorescence intensity in the midline-crossing region. No significant differences were found between control and traF-expressing males with either GAL4 lines. Scale bars: 25µm.
The simplest possible explanation for our findings is that feminization of ppk23-expressing GRNs lead to increased male chaining behavior was due to their reduced detection of a inhibitory signals from other males but without affecting their response to excitatory signals from females. To test this hypothesis we examined the behavioral response of males to the inhibitory pheromone 7-T, which is sufficient to inhibit male–male courtship (Billeter et al., 2009; Fernández et al., 2010; Ferveur and Sureau, 1996; Krupp et al., 2008). Therefore, we examined the effect of feminization of ppk23-expressing GRNs on the behavioral response of manipulated and control males to 7-T. Our data show that in contrast to our hypothesis, feminized males were still sensitive to the inhibitory effects of 7-T when responding to perfumed decoys (supplementary material Fig. S4A,B). These data suggested that the increase in male–male courtship behavior was not due to a reduced sensing of the principle inhibitory pheromone 7-T, and may suggest that feminized males are actively attracted to other males due to ectopic changes in chemosensory functions.
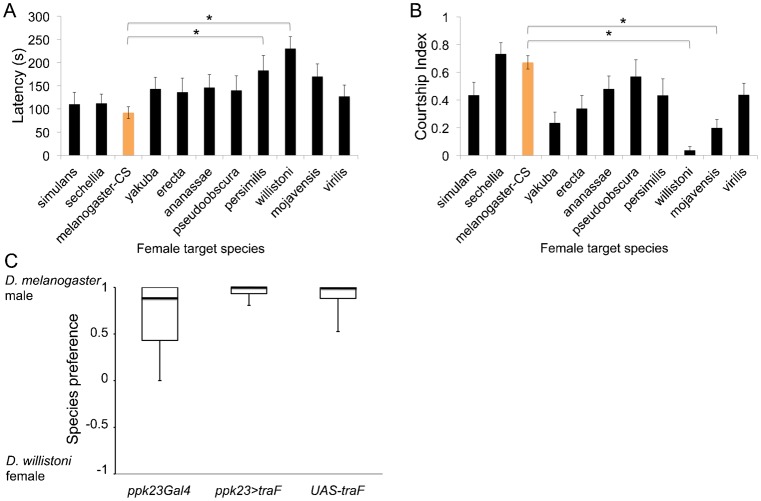
Our data indicate that males with feminized ppk23-expressing cells court conspecific males, but when given a choice between the sexes, still prefer to court conspecifc females. Thus, these data could not resolve whether courting wild-types males by ppk23-feminized males is an active choice or whether these males will court any possible target in the absence of females. To better distinguish between these two possible explanations we next provided D. melanogaster wild-type males with heterospecific females from diverse Drosophila species of varying phylogenetic distances. Our data indicated that wild-type D. melanogaster males promiscuously courted most single female targets, independent of phylogenetic distances [ANOVA, n = 15–20 for each species except D. melanogaster (n = 61), *p<0.05] (Fig. 3A,B). However, females from D. persimilis, D. willistoni as measured by courtship latency, and D. willistoni and D. mojavensis as measured by courtship index, were significantly less attractive than other species. As a result, we hypothesized that if ppk23-feminized males court other D. melanogaster males because they actively find them attractive then when presented with a choice between a D. melanogaster male and an unattractive female from a different species then they will still court conspecfic males. Alternatively, if in the absence of D. melanogaster female, ppk23-femized males will court any targets without discrimination then they should court both targets equally. To test this we asked ppk23-feminized males to choose between between a D. melanogaster male and the unattractive D. willistoni female. To our surprise, both feminized and wild-type control males preferred D. melanogaster males to D. willistoni females as courtship targets (Kruskal–Wallis rank sum test, n = 10–15, p = 0.44) (Fig. 3C). These data further supported a model in which male sexual preferences are strongly affected by the available pool of mating targets, and that the decision to court a specific target depends on its relative attractiveness to other possible targets. Furthermore, our data indicate that the feminization of ppk23-expressing GRNs leads to an active choice of males as possible targets by shifting how males interpret their social environment when making courtship decisions in complex social environments.
Fig. 3. D. melanogaster males prefer to court conspecific males over females of a distant species.
Wild-type D. melanogaster males courted females from other species with varying degrees of intensity as measured by the courtship latency (A) and the courtship index (B). (C) In choice assays, D. melanogaster males of all tested genotypes chose to court conspecific males over females of D. willistoni. Boxplots represent the distribution of male mating choices. No significant differences were found between feminized flies and the parental controls. *p<0.05.
Feminization of ppk23-expressing GRNs leads to changes in the sensory transcriptome in the male appendages
Feminization of ppk23-expressing GRNs did not affect the overall cell number in the forelegs of males and females (supplementary material Fig. S3), or their axonal projection patterns (Fig. 2). Therefore, we hypothesized that an alternative explanation for the observed effects of feminization on male behavior were transcriptional changes in ppk23-expressing GRNs. To test this hypothesis we used real-time quantitative RT-PCR to study changes in the expression of fruF and candidate genes in the male appendages in response to ectopic feminization. We focused our analysis on several genes from the Gr and ppk families, which have been previously implicated in mediating the gustatory response to contact pheromones (Ben-Shahar et al., 2010; Ben-Shahar et al., 2007; Bray and Amrein, 2003; Lin et al., 2005; Liu et al., 2012; Lu et al., 2012; Miyamoto and Amrein, 2008; Moon et al., 2009; Starostina et al., 2012; Thistle et al., 2012; Toda et al., 2012; Watanabe et al., 2011), as well genes that encode for feeding related sweet and bitter receptors (Gr5a and Gr66a, respectively) (Dahanukar et al., 2001; Marella et al., 2006; Moon et al., 2006). Although we observed statistically significant changes in the expression levels of several members of the Gr and ppk families, none of the studied receptor genes showed a dramatic change that may explain the robust behavioral outcome of ppk23-feminization (Fig. 4A) (Independent sample t-test; n = 4 for each bar; *p<0.05; **p<0.01; ***p<0.001). Furthermore, although we have previously shown that ppk23-expressing GRNs do not overlap anatomically with either sweet (Gr5a-expressing GRNs) or bitter (Gr66a-expressing GRNs) (Lu et al., 2012), we observed a small but significant increase in Gr5a expression in the appendages of feminized males relative to wild-type controls (Fig. 4A). Feminized males also showed a significant increase in their sensory sensitivity to sugar (supplementary material Fig. S5), suggesting that feminization of one GRN type may have indirectly affected the physiology of other feeding related GRNs.
