Abstract
Emerging data suggest that mechanisms to evade the human immune system may be shared by the conceptus, tumour cells, persistent pathogens and viruses. It is therefore timely to revisit the human fetoembryonic defense system (Hu-FEDS) hypothesis that was proposed in two papers in the 1990s. The initial paper suggested that glycoconjugates expressed in the human reproductive system inhibited immune responses directed against gametes and the developing human by employing their carbohydrate sequences as functional groups. These glycoconjugates were proposed to block specific binding interactions and interact with lectins linked to signal transduction pathways that modulated immune cell functions. The second article suggested that aggressive tumour cells and persistent pathogens (HIV, H. pylori, schistosomes) either mimicked or acquired the same carbohydrate functional groups employed in this system to evade immune responses. This subterfuge enabled these pathogens and tumour cells to couple their survival to the human reproductive imperative. The Hu-FEDS model has been repeatedly tested since its inception. Data relevant to this model have also been obtained in other studies. Herein, the Hu-FEDS hypothesis is revisited in the context of these more recent findings. Far more supportive evidence for this model now exists than when it was first proposed, and many of the original predictions have been validated. This type of subterfuge by pathogens and tumour cells likely applies to all sexually reproducing metazoans that must protect their gametes from immune responses. Intervention in these pathological states will likely remain problematic until this system of immune evasion is fully understood and appreciated.
Keywords: AIDS, galectins, Siglecs, Lewis antigens, immune evasion
Introduction
Recent data suggest that mechanisms to evade the human immune system may be shared by the conceptus, tumour cells, persistent pathogens and viruses. It is therefore timely to revisit the human fetoembryonic defense system (Hu-FEDS) hypothesis that was proposed in two papers in the 1990s (Clark et al., 1996; 1997). At that time there was little evidence to support this hypothesis. Herein, the Hu-FEDS hypothesis is revisited in the context of more recent studies that are relevant to this experimental model.
In 1953, Sir Peter Medawar defined one of the greatest enigmas in immunology. His specific question was ‘how does the pregnant mother nourish within itself for many weeks or months a fetus that is antigenically a foreign body?’ (Medawar, 1953). He cited three possible reasons why the ‘fetal transplant’ was not rejected by the mother: (i) antigenic immaturity of the fetus; (ii) immunological indolence or inertness of the mother and (iii) anatomical separation from the mother (Medawar, 1953).
Immunity is readily induced in skin transplantation tests by injections of fetal tissue, confirming that the fetus is antigenically mature (Billingham et al., 1956). Women respond to pathogens during pregnancy, arguing against maternal indolence (Head and Billingham, 1986). Human endovascular trophoblasts of placental origin invade and remodel the maternal spiral arteries to enable increased blood flow to the placenta, indicating intimate fetomaternal contact (Burton and Jauniaux, 2004). In short, the hypotheses proposed by Medawar were not supported by subsequent investigations.
In the first Hu-FEDS hypothesis article (Clark et al., 1996), a specific glycoprotein [glycodelin-A (GdA)] and mucins present in the placenta, amniotic fluid and decidua were implicated as factors that suppress the maternal immune response in the pregnant uterus. These glycoconjugates were suggested to manifest their effects by employing their glycans as functional groups to block immune cell binding or interact with lectin-like receptors coupled to signal transduction proteins that modulate immune responses. Another major emphasis of the Hu-FEDS model was the concept that the carbohydrate sequences on the surface of human gametes are also employed as functional groups for immune deviation (Clark et al., 1996). Factors in human seminal plasma and the pregnant uterus had previously been shown to inhibit immune responses in vitro (Bolton et al., 1987; Kelly and Critchley, 1997). However, the concept that human gametes could present specific signals to modulate immune responses was novel.
In the second Hu-FEDS hypothesis article, this model was further expanded (Clark et al., 1997). The suggestion was made that persistent pathogens and aggressive tumour cells either acquired or mimicked the same carbohydrate functional groups that were employed to modulate immune responses directed against gametes and the developing human in utero. HIV, schistosomes and H. pylori were presented as major pathogens that exploited this system of protection (Clark et al., 1997).
Since 1996, many different pathways of immune modulation have been implicated in the induction of tolerance to the developing eutherian in utero that are independent of carbohydrate recognition. They have been extensively reviewed (Moffett and Loke, 2006; Trowsdale and Betz, 2006; Prins et al., 2012; Arck and Hecher, 2013; Erlebacher, 2013). Similarly, pathogens and tumour cells employ many pathways of immune evasion that do not rely on lectin-like interactions (Cohen, 1985; Whiteside, 2009; Wilson, 2012; Matsuura, 2013). The Hu-FEDS hypothesis is concerned primarily with the roles of glycoconjugates and lectins in the induction of tolerance required to fulfill the reproductive imperative and their linkages to pathogenesis. This model has been repeatedly tested since it was initially proposed. The results of many other studies have also greatly impacted this paradigm, and this evidence will be presented in this review.