Fig. 4. Feminization of ppk23-expressing cells leads to significant shifts in the chemosensory transcriptome in male appendages.
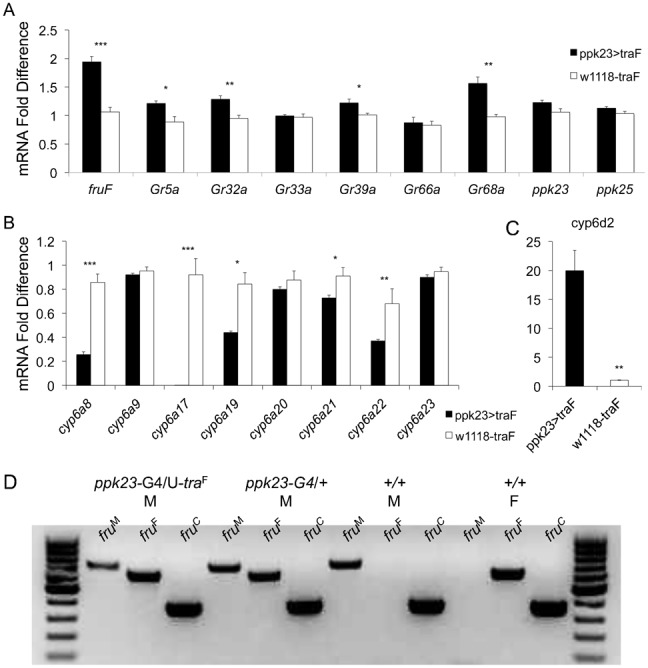
(A) Real-time quantitative RT-PCR analyses of chemosensory genes that have been previously implicated in pheromonal sensing. Analyses were of total RNA extracted from male appendages from feminized flies (w1118;ppk23-GAL4/UAS-traF) and wild-type controls (w1118/UAS-traF). (B) Real-time quantitative RT-PCR analyses of members of the Cytochrome P450 family, subfamily 6. The expression of Cyp6d2 is shown separately since this gene was regulated in the opposite direction relative to all other Cyp6 genes (C). (D) PCR analyses of sex-specific fru transcripts in appendages. fruM, male-specific; fruF, female-specific; fruC, common exons. M = male, F = female, +/+ = w1118. *p<0.05; **p<0.01.
The perception of pheromones by the chemosensory system also depends on rapid enzymatic removal of the perceived chemicals (Feyereisen, 2006; Oakeshott et al., 2010; Wang et al., 2008). In support of this, a gene encoding for a cytochrome P450 enzyme (Cyp6a20) was recently implicated in chemosensory functions underlying male–male interactions in Drosophila (Wang et al., 2008). Although the exact role of these enzymes in chemosensory biology is not fully understood, it is likely that secreted members of the family play a role in the breakdown of cuticular contact pheromones once they enter the lumen of chemosensory sensillum (Feyereisen, 2006; Willingham and Keil, 2004), where they possibly play a role in the removal or modifications of the sensed pheromones. However, we did not find that Cyp6a20 was significantly regulated by the feminization of ppk23-expressing cells (Fig. 4B) (Independent sample t-test; n = 4 for each bar; *p<0.05; **p<0.01; ***p<0.001). Nonetheless, several other related family members that cluster in the same genomic region as Cyp6a20 showed dramatic changes in their expression levels in male appendages in response to feminization, with the most dramatic patterns shown by Cyp6a17 (Fig. 4B) and Cyp6d2 (Fig. 4C) (Independent sample t-test; n = 4 for each bar; *p<0.05; **p<0.01; ***p<0.001). Thus, the expression of traF in ppk23-expressing sensory neurons in males has likely led to major qualitative and quantitative changes in the expression patterns of chemosensory receptors and other genes associated with contact pheromonal signal transduction pathways.
Unexpectedly, we found that the expression of fruF in the appendages of ppk23-feminized males was only about 2-fold higher than in our control line (Fig. 4A). Since our control flies included one copy of the UAS-traF transgene, these data suggested that this UAS line might be expressing some levels of traF even when GAL4 is not present. To test this, we used PCR to amplify male-specific, female-specific, and common fru exons in control and ppk23-feminized males, as well as wild-type males and females as positive controls. We found that males carrying one copy of the UAS-traF transgene expressed fruM and fruF (Fig. 4D), indicating partial level of feminization, which is likely due to a “leaky” UAS transgene.
Discussion
Courtship in Drosophila melanogaster is one of the best-characterized animal mating behaviors at the molecular and cellular levels (Villella and Hall, 2008). However, we still know relatively little about how flies sense and integrate sex-specific sensory signals (Dickson, 2008). Previous studies of one of the primary sex-determination factors fru indicated that mutations in this gene lead to male chaining behavior (Anand et al., 2001; Demir and Dickson, 2005; Gailey and Hall, 1989; Goodwin et al., 2000; Lee et al., 2000; Manoli et al., 2005; Ryner et al., 1996). In this study we show that genetic feminization of the contact pheromone chemosensory neurons in the male fruit fly appendages is sufficient to phenocopy the classic fru behavioral male chaining phenotype (Fig. 1A). However, in contrast to fru mutant males who do not discriminate between males and females (Villella et al., 1997), ppk23-feminized males still retained their overall preference for courting females over males (Fig. 1B). Thus, our studies indicate that the behavioral impact of feminizing pheromone-sensing neurons on male courtship behavior cannot be explained solely by changes in fru-dependent processes. Nevertheless, our data clearly demonstrate that qualitative changes in the expression of chemosensory-related genes are associated with sensory feminization, suggesting that the transcription of some molecular sensory receptors is under the influence of the sex-determination pathway, and may explain some of the differences in pheromone driven behaviors in males and females (Fig. 4).
Previous studies indicated that the decision of a male to court a specific target is mediated by both attractive and repulsive signals (Billeter et al., 2009; Fernández et al., 2010; Kent et al., 2008; Krupp et al., 2008), and it is the summation of these two opposing forces that determines the length of courtship latency and the intensity of the courtship behavior once a male is committed to a specific target (Ferveur and Sureau, 1996). We found that males with feminized ppk23-expressing sensory neurons courted other males, but when given a choice between a male or a female D. melanogaster they still preferred to court a female (Fig. 1). These data indicate that feminization did not abolish the ability of these males to discriminate between males and females but rather reduced the inhibition of male–male attraction. A previous study indicated that wild-type males find animals that do not produce any cuticular hydrocarbons, and hence do not have a pheromonal signature, as sexually attractive (Billeter et al., 2009). Thus, the simplest explanation for these data is that feminized males could not sense a male-specific inhibitory pheromone, which resulted in high male–male courtship (Fig. 1). However, feminization of ppk23-expresssing neurons did not affect the ability of males to sense excitatory signals present in the female. Thus, when presented with a choice between a male and a female, feminized males still preferred to court females over males. In spite of the simplicity of the above model, further investigations indicated that the increased courtship toward other males by males with feminized ppk23-expressing cells was not purely due to the lack of sensing of an inhibitory signal. This is based on data that indicated feminized males still avoided females that were perfumed with 7-T, the main inhibitory cuticular pheromone in D. melanogaster (Billeter et al., 2009; Ferveur and Sureau, 1996; Lacaille et al., 2007; Wang et al., 2011) (supplementary material Fig. S4). Since the CHC profile of males is typically enriched with 7-T, our data suggest that although feminized males can sense and are repulsed by 7-T, they still find wild-type males attractive. These data showed that feminized males were actively attracted to wild-type males rather than passively defaulting to males due to the lack of an inhibitory signal, but to a lesser extent relative to their attraction to females. Furthermore, when we gave feminized and wild-type males the choice between a D. melanogaster male and a D. willistoni female, males from all genotypes (including wild-type males) preferred to court conspecific males relative to heterospecific females (Fig. 3). Together, these data suggest that males interpret the sensory input into ppk23-expresing cells in the context of the social environment they are exposed to. One limitation of our study is the differing strain backgrounds of our transgenic lines, and we cannot exclude that these differences may have an influence on our results. Nevertheless, our data indicate that male sexual decision-making is strongly influenced by the available mating pool. There is a possibility that the manipulations we employed in our study may have resulted in an intersex phenotype rather than full feminization. However, this would still fit our hypothesis that ppk23-expressing cells integrate their own sexual genetic identity with social signals to drive sexual behaviors in males. In addition, the feminization of ppk23-expressing neurons can lead to erroneous interpretations of the mating targets pool. These data are in further support of previous studies that showed that the social context of both males and females could affect their courtship behavior as well as the production of pheromones (Billeter et al., 2012; Kent et al., 2008; Krupp et al., 2008).
While our experimental data cannot completely exclude the possibility that feminized males were able to recognize female via non-gustatory pathways, our use of decapitated males and females as targets under red light conditions eliminated vision and the possibility that the courting males recognized sex-specific active behavioral patterns initiated by the courtship targets. Together, these data suggest that changes in the perception of contact pheromones played a role in the abnormal mating behaviors of manipulated males.
Although we have previously shown that ppk23-expressing cells do not overlap with sweet sensing (Gr5a-expressing) neurons (Lu et al., 2012), males with feminized ppk23-expressing neurons showed a small but significant increase in the expression of Gr5a receptor in their appendages. Furthermore, feminized males showed higher behavioral sensitivity to sugar stimuli. These data suggest that feminization of pheromone-sensing neurons can affect other classes of gustatory receptor neurons, possibly via indirect mechanisms. These data also further support the possible sensory crosstalk between canonical taste sensory pathways and the pheromonal input pathways as has been shown for the bitter receptors Gr66a, Gr33a, and Gr32a (Koganezawa et al., 2010; Lacaille et al., 2009; Miyamoto and Amrein, 2008; Moon et al., 2009; Wang et al., 2011).
Previously, we have shown that sexually-dimorphic ppk23-expressing neurons represent the primary fru-expressing GRNs in the male appendages (Lu et al., 2012). These data suggested that ppk23-expressing cells represent the primary subpopulation of contact pheromone-sensing GRNs. In agreement with these data, we found that feminization of all GRNs by using the pan-gustatory driver Poxn (Boll and Noll, 2002; Dambly-Chaudière et al., 1992) also led to male chaining behavior (supplementary material Fig. S1). However, in stark contrast to male–male courtship behaviors of fru mutant males (Gailey and Hall, 1989; Villella et al., 1997) and in males with feminized ppk23-expressing cells, males with a feminized gustatory system preferred males to females (supplementary material Fig. S1). Since Poxn-GAL4 is expressed in all gustatory receptor neurons including fru-expressing neurons in the proboscis, these data suggest that additional gustatory neurons that do not express ppk23 are also likely to play a role in the sexual decision making process of male Drosophila.