Immune recognition of human eggs
Human zona pellucida (ZP) glycans were predicted to mediate both sperm binding and immune recognition (Clark et al., 1996). This suggestion was based on the ability of fucoidan and the sialyl-Lewisx tetrasaccharide (sLex) to inhibit sperm binding in the human hemizona assay (Table I) (Huang et al., 1982; Clark et al., 1995). sLex is the universal ligand for selectins, cell adhesion molecules that mediate initial neutrophil binding to inflamed endothelium (Foxall et al., 1992). Fucoidan is a potent inhibitor of lymphocyte homing, a process that also relies on selectin-mediated adhesions (Imai et al., 1993). However, selectins were not detected on human sperm, leading to the proposal that human sperm–egg binding involved a ‘selectin-like interaction’ (Patankar et al., 1993; Clark et al., 1995). Though not a selectin, the binding specificity of the human egg binding protein was anticipated to overlap with these cell adhesion molecules. The possibility that sLex was being employed for both immune and gamete adhesions suggested that immune cells could also recognize the human egg, perhaps evoking protective responses (Clark et al., 1996).
Table I.
Terminal carbohydrate sequences referred to in the text.
| Name | Symbol | Sequence |
|---|---|---|
| Sialyl-Lex | 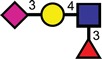 |
 |
| 3-Sulpho-Lex | 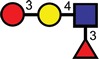 |
 |
| Lewisx | 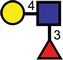 |
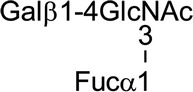 |
| Lewisy | 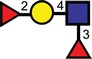 |
 |
| Fucosylated LacdiNAc | 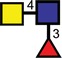 |
 |
| Pseudo-Lewisy | 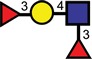 |
 |
 , N-acetylneuraminic acid;
, N-acetylneuraminic acid;  , galactose;
, galactose;  , N-acetylglucosamine;
, N-acetylglucosamine;  , fucose;
, fucose;  , sulphate (S);
, sulphate (S);  , N-acetylgalactosamine.
, N-acetylgalactosamine.
Recent studies have confirmed that the human ZP is profusely coated with sLex on both N- and O-glycans (Figs 1 and 2) (Pang et al., 2011). A minor amount of N-glycans terminated with another selectin ligand (sulpho-Lewisx) was also detected (Table I). sLex also inhibited human sperm–ZP binding in either the intact or multivalent neoglycoprotein form (Pang et al., 2011). These results confirmed the carbohydrate binding specificity for the human sperm–egg interaction that was originally proposed two decades ago (Patankar et al., 1993). sLex also binds to Siglec-9, an immunoglobulin-like lectin receptor that bears an immunoreceptor tyrosine-based inhibitory motif associated with many types of immune cells (Angata and Varki, 2000; Avril et al., 2004). The binding of sLex to Siglec-9 could induce an inhibitory signal in immune cells that encounter an egg. Activated neutrophils or other immune cells in the infected or inflamed uterus could bind to multivalent sLex on the human ZP, competing with sperm binding and inhibiting fertilization. Under normal quiescent conditions, multivalent sLex on the ZP could mediate human sperm binding and inhibit potential responses by Siglec-9 expressing immune cells. The presentation of sLex on the human ZP in this context could ensure that pregnancy proceeds only within a healthy uterine environment (Clark, 2013).
Figure 1.
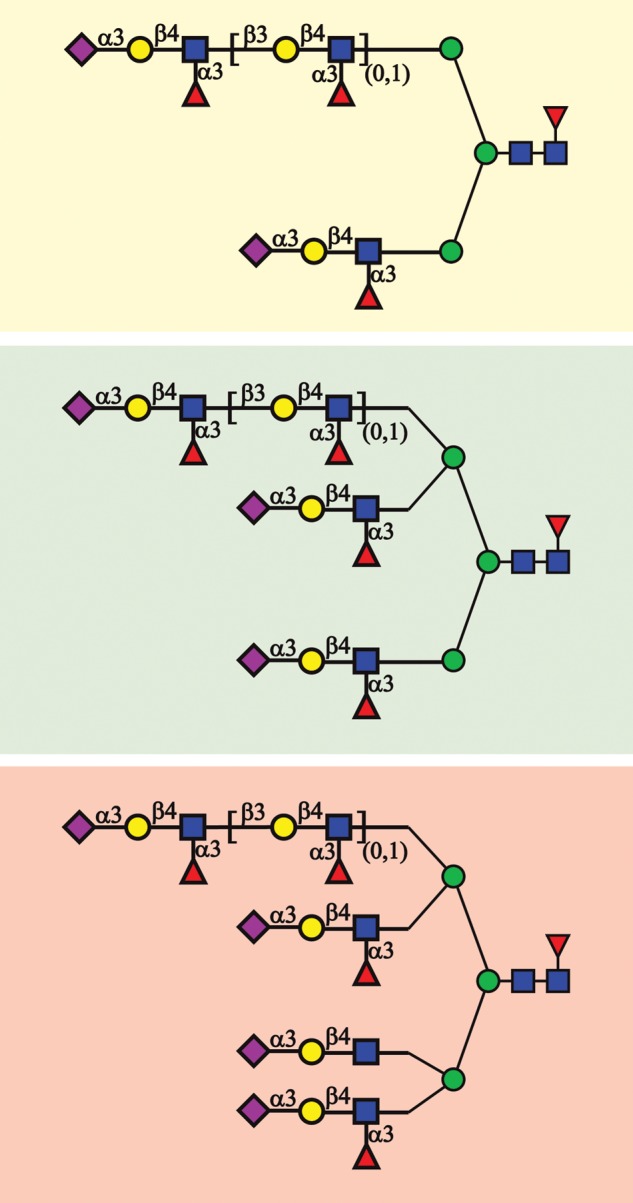
Major human ZP N-glycans. sLex sequences are in dashed boxes. This figure is adapted from a previous study (Pang et al., 2011).