Although we have previously shown that contact pheromone sensory neurons are sexually dimorphic in terms of their axonal projection patterns (Lu et al., 2012), feminization of gustatory receptor cells affected the behavior of males without an obvious gross impact on male-specific axonal patterns (Fig. 2). This outcome was surprising since previously published studies showed that manipulation of the fru-dependent sex determination pathway had a significant effect on axonal midline crossing of gustatory neurons in males and females (Mellert et al., 2010; Possidente and Murphey, 1989). It is possible that the lack of effect of traF with the ppk23-GAL4 driver is due to the late onset of ppk23 transcription during development. ppk23 expression begins in the late pupal stages (supplementary material Fig. S6A), and therefore ppk23-GAL4 may not affect midline crossing in the nervous system. Rather, ppk23 may act in the maintenance of sex-specific circuits post-developmentally. Poxn expression, however, begins in the embryonic stage (supplementary material Fig. S6B) and so it remains unclear why the expression of traF with the poxn driver did not alter neuronal wiring patterns. Consequently, based on the current understanding of the sex-determination pathway in Drosophila, we expected that ectopic expression of traF in males would phenocopy what was reported in previous studies since traF signaling is upstream from fru. Furthermore, ppk23-feminized males did show a significant increase in the fruF specific transcripts in their appendages (Fig. 4A). One possible genetic explanation to the discrepancy in our findings is that we ectopically expressed traF in the background of wild-type tra locus. Therefore, it is possible that the endogenous male-specific sex-determination genetic cascade was sufficient to maintain the male-specific axonal projection pattern. Nevertheless, our data strongly support the hypothesis that certain aspects of the sexual dimorphism observed in the ppk23-expressing cells do not depend on their abundance in males versus females or their sexually dimorphic axonal midline crossing.
The studies we report here contribute to a better understanding of the role of the sex-determination pathway in regulating the sensory inputs used by males to make mating related decisions. Our data support a model in which ppk23 pheromone sensing neurons represent a focal element in the sex circuit, which determines how males respond to their social environment to achieve adaptive mating decisions. Our approach indicates that by taking advantage of mosaic males in which only one class of sensory neurons is female-like in otherwise intact males would enable us to start dissecting in high detail the genetic networks that determine sexual decision making in males and females, independent of higher central neuronal functions.
Materials and Methods
Fruit fly strains and genetics
All fly stocks were maintained on standard cornmeal medium at 25°C under 12:12 light–dark cycle. The ppk23 promoter-GAL4 line was described previously (Lu et al., 2012). UAS-traF flies were from Ralph Greenspan. Unless mentioned, all other fly strains used in our studies were obtained from the Bloomington Stock Center. Non-D. melanogaster fruit fly species were obtained from the San Diego Species Stock Center. Specific lines used were: D. simulans 14011-0251.192, D. sechellia 14021-0248.03, D. yakuba 14021-0261.01, D. erecta 14021-0224.00, D. ananassae 14024-0371.16, D. pseudoobscura 14011-0121.104, D. persimilis 14011-0111.50, D. willistoni 14030-0811.35, D. mojavensis 15081-1352.23, and D. virilis 15010-1051.118. The used species were chosen based on whole genome availability as well as coverage of the major groups across the Drosophila lineage. All species were maintained on standard cornmeal medium except for D. mojavensis, D. persimilis and D. pseudoobscura, which were supplemented with banana, and D. sechellia, which was supplemented with noni fruit leather (Morinda citrifolia).
Real-time quantitative RT-PCR assays
qRT-PCR was assayed as previously described (Lu et al., 2012). Briefly, fly appendages were separated by repeated vortexing of whole flies frozen in liquid nitrogen. Total RNA extraction and cDNA synthesis were performed by using Trizol and SuperScript II reverse transcriptase, respectively (Invitrogen). qPCR assays were performed on an ABI7500 machine with ABI SYBRGreen chemistry. The housekeeping gene rp49 was used as an RNA loading control. Ct data were transformed according to the ΔΔCt method and are represented as relative values (Ben-Shahar et al., 2002). See supplementary material Table S1 for gene-specific primers used in our study.
RT-PCR assays
Total RNA extraction and cDNA synthesis from fly appendages were performed as described above. To identify the presence of fru transcripts in our samples we conducted a PCR-based screen using the following forward primers: male specific fruM (GGCGACGTCACAGGATTATT), female specific fruF (TCAATCAACACTCAACCCGA), common fruC (TGGAACAATCATCCCACAAA), and a common fruR reverse primer (AGTCGGAGCGGTAGTTCAGA). PCRs were performed with Taq supermix (Lamda) in 25 µL reactions, and then separated on a 1.0% agarose gel (Fig. 4D).
Chemical analysis of cuticular hydrocarbons (CHC)
Male flies that were 4–7 days old were kept frozen in −80°C until extraction. Parental genotypes ppk23-GAL4 and UAS-traF were used as controls. For CHC extraction, groups of 5 frozen flies were shaken in a glass vial with 200 µL of Hexane. 100 ng n-octadecane was added to the extracts (C-18), as an internal standard. Samples from the extract were analyzed using gas chromatography (CP 3900; Varian). Quantitative analyses of CHCs were done with a DB-1 fused silica column that was temperature-programmed from 150°C (1 min. of initial hold) at 5°C/min to 300°C. Compound quantification was done by peak integration in comparison with the internal standard. Peaks identity was verified by using a 5975 Supersonic Molecular Beam (SMB) GC-MS with cold EI (Amirav et al., 2008) (Aviv Analytical model 5975-SMB, http://www.avivanalytical.com), which provides an unambiguous molecular ion as well as pronounced ion fragments at the branching points of branched hydrocarbons. The identities of the compounds in the extracts were in agreement with previously published data (Everaerts et al., 2010).
Histochemistry and microscopy
Immunostainings of thoracic ganglia was done as previously described (Lu et al., 2012). In short, freshly dissected brain and thoracic ganglia from flies that express a membrane tethered version of EGFP (CD8::GFP) in either ppk23 or Poxn expressing neurons were fixed in 4% paraformaldehyde and washed in PBT. The specimens were co-stained with anti-GFP (Invitrogen) and the neuropil marker anti-nc82 (Developmental Studies Hybridoma Bank, University of Iowa) and mounted on slides with Slowfade Gold antifade reagent (Invitrogen) according to well-established protocols (Wu and Luo, 2006). All images were taken with a Nikon A1 confocal microscope. Shown images were constructed from optical Z-stacks and analyzed using the Nikon NIS-Elements software package.
Courtship behavior
Courtship was assayed with 4�–7-day-old males as previously described (Ben-Shahar et al., 2010; Lu et al., 2012). In short, courtship assays were done under red light conditions unless differently stated and targets were decapitated. Courtship latency was calculated as the time from female introduction until the male showed obvious courtship behavior such as orientation coupled with wing extensions. Once courtship began, courtship index was calculated as the proportion of time a male spent in any courtship-related activity during a 10 min. period or until mating occurred. For the 7-T treatment, groups of CO2 anesthetized virgin 4–5-day-old females were placed in small glass vials that were coated with a thin layer of the compound. Females were then perfumed by three repeats of 20 seconds of gentle vortexing followed by a 20-second rest interval according to previously published protocols (Billeter et al., 2009). The 7-T courtship assays were performed under white light in a circular courtship arena (22 mm in diameter).
Interspecific single-pair tests
D. melanogaster virgin males were collected upon eclosion and kept separately in small vials (12×75 mm). Female virgin flies of all species were collected upon eclosion and kept in groups of up to 10 flies from a single-species. All vials contained standard cornmeal medium. Flies were aged 4–7 days under constant conditions of 25°C and a 12:12 light–dark cycle before behavioral experiments to ensure reproductive maturation. Interspecific no-choice tests were then carried out in behavioral chambers as previously described (Lu et al., 2012).
Chaining behavior
Male chaining was assayed with eight male in a 22 mm diameter circular arena as previously described (Lu et al., 2012). Chaining index was calculated as the proportion of time in which at least three males showed chaining courtship to each other during a 10 min. observation.
Choice behavior
Choice was assayed by introducing a single focal male and two decapitated targets. Flies were videotaped and analyzed for the duration of time the focal male spent courting each of the two targets. The courtship choice index was calculated [(duration of courtship of target A – duration of courtship of target B)/total courtship time]. Courtship time was measured from the moment the male started courting one of the targets. Total assay time was kept at 10 min.
Proboscis extension reflex
Proboscis extension reflex (PER) assays were as previously described (Lu et al., 2012). In short, 1-day-old flies were starved for approximately 24 hours, then immobilized by chilling on ice and mounted ventral-side-up using myristic acid. Flies were allowed to recover for two hours under humid conditions. Flies were satiated with water prior to the PER training. Flies were tested by introducing a drop of the test solution to a foreleg. Only full PER responses were recorded as positive. Each fly was exposed three times to the same stimulus in each concentration with water application between each trial. ‘Responders’ were classified as such if they responded to at least 2 out of 3 trials. The responding index represents the sum of all positive responses of an individual animal to a specific sequence of tarsal stimuli.
Statistical procedures
All statistical tests were performed using the R statistical package. Data were tested for normality by using the Shapiro–Wilk test. Two-sample t-tests and one-way ANOVA tests were used for parametric statistics and the two-samples Wilcoxon test and Kruskal–Wallis rank sum test were used for non-parametric tests. Chi-square tests were used for frequency-based data.
Supplementary Material
Acknowledgments
We thank Wilson Lin for assistance with PER assays. We thank Sam Funderburk for assistance with fly husbandry. We also thank Prof. A. Amirav of the Chemistry Department at Tel Aviv University for the use of the SMB GC/MS. We thank members of the Ben-Shahar lab for useful comments on earlier versions of the manuscript.
Footnotes
Author Contributions: B.L. and Y.B.-S. designed experiments. B.L., K.M.Z., R.S. and A.H. executed experiments and analyzed data. B.L., K.M.Z., A.H. and Y.B.-S. wrote the paper.
Competing interests: The authors have no competing interests to declare.
Funding
This work was supported in part by the Children's Discovery Institute of Washington University and St Louis Children's Hospital [grant number MD-II-2009-170 to Y.B.-S.]; and the National Institute on Deafness and Other Communication Disorders [grant number 5R03DC010244 to Y.B.-S.].