Figure 2.
Human ZP-associated O-glycans. sLex sequences are in the dashed boxes. This figure is adapted from a previous study (Pang et al., 2011).
Immune recognition of human sperm
Testicular germ cells differentiate into sperm following the initiation of puberty, long after the period of thymic education (Fijak and Meinhardt, 2006). Sperm-specific proteins that are not tolerized during early development are autoantigens (neoantigens). The induction of autoimmune orchitis following the injection of autologous testicular homogenates distal to the testes confirms that such antigens are foreign (Tung et al., 1981). However, the testis is an immune privileged site due to its ability to tolerate allografts and xenografts (Head and Billingham, 1985). This immune privilege was initially explained by a blood–testis barrier (Setchell, 1967; Dym, 1973). However, autoantigens are present in the basal compartment of the testis, which lack this barrier (Yule et al., 1988; Saari et al., 1996).
Despite repeated challenge with sperm proteins, only ∼2–3% of women will ever develop antisperm antibodies associated with subfertility or infertility (Rumke and Hellinga, 1959; Lombardo et al., 2001). The incidence of allergic reactions to human sperm is also rare (Sublett and Bernstein, 2011). These results indicate that human sperm and seminal plasma must have very powerful means of attenuating immune responses directed against sperm autoantigens in the male and female reproductive systems.
Major histocompatibility (MHC) antigens in humans are referred to as human leukocyte antigens (HLAs). Sperm and eggs completely lack HLA class I and II antigens (Hutter and Dohr, 1998). Natural killer (NK) cells lyse cells lacking HLA class I antigens, a concept known as ‘missing self’ (Karre, 2002). NK cells are the predominant immune cell type in the human uterus, indicating that they could target sperm (King et al., 1991). Structural analysis of the oligosaccharides derived from classical HLA class I molecules confirms that 35–92% are biantennary bisecting type N-glycans (Fig. 3) (Barber et al., 1996). HLA class I negative K562 erythroleukemia cells evade lysis by NK cells if they express a sufficient level of these N-glycans on their plasma membranes, indicating that NK cells also survey the oligosaccharides on target cells (el Ouagari et al., 1995; Yoshimura et al., 1996).
Figure 3.
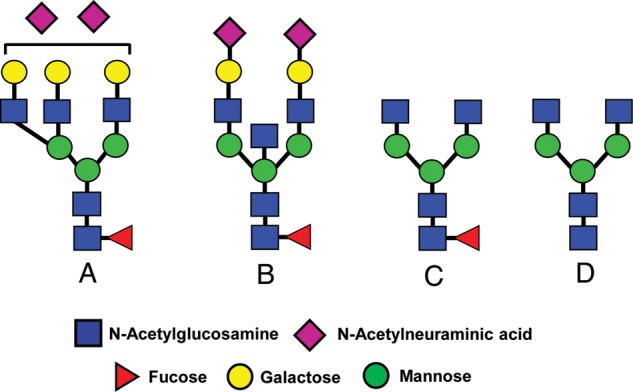
Restricted heterogeneity of N-glycans associated with classical class I molecules (HLA-A, -B, -C). These structures are referred to as triantennary N-glycan (A), biantennary bisecting type N-glycans (B) or truncated N-glycans (A and B). In A, the exact positions of the N-acetylneuraminic residues were not defined.
Glycomic analysis of human sperm has now confirmed the substantial expression of biantennary bisecting type N-glycans on these cells (Pang et al., 2007). These N-glycans bind to erythroagglutinating phytohemagglutinin (E-PHA) (Cummings and Kornfeld, 1982; Yamashita et al., 1983). The binding of E-PHA to the plasma membrane of human sperm indicates that these N-glycans are localized to the surface of these gametes (Lee and Damjanov, 1985; Cross and Overstreet, 1987). Sequencing of human sperm oligosaccharides also revealed the profligate expression of Lewis antigens (Lex, Ley), many in tri- and tetravalent presentations on a single N-glycan (Table I, Fig. 4) (Pang et al., 2007). Most human sperm are immunostained with anti-Ley monoclonal antibody (mAb), and there is uniform binding of this mAb to the inner acrosomal membrane and acrosomal contents (Pang et al., 2007). Lex and Ley are carbohydrate ligands for DC-SIGN, a C-type lectin receptor (CLR) expressed on dendritic cells that is associated with potent immune tolerizing effects (Gringhuis et al., 2009).
Figure 4.
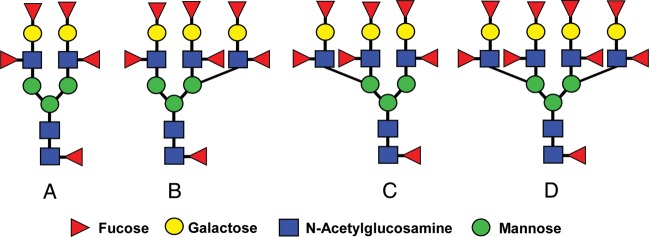
The expression of N-glycans with multivalent Ley on human sperm and seminal plasma glycoproteins. Biantennary, (A), triantennary (B and C) and tetraantennary N-glycans (D) terminated on each antenna with Ley (Table I) are expressed on human sperm and seminal plasma glycoproteins (Pang et al., 2007, 2009). Heterogeneous intermediates terminated with different combinations of Lex, Ley or lacNAc (Galβ1-4 GlcNAc) on their antenna are also present.