References
- Amirav A., Gordin A., Poliak M., Fialkov A. B. (2008). Gas chromatography–mass spectrometry with supersonic molecular beams. J. Mass Spectrom. 43, 141–163 10.1002/jms.1380 [DOI] [PubMed] [Google Scholar]
- Anand A., Villella A., Ryner L. C., Carlo T., Goodwin S. F., Song H. J., Gailey D. A., Morales A., Hall J. C., Baker B. S. et al. (2001). Molecular genetic dissection of the sex-specific and vital functions of the Drosophila melanogaster sex determination gene fruitless. Genetics 158, 1569–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasaki T., Kimura K. (1997). pox-neuro is required for development of chemosensory bristles in Drosophila. J. Neurobiol. 32, 707–721 [DOI] [PubMed] [Google Scholar]
- Ben-Shahar Y., Robichon A., Sokolowski M. B., Robinson G. E. (2002). Influence of gene action across different time scales on behavior. Science 296, 741–744 10.1126/science.1069911 [DOI] [PubMed] [Google Scholar]
- Ben-Shahar Y., Nannapaneni K., Casavant T. L., Scheetz T. E., Welsh M. J. (2007). Eukaryotic operon-like transcription of functionally related genes in Drosophila. Proc. Natl. Acad. Sci. USA 104, 222–227 10.1073/pnas.0609683104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar Y., Lu B., Collier D. M., Snyder P. M., Schnizler M., Welsh M. J. (2010). The Drosophila gene CheB42a is a novel modifier of Deg/ENaC channel function. PLoS ONE 5, e9395 10.1371/journal.pone.0009395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeter J. C., Atallah J., Krupp J. J., Millar J. G., Levine J. D. (2009). Specialized cells tag sexual and species identity in Drosophila melanogaster. Nature 461, 987–991 10.1038/nature08495 [DOI] [PubMed] [Google Scholar]
- Billeter J. C., Jagadeesh S., Stepek N., Azanchi R., Levine J. D. (2012). Drosophila melanogaster females change mating behaviour and offspring production based on social context. Proc. Biol. Sci. 279, 2417–2425 10.1098/rspb.2011.2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boll W., Noll M. (2002). The Drosophila Pox neuro gene: control of male courtship behavior and fertility as revealed by a complete dissection of all enhancers. Development 129, 5667–5681 10.1242/dev.00157 [DOI] [PubMed] [Google Scholar]
- Bray S., Amrein H. (2003). A putative Drosophila pheromone receptor expressed in male-specific taste neurons is required for efficient courtship. Neuron 39, 1019–1029 10.1016/S0896-6273(03)00542-7 [DOI] [PubMed] [Google Scholar]
- Dahanukar A., Foster K., van der Goes van Naters W. M., Carlson J. R. (2001). A Gr receptor is required for response to the sugar trehalose in taste neurons of Drosophila. Nat. Neurosci. 4, 1182–1186 10.1038/nn765 [DOI] [PubMed] [Google Scholar]
- Dambly-Chaudière C., Jamet E., Burri M., Bopp D., Basler K., Hafen E., Dumont N., Spielmann P., Ghysen A., Noll M. (1992). The paired box gene pox neuro: a determinant of poly-innervated sense organs in Drosophila. Cell 69, 159–172 10.1016/0092-8674(92)90127-X [DOI] [PubMed] [Google Scholar]
- Demir E., Dickson B. J. (2005). fruitless splicing specifies male courtship behavior in Drosophila. Cell 121, 785–794 10.1016/j.cell.2005.04.027 [DOI] [PubMed] [Google Scholar]
- Dickson B. J. (2008). Wired for sex: the neurobiology of Drosophila mating decisions. Science 322, 904–909 10.1126/science.1159276 [DOI] [PubMed] [Google Scholar]
- Everaerts C., Farine J. P., Cobb M., Ferveur J. F. (2010). Drosophila cuticular hydrocarbons revisited: mating status alters cuticular profiles. PLoS ONE 5, e9607 10.1371/journal.pone.0009607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández M. P., Chan Y. B., Yew J. Y., Billeter J. C., Dreisewerd K., Levine J. D., Kravitz E. A. (2010). Pheromonal and behavioral cues trigger male-to-female aggression in Drosophila. PLoS Biol. 8, e1000541 10.1371/journal.pbio.1000541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferveur J. F. (2005). Cuticular hydrocarbons: their evolution and roles in Drosophila pheromonal communication. Behav. Genet. 35, 279–295 10.1007/s10519-005-3220-5 [DOI] [PubMed] [Google Scholar]
- Ferveur J. F., Sureau G. (1996). Simultaneous influence on male courtship of stimulatory and inhibitory pheromones produced by live sex-mosaic Drosophila melanogaster. Proc. Biol. Sci. 263, 967–973 10.1098/rspb.1996.0143 [DOI] [PubMed] [Google Scholar]
- Ferveur J. F., Störtkuhl K. F., Stocker R. F., Greenspan R. J. (1995). Genetic feminization of brain structures and changed sexual orientation in male Drosophila. Science 267, 902–905 10.1126/science.7846534 [DOI] [PubMed] [Google Scholar]
- Ferveur J. F., Savarit F., O'Kane C. J., Sureau G., Greenspan R. J., Jallon J. M. (1997). Genetic feminization of pheromones and its behavioral consequences in Drosophila males. Science 276, 1555–1558 10.1126/science.276.5318.1555 [DOI] [PubMed] [Google Scholar]
- Feyereisen R. (2006). Evolution of insect P450. Biochem. Soc. Trans. 34, 1252–1255 10.1042/BST0341252 [DOI] [PubMed] [Google Scholar]
- Gailey D. A., Hall J. C. (1989). Behavior and cytogenetics of fruitless in Drosophila melanogaster: different courtship defects caused by separate, closely linked lesions. Genetics 121, 773–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin S. F., Taylor B. J., Villella A., Foss M., Ryner L. C., Baker B. S., Hall J. C. (2000). Aberrant splicing and altered spatial expression patterns in fruitless mutants of Drosophila melanogaster. Genetics 154, 725–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoxha V., Lama C., Chang P. L., Saurabh S., Patel N., Olate N., Dauwalder B. (2013). Sex-specific signaling in the blood-brain barrier is required for male courtship in Drosophila. PLoS Genet. 9, e1003217 10.1371/journal.pgen.1003217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent C., Azanchi R., Smith B., Formosa A., Levine J. D. (2008). Social context influences chemical communication in D. melanogaster males. Curr. Biol. 18, 1384–1389 10.1016/j.cub.2008.07.088 [DOI] [PubMed] [Google Scholar]
- Koganezawa M., Haba D., Matsuo T., Yamamoto D. (2010). The shaping of male courtship posture by lateralized gustatory inputs to male-specific interneurons. Curr. Biol. 20, 1–8 10.1016/j.cub.2009.11.038 [DOI] [PubMed] [Google Scholar]
- Krupp J. J., Kent C., Billeter J. C., Azanchi R., So A. K., Schonfeld J. A., Smith B. P., Lucas C., Levine J. D. (2008). Social experience modifies pheromone expression and mating behavior in male Drosophila melanogaster. Curr. Biol. 18, 1373–1383 10.1016/j.cub.2008.07.089 [DOI] [PubMed] [Google Scholar]
- Kuo T. H., Yew J. Y., Fedina T. Y., Dreisewerd K., Dierick H. A., Pletcher S. D. (2012). Aging modulates cuticular hydrocarbons and sexual attractiveness in Drosophila melanogaster. J. Exp. Biol. 215, 814–821 10.1242/jeb.064980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacaille F., Hiroi M., Twele R., Inoshita T., Umemoto D., Manière G., Marion-Poll F., Ozaki M., Francke W., Cobb M. et al. (2007). An inhibitory sex pheromone tastes bitter for Drosophila males. PLoS ONE 2, e661 10.1371/journal.pone.0000661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacaille F., Everaerts C., Ferveur J. F. (2009). Feminization and alteration of Drosophila taste neurons induce reciprocal effects on male avoidance behavior. Behav. Genet. 39, 554–563 10.1007/s10519-009-9286-8 [DOI] [PubMed] [Google Scholar]
- Lazareva A. A., Roman G., Mattox W., Hardin P. E., Dauwalder B. (2007). A role for the adult fat body in Drosophila male courtship behavior. PLoS Genet. 3, e16 10.1371/journal.pgen.0030016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G., Foss M., Goodwin S. F., Carlo T., Taylor B. J., Hall J. C. (2000). Spatial, temporal, and sexually dimorphic expression patterns of the fruitless gene in the Drosophila central nervous system. J. Neurobiol. 43, 404–426 [DOI] [PubMed] [Google Scholar]
- Lin H., Mann K. J., Starostina E., Kinser R. D., Pikielny C. W. (2005). A Drosophila DEG/ENaC channel subunit is required for male response to female pheromones. Proc. Natl. Acad. Sci. USA 102, 12831–12836 10.1073/pnas.0506420102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., Starostina E., Vijayan V., Pikielny C. W. (2012). Two Drosophila DEG/ENaC channel subunits have distinct functions in gustatory neurons that activate male courtship. J. Neurosci. 32, 11879–11889 10.1523/JNEUROSCI.1376-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B., LaMora A., Sun Y., Welsh M. J., Ben-Shahar Y. (2012). ppk23-dependent chemosensory functions contribute to courtship behavior in Drosophila melanogaster. PLoS Genet. 8, e1002587 10.1371/journal.pgen.1002587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoli D. S., Foss M., Villella A., Taylor B. J., Hall J. C., Baker B. S. (2005). Male-specific fruitless specifies the neural substrates of Drosophila courtship behaviour. Nature 436, 395–400 10.1038/nature03859 [DOI] [PubMed] [Google Scholar]
- Marella S., Fischler W., Kong P., Asgarian S., Rueckert E., Scott K. (2006). Imaging taste responses in the fly brain reveals a functional map of taste category and behavior. Neuron 49, 285–295 10.1016/j.neuron.2005.11.037 [DOI] [PubMed] [Google Scholar]
- Mellert D. J., Knapp J. M., Manoli D. S., Meissner G. W., Baker B. S. (2010). Midline crossing by gustatory receptor neuron axons is regulated by fruitless, doublesex and the Roundabout receptors. Development 137, 323–332 10.1242/dev.045047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T., Amrein H. (2008). Suppression of male courtship by a Drosophila pheromone receptor. Nat. Neurosci. 11, 874–876 10.1038/nn.2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon S. J., Köttgen M., Jiao Y., Xu H., Montell C. (2006). A taste receptor required for the caffeine response in vivo. Curr. Biol. 16, 1812–1817 10.1016/j.cub.2006.07.024 [DOI] [PubMed] [Google Scholar]
- Moon S. J., Lee Y., Jiao Y., Montell C. (2009). A Drosophila gustatory receptor essential for aversive taste and inhibiting male-to-male courtship. Curr. Biol. 19, 1623–1627 10.1016/j.cub.2009.07.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottebohm E., Dambly-Chaudière C., Ghysen A. (1992). Connectivity of chemosensory neurons is controlled by the gene poxn in Drosophila. Nature 359, 829–832 10.1038/359829a0 [DOI] [PubMed] [Google Scholar]
- Nottebohm E., Usui A., Therianos S., Kimura K., Dambly-Chaudière C., Ghysen A. (1994). The gene poxn controls different steps of the formation of chemosensory organs in Drosophila. Neuron 12, 25–34 10.1016/0896-6273(94)90149-X [DOI] [PubMed] [Google Scholar]