Human seminal plasma glycoproteins
Seminal plasma contains several immune-deviating factors that do not rely on carbohydrate recognition (Kelly, 1999; Robertson et al., 2009). The possibility was considered that glycoproteins in this fluid could also evoke immune tolerance. Carbohydrate sequencing confirmed that seminal plasma glycoproteins are decorated with exactly the same types of unusual multivalent Lex and Ley type N-glycans observed in sperm (Pang et al., 2009). Mucin-associated O-glycans and free oligosaccharides in seminal plasma are also abundantly decorated with Lex and Ley (Hanisch et al., 1986; Chalabi et al., 2002). Unlike other normal tissues, these Lewis carbohydrate antigens are prevalent in the male reproductive system.
Glycoprotein ligands for CLRs like DC-SIGN have been implicated as the preferred mediators of immune homeostasis (Garcia-Vallejo and van Kooyk, 2009). Clusterin, galectin-3-binding protein, prostatic acid phosphatase and protein C inhibitor were recently identified as the major endogenous glycoprotein ligands for DC-SIGN in human seminal plasma (Clark et al., 2012). These glycoproteins likely supplement transforming growth factor (TGF)-β, prostaglandins, spermine and prostasomes to evoke immune privilege in the male reproductive system (Kelly, 1999; Robertson et al., 2009). They also modulate immune responses in the female reproductive system after insemination (Clark and Schust, 2013).
Glycodelin-A
GdA was originally designated as a major component of the Hu-FEDS hypothesis (Clark et al., 1996). GdA is a 27 kDa endometrial glycoprotein that is secreted from the mid-luteal phase until the end of the first trimester (Julkunen et al., 1985; Dalton et al., 1995). It is a major decidual product between 7 and 11 weeks of gestation, constituting 4–16% of the total protein in this tissue (Julkunen et al., 1991). GdA is secreted into the amniotic fluid, and taken up and concentrated in the placenta (Julkunen et al., 1986). The expression of GdA declines precipitously in the uterus after the 20th week of gestation, becoming a minor decidual component at term (Julkunen et al., 1991).
The immune-deviating effects of GdA have been the subject of a recent review (Clark and Schust, 2013). This glycoprotein: (i) blocks mitogen-induced proliferation of T cells; (ii) inhibits IL-2 production in activated T cells; (iii) promotes apoptosis in activated T cells; (iv) interacts with CD45 on T cells by employing a lectin-like activity; (v) blocks NK cell lysis of K562 erythroleukemia cells; (vi) decreases the release of IgM and the expression of MHC class II molecules by B lymphocytes; (vii) inhibits the chemoattractant-stimulated migration of monocytes; (viii) blocks E-selectin-mediated adhesion of neutrophils and (ix) induces the release of IL-6 from monocytes and macrophages by binding to L-selectin and an extracellular signal-regulated kinase. This glycoprotein is also a ligand for Siglec-6, an immune lectin expressed on the surface of human syncytiotrophoblasts and cytotrophoblasts (Lam et al., 2011). GdA is also a potent inhibitor of sperm binding in the human hemizona assay (Oehninger et al., 1995).
Seminal plasma also contains an isoform of GdA designated GdS (Julkunen et al., 1984). This isoform has the same protein backbone as GdA, but does not induce any of the same immune-deviating effects. GdA and GdS are also decorated with very different oligosaccharides, implicating the N-glycans linked to GdA as functional groups (Fig. 5) (Dell et al., 1995; Morris et al., 1996; Lee et al., 2009). The major antennae on GdA-associated N-glycans are α2–6 sialylated or fucosylated lacdiNAc and Sda sequences (Dell et al., 1995; Lee et al., 2009). The fucosylated lacdiNAc sequence was previously implicated as a selectin ligand, consistent with the observation that GdA inhibits E-selectin-mediated adhesions (Table I) (Grinnell et al., 1994; Jeschke et al., 2003). Like Lex and Ley, the fucosylated lacdiNAc sequence is also a carbohydrate ligand for DC-SIGN, as noted previously a CLR associated with several immunomodulatory effects (van Liempt et al., 2006; Gringhuis et al., 2009).
Figure 5.
Glycans associated with GdA and GdS. A total of 44 different N-glycans were previously identified in GdA (Lee et al., 2009). This glycoprotein is decorated primarily with complex type N-glycans and only very marginal amounts of high mannose/hybrid type N-glycans. Antenna are attached to biantennary (Bi), triantennary (Tri) and tetraantennary N-glycans (Tetra) via β1–2, β1–4 or β1–6 linkages to the trimannosyl core (L1–L3, respectively). The majority of the antennae contain the unusual lacdiNAc sequence (GalNAcβ1-4 GlcNAc) in intact, fucosylated or sialylated forms (A1–A3). The remaining antennae (A4–A8) are based on the conventional lacNAc sequence (Galβ1-4GlcNAc). The terminal lacNAc sequence on A8 is also modified with fucose, N-acetylgalactosamine and sialic acid to generate the same sequences shown in A5–A7. In contrast, the N-glycans linked to GdS are high mannose and biantennary complex types with only nine structures identified (Morris et al., 1996). GdS does not bind to DC-SIGN, even though this glycoprotein bears high mannose type N-glycans, Lewisx and Lewisy. This CLR displays preferential binding to seminal plasma glycoproteins bearing triantennary and tetraantennary N-glycans terminated with multivalent Lewisx and Lewisy sequences (Clark et al., 2012).