- Oakeshott J. G., Johnson R. M., Berenbaum M. R., Ranson H., Cristino A. S., Claudianos C. (2010). Metabolic enzymes associated with xenobiotic and chemosensory responses in Nasonia vitripennis. Insect Mol. Biol. 19, Suppl. 1147–163 10.1111/j.1365-2583.2009.00961.x [DOI] [PubMed] [Google Scholar]
- Possidente D. R., Murphey R. K. (1989). Genetic control of sexually dimorphic axon morphology in Drosophila sensory neurons. Dev. Biol. 132, 448–457 10.1016/0012-1606(89)90241-8 [DOI] [PubMed] [Google Scholar]
- Rideout E. J., Dornan A. J., Neville M. C., Eadie S., Goodwin S. F. (2010). Control of sexual differentiation and behavior by the doublesex gene in Drosophila melanogaster. Nat. Neurosci. 13, 458–466 10.1038/nn.2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinett C. C., Vaughan A. G., Knapp J. M., Baker B. S. (2010). Sex and the single cell. II. There is a time and place for sex. PLoS Biol. 8, e1000365 10.1371/journal.pbio.1000365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryner L. C., Goodwin S. F., Castrillon D. H., Anand A., Villella A., Baker B. S., Hall J. C., Taylor B. J., Wasserman S. A. (1996). Control of male sexual behavior and sexual orientation in Drosophila by the fruitless gene. Cell 87, 1079–1089 10.1016/S0092-8674(00)81802-4 [DOI] [PubMed] [Google Scholar]
- Shirangi T. R., Stern D. L., Truman J. W. (2013). Motor control of Drosophila courtship song. Cell Rep. 5, 678–686 10.1016/j.celrep.2013.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siwicki K. K., Kravitz E. A. (2009). Fruitless, doublesex and the genetics of social behavior in Drosophila melanogaster. Curr. Opin. Neurobiol. 19, 200–206 10.1016/j.conb.2009.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starostina E., Liu T., Vijayan V., Zheng Z., Siwicki K. K., Pikielny C. W. (2012). A Drosophila DEG/ENaC subunit functions specifically in gustatory neurons required for male courtship behavior. J. Neurosci. 32, 4665–4674 10.1523/JNEUROSCI.6178-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger P., Kvitsiani D., Rotkopf S., Tirián L., Dickson B. J. (2005). Neural circuitry that governs Drosophila male courtship behavior. Cell 121, 795–807 10.1016/j.cell.2005.04.026 [DOI] [PubMed] [Google Scholar]
- Thistle R., Cameron P., Ghorayshi A., Dennison L., Scott K. (2012). Contact chemoreceptors mediate male–male repulsion and male–female attraction during Drosophila courtship. Cell 149, 1140–1151 10.1016/j.cell.2012.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda H., Zhao X., Dickson B. J. (2012). The Drosophila female aphrodisiac pheromone activates ppk23+ sensory neurons to elicit male courtship behavior. Cell Rep. 1, 599–607 10.1016/j.celrep.2012.05.007 [DOI] [PubMed] [Google Scholar]
- Verhulst E. C., van de Zande L., Beukeboom L. W. (2010). Insect sex determination: it all evolves around transformer. Curr. Opin. Genet. Dev. 20, 376–383 10.1016/j.gde.2010.05.001 [DOI] [PubMed] [Google Scholar]
- Vervoort M., Zink D., Pujol N., Victoir K., Dumont N., Ghysen A., Dambly-Chaudière C. (1995). Genetic determinants of sense organ identity in Drosophila: regulatory interactions between cut and poxn. Development 121, 3111–3120. [DOI] [PubMed] [Google Scholar]
- Villella A., Hall J. C. (2008). Neurogenetics of courtship and mating in Drosophila. Adv. Genet. 62, 67–184 10.1016/S0065-2660(08)00603-2 [DOI] [PubMed] [Google Scholar]
- Villella A., Gailey D. A., Berwald B., Ohshima S., Barnes P. T., Hall J. C. (1997). Extended reproductive roles of the fruitless gene in Drosophila melanogaster revealed by behavioral analysis of new fru mutants. Genetics 147, 1107–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Anderson D. J. (2010). Identification of an aggression-promoting pheromone and its receptor neurons in Drosophila. Nature 463, 227–231 10.1038/nature08678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Dankert H., Perona P., Anderson D. J. (2008). A common genetic target for environmental and heritable influences on aggressiveness in Drosophila. Proc. Natl. Acad. Sci. USA 105, 5657–5663 10.1073/pnas.0801327105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Han X., Mehren J., Hiroi M., Billeter J. C., Miyamoto T., Amrein H., Levine J. D., Anderson D. J. (2011). Hierarchical chemosensory regulation of male–male social interactions in Drosophila. Nat. Neurosci. 14, 757–762 10.1038/nn.2800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K., Toba G., Koganezawa M., Yamamoto D. (2011). Gr39a, a highly diversified gustatory receptor in Drosophila, has a role in sexual behavior. Behav. Genet. 41, 746–753 10.1007/s10519-011-9461-6 [DOI] [PubMed] [Google Scholar]
- Willingham A. T., Keil T. (2004). A tissue specific cytochrome P450 required for the structure and function of Drosophila sensory organs. Mech. Dev. 121, 1289–1297 10.1016/j.mod.2004.04.017 [DOI] [PubMed] [Google Scholar]
- Wu J. S., Luo L. (2006). A protocol for dissecting Drosophila melanogaster brains for live imaging or immunostaining. Nat. Protoc. 1, 2110–2115 10.1038/nprot.2006.336 [DOI] [PubMed] [Google Scholar]
- Yew J. Y., Dreisewerd K., Luftmann H., Müthing J., Pohlentz G., Kravitz E. A. (2009). A new male sex pheromone and novel cuticular cues for chemical communication in Drosophila. Curr. Biol. 19, 1245–1254 10.1016/j.cub.2009.06.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.