The highly elevated expression of GdA plus its known activities should confirm that this glycoprotein is a major immunomodulatory factor during pregnancy. Nonetheless, GdA is virtually never mentioned in reviews focused on the induction of the tolerant state during human pregnancy (Moffett and Loke, 2006; Trowsdale and Betz, 2006; Arck and Hecher, 2013; Erlebacher, 2013). GdA is not a classical immune molecule nor is there a murine analogue, precluding knockout strategies that test its physiological significance in mice. However, there are numerous anatomical, biochemical and physiological differences between murine and human reproduction (Duc-Goiran et al., 1999; Arck and Hecher, 2013).
Humans and other higher primates display a deeper level of haemochorial implantation than other eutherians (Duc-Goiran et al., 1999; Clancy, 2009; Pijnenborg et al., 2011). Pre-eclampsia is a disease of pregnant women and a small number of chimpanzees and gorillas (Pijnenborg et al., 2011). This pathological condition has been linked to an inadequate depth of haemochorial implantation and deficient remodeling of the spiral arteries (Pennington et al., 2012). GdA could play a crucial role in human implantation. Surplus chorionic villous sampling tissues were collected at 10–12 week of gestational age, banked and analysed for gene expression by microarray analysis after pregnancy outcomes were determined. The mRNA for GdA was decreased by 15.6-fold in the decidua of women who developed pre-eclampsia compared with term pregnancies (Founds et al., 2009). This finding indicates that GdA could be a key factor that promotes deep haemochorial implantation in humans, and explain why a functional analogue is not present in mice. Aberrant glycosylation of GdA could also result in the development of pre-eclampsia. Clearly, this evidence indicates that GdA is essential for the development of normal human pregnancies.
Cancer antigen 125 (CA125, MUC16)
CA125 was not discussed at all in the Hu-FEDs papers because its glycosylation was not defined at that time. CA125 was initially identified as a tumour-associated antigen in ovarian cancer patients (Bast et al., 1981). It is an enormous mucinous glycoprotein (24 000 amino acids) that is highly decorated with both N- and O-glycans (Kui Wong et al., 2003). Like GdA, CA125 is an endometrial product that is highly up-regulated in uterine flushings during the mid-luteal phase and first trimester, placing it in a temporospatial position where it could act as a major immune-deviating factor during the early stages of pregnancy (Dalton et al., 1995).
The initial question that arose during preliminary studies was which immune cell type could be targeted by CA125 during both early pregnancy and ovarian tumour development. Uterine NK (uNK) cells are present in small numbers in the proliferative and early secretory phase endometrium, but their numbers increase substantially during the late secretory phase, becoming the predominant immune cell type (King et al., 1991). NK cells in the peripheral blood can be divided into two different populations, CD16posCD56dim cytotoxic cells that constitute 90% of the total and CD16dim/negCD56bright cells lacking cytotoxic activity that make up the remaining 10% (Nagler et al., 1989). uNK cells are also CD16dim/negCD56bright cells with low cytotoxic activity (King et al., 1991; Kopcow et al., 2005).
Trophoblasts secrete a chemokine (MIP-1α), which sequesters uNK cells at the site of implantation, where they come into direct contact with the human embryo (Drake et al., 2001). Syncytiotrophoblasts on the surface of the implanting human embryo lack HLA class I molecules, which could make them sensitive to lysis by uNK cells based on the ‘missing self’ hypothesis (Carbone et al., 1996; Blaschitz et al., 2001). However, syncytiotrophoblasts are completely resistant to killing by uNK cells, and only partially susceptible to lymphokine-activated uNK cells (King and Loke, 1990). These changes in marker expression and lytic activity led to the hypothesis that CA125 could be a major factor that affects the cytotoxicity of uNK cells and NK cells in the peritoneal cavity of ovarian cancer patients.
CA125 was isolated from an ovarian carcinoma cell line (OVCAR-3) and analysed for its carbohydrate expression (Kui Wong et al., 2003). Incubation of peripheral blood NK cells with CA125 at physiological concentrations present in the pregnant uterus for 3 days led to a 50–70% decline in their ability to kill K562 target cells (Patankar et al., 2005). Lymphokine-activated NK cells were inhibited to the same extent as circulating NK cells. This exposure did not change the expression of any NK cell marker except for CD16, which was reduced by 40–70% (Patankar et al., 2005). As noted previously, uNK cells are CD16dim/neg cells (King et al., 1991). The cytolytic activity of uNK cells is decreased by 85% compared with peripheral blood NK cells (Kopcow et al., 2005). These findings indicate that CA125 promotes the uNK phenotype.
The immune-deviating activity of CA125 on NK cell cytotoxicity and CD16 marker expression relies completely on its ability to bind to Siglec-9 on the surface of NK cells (Belisle et al., 2010). CA125 does not bind to NK cells after the removal of its terminal sialic acid residues. These results indicate that CA125 employs its carbohydrate sequences as functional groups to induce immune deviation in NK cells.
Uromodulin
Uromodulin was also not discussed in the original Hu-FEDS papers. This immune-deviating glycoprotein was initially detected in the urine of pregnant women (Muchmore and Decker, 1985). Subsequent studies confirmed that uromodulin is a pregnancy-associated isoform of Tamm–Horsfall glycoprotein (THP) (Tamm and Horsfall, 1952; Hession et al., 1987). Uromodulin is a considerably more potent inhibitor of antigen-induced T cell proliferation than THP derived from either men or non-pregnant women (Hession et al., 1987).
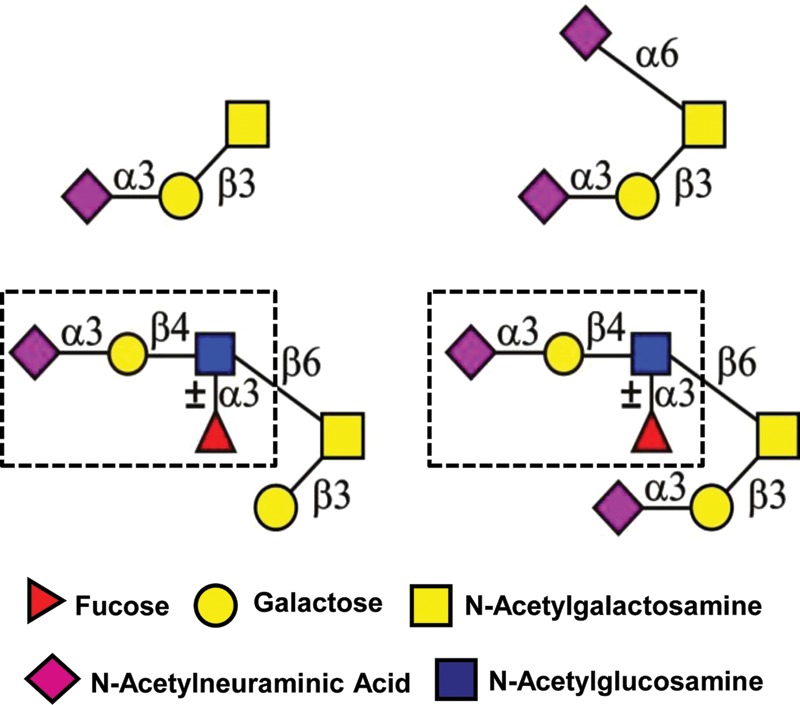
In early studies, the glycans linked to uromodulin were implicated as potential functional groups that enabled its immune-deviating activities (Muchmore et al., 1987). However, this claim was not confirmed in 1996. Dell and coworkers later performed extensive structural analysis of uromodulin and THP to determine if any differences existed between the isoforms (Easton et al., 2000). They observed no major changes in N-glycosylation between them. However, unusual core 2 type O-glycans bearing one, two or three sLex terminals were detected on uromodulin (Fig. 6). THP obtained from non-pregnant females and males was decorated with simple core 1 type O-glycans (Easton et al., 2000). The glycosylation of uromodulin shifted almost completely back to the THP glycoforms 2 months after parturition (Easton et al., 2000). No other changes were detected in uromodulin compared with THP, confirming that differential O-glycosylation was responsible for the greatly enhanced immune-deviating activity of uromodulin.
Figure 6.
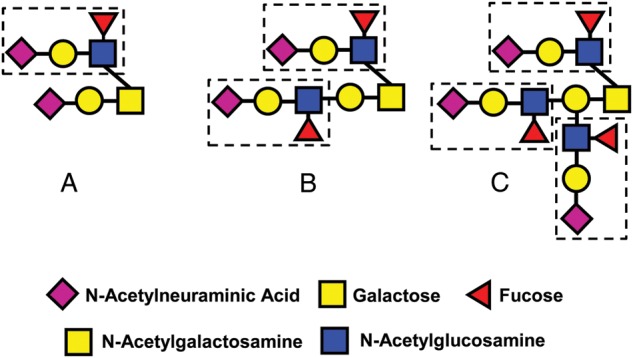
O-Glycans associated with uromodulin. sLex is in each dashed box. O-Glycans bearing one (A), two (B) or three of these sequences are present in uromodulin, but not in THP.
The precise functional role of uromodulin during pregnancy remains to be determined. Nonetheless, these results support the concept that uromodulin O-glycans act as functional groups to enable its immune-deviating activities during pregnancy.
Galectins
Galectins are a family of small soluble lectins that have one or two carbohydrate recognition domains with affinity for lactose and/or N-acetyllactosamine (lacNAc) (Barondes et al., 1994). Galectins often possess domains that accommodate modifications of lacNAc with other monosaccharides (Cummings and Liu, 2009). Multivalent presentations of lacNAc or polyvalent forms of this disaccharide (polylactosamine) substantially increase the affinity of some galectins for specific glycans bearing such sequences (Hirabayashi et al., 2002; Stowell et al., 2008a; Vasta et al., 2012). Because they often dimerize and in some cases oligomerize, galectins form lattices between glycoproteins on the surface of cells and between cells (Demetriou et al., 2001; Vasta et al., 2012). This property enables them to promote a plethora of biological activities in many different biological pathways (Vasta et al., 2012).
Before 1999, galectins had not been detected in the eutherian uterus. Interest in the role of galectins during development was heightened by the observation that human placental protein (PP13) is a galectin (Gal-13) (Than et al., 1999). There is currently evidence for the expression of 16 different human galectin genes at the fetomaternal interface (Than et al., 2009). Galectins induce many immune-deviating effects in T cells, B cells, neutrophils, macrophages, eosinophils, mast cells and basophils (Cummings and Liu, 2009; Than et al., 2012).
Gal-1 is the best studied galectin among those up-regulated in the human placenta during pregnancy (Blidner and Rabinovich, 2013). Serum levels of Gal-1 increase during the first trimester, peak during the second trimester and remain elevated until parturition in pregnant women (Tirado-Gonzalez et al., 2013). Gal-1 promotes the apoptosis of alloreactive T cells as well as the development of tolerogenic DCs and T regulatory cells (Blois et al., 2007; Kopcow et al., 2008). This galectin also induces the expression of HLA-G on trophoblasts during the initial stages of human pregnancy (Tirado-Gonzalez et al., 2013). Another galectin (Gal-3) is present in human semen (Jones et al., 2010). This galectin promotes the apoptosis of primary-activated human T cells, indicating that it could induce immune deviations in the male and female reproductive systems (Stowell et al., 2008b). Ligands for galectins are also expressed on the surface of the murine ZP, suggesting that these gametes could be protected by these immune modulators (Clark and Dell, 2006). Galectins are up-regulated in tumour cells and bind to pathogens, indicating that they could be employed for immune deviations that promote their survival (Vasta, 2009; Cedeno-Laurent and Dimitroff, 2012; Rabinovich and Croci, 2012).
HIV and AIDS
A major focus of the second Hu-FEDS paper was to define how HIV could employ its carbohydrate functional groups to modify immune responses leading to the development of AIDS (Clark et al., 1997). The key linchpin was considered to be the ability of HIV to specifically infect CD4+ T cells, providing access to the glycosylation machinery in this cell type (Barre-Sinoussi et al., 1983). This integration enabled HIV glycoproteins to acquire glycans that normally acted as functional groups during T cell interactions. In 1996, there were many reports indicating that structural similarities existed between HIV glycoproteins and native T cell glycoproteins (Golding et al., 1988, 1989; Imberti et al., 1991; Dalgleish et al., 1992; Levy, 1993). The three-dimensional structure of a protein combined with the location of its specific glycosylation sites are essential for determining the sequence of the glycans linked to a glycoprotein. This complementarity meant that HIV could direct the synthesis of proteins that could aberrantly activate or suppress immune responses. Because of the high mutation rate of HIV, many glycoproteins with slightly different protein structures could be generated, thus enabling mutant viruses to acquire different carbohydrate functional groups (Clark et al., 1997).
Several effects of this manoeuvering were predicted including: (i) HIV isotypes expressing the ‘right’ carbohydrate sequences could suppress immune responses directed against them; (ii) the glycans on these variant HIV could bind to the carbohydrate receptors of different cell types, enabling them to infect other immune and non-immune cell types and (iii) soluble or cell surface-associated HIV glycoproteins could inhibit normal immune functions or induce aberrant activation of the immune responses. HIV mutants would be selected for appropriate composite glycoproteins that could overcome the human immune system by combining their high mutational rate with the T cell lineage glycosylation system.
Another interesting linkage was also suggested in the original Hu-FEDS model. Ley is normally expressed on a low percentage (5–8%) of CD4+ and CD8+ T lymphocytes in the circulation (Table I). However, after HIV infection, the proportion of Ley positive CD4+ and CD8+ T lymphocytes gradually increases over time to 20–25% (Adachi et al., 1988; Kashiwagi et al., 1994). This elevation is positively correlated with the severity of the immune suppression observed in patients. This increased expression is likely directly induced by HIV, since the percentage of human H9 lymphoblastoid cells bearing Ley increases from 12 to 97% after infection (Adachi et al., 1988; Kashiwagi et al., 1994). This aberrant expression of a DC-SIGN ligand could promote inappropriate T cell binding and signalling interactions with dendritic cells. Such interactions could also selectively protect HIV-infected cells, providing a reservoir for the virus. As outlined in this review, Ley or a close structural analogue is expressed on many persistent pathogens and human sperm.
The glycans associated with the major capsid glycoprotein of HIV (gp120) were subsequently implicated in the promotion of viral infection. gp120 contains a very high percentage of high mannose type N-glycans (Geyer et al., 1988; Mizuochi et al., 1990; Bonomelli et al., 2011). DC-SIGN binds not only to fucosylated sequences like Lex and Ley, but also to high mannose type N-glycans (Feinberg et al., 2001). HIV binds to dendritic cells via the interaction of its high mannose type N-glycans on gp120 with DC-SIGN (Geijtenbeek et al., 2000). This presentation promotes efficient HIV infection of CD4+ T cells. The interaction of DC-SIGN with different carbohydrate sequences on gp120 could promote signalling that leads to either immune suppression or activation (Geijtenbeek and Gringhuis, 2009). This flexibility provides HIV with the ability to modulate adaptive immune responses depending on the glycosylation of its glycoproteins. HIV promotes immune activation following initial infection, but induces immune suppression during the development of AIDS (Fauci, 1993). Viral capsid-associated gp120 almost exclusively acquires high mannose type N-glycans in human and monkey cell types infected in vitro (Geyer et al., 1988; Bonomelli et al., 2011). However, the N-glycans acquired by gp120 in vivo when AIDS is manifested have not been defined.
Simian immunodeficiency virus
Connections to simian immunodeficiency virus (SIV) infections were not considered in the second Hu-FEDS article. However, they were discussed in another paper published later that same year (Clark and Patankar, 1997). Modern AIDS vaccination strategies seek to block HIV infection, but this pathway is not required to prevent the development of AIDS in the natural hosts of SIV. The predominant mechanism for escaping the pathological effects of SIV is the induction of tolerance. Many species of African monkeys are infected with their own species-specific variant of SIV, but very few ever develop symptoms associated with AIDS (Daniel et al., 1987; Aghokeng and Peeters, 2005). The two major experimental animal models for investigating the effects of homologous SIV infection are African green monkeys (AGM) and sooty mangabeys (SM) infected with SIVagm and SIVsm, respectively (Kraus et al., 1989; Silvestri et al., 2003). AGMs and SMs display a profound lifelong viremia that is greater than or equal to the highest levels of circulating HIV observed during infection in humans without developing AIDS (Broussard et al., 2001).
There are currently four hypotheses that have been proposed to explain how SIVsm fails to induce simian AIDS in its natural hosts (Chahroudi et al., 2012). However, differential glycosylation of SIV glycoproteins could contribute to this tolerizing effect. The possibility was suggested that SIVagm and SIVsm acquire the same carbohydrate functional groups that are employed to induce tolerance to gametes or the developing monkey in utero (Clark and Patankar, 1997). The immune system of African monkeys would be activated only during the initial stages of infection with their own SIV subtype, and subsequently develop only mild responses to these virions.
There is inferential evidence that supports this hypothesis. Human H9 lymphoblastoid cells were infected with either HIV-1 or SIVsm in previous studies (Geyer et al., 1988; Holschbach et al., 1990). The relative proportion of high mannose type N-glycans was substantially elevated in gp120 isolated from the HIV capsid compared with the corresponding SIVsm glycoprotein (gp130) (92 versus 24%). More importantly, 37% of the N-glycans linked to gp130 were identified as tetraantennary types, in some cases bearing one or two additional lacNAc sequences (Fig. 7) (Holschbach et al., 1990). N-glycans with this structure are high affinity ligands for galectins designated Gal-1 and Gal-3 (Hirabayashi et al., 2002; Stowell et al., 2008a; Song et al., 2009). As discussed previously, galectins possess diverse immune modulating activities (Cummings and Liu, 2009; Than et al., 2012). No tetraantennary N-glycans were detected in gp120 isolated from HIV propagated in H9 cells (Geyer et al., 1988; Mizuochi et al., 1990). However, definitive glycomic analysis of gp130 isolated from SIVsm in the circulation of SMs must be performed to determine if these virions actually acquire galectin ligands. If this acquisition is confirmed, it could help to explain how tolerance to SIVsm is induced in its natural host.
Figure 7.
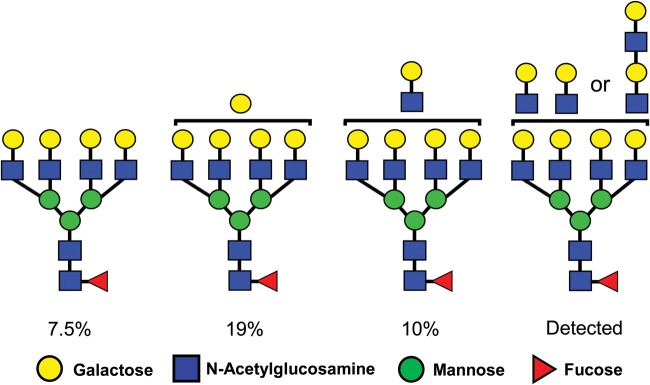
Galectin ligands are abundantly expressed on the major viral capsid glycoprotein isolated from SIVsm produced by infected human H9 lymphoblastoid cells. N-Glycans were isolated from the major viral coat glycoprotein (gp130) of SIVsm propagated in human H9 lymphoblastoid cells (Holschbach et al., 1990). Complex type N-Glycans were digested with neuraminidase to remove sialic acid in α2–3 and α2–6 linkage and sequenced. The percentage of the total N-Glycan fraction is indicated for each oligosaccharide.
In contrast, heterologous infection of macaques with SIVsm or SIVagm results in the development of simian AIDS (Hirsch et al., 1995; Villinger et al., 1996). This differential response was proposed to occur because SIVagm and SIVsm do not acquire the appropriate carbohydrate functional groups necessary to evoke tolerance in macaques (Clark and Patankar, 1997). Comparative glycomic analysis of gp130 isolated from SIVsm propagated in rhesus monkeys and SMs must be performed to determine if they are differentially glycosylated. Heterologous infection with chimpanzee SIV (SIVcpz) is considered to be how HIV-1 was initially introduced into the human population (Sharp and Hahn, 2011).
In conclusion, the results that have been obtained during the investigation of SIV infection of natural and heterologous hosts are consistent with the Hu-FEDS model for AIDS pathogenesis. However, more careful experimentation is necessary to validate the potential linkages discussed here.
Helicobacter pylori
This bacterial species was also proposed to be a Hu-FEDS pathogen (Clark et al., 1997). Infection with H. pylori is the major cause of gastric ulcers and cancers in humans (Marshall, 1983; 1993). This bacterium infected modern humans before they migrated out of Africa, indicating an ancient association with this pathogen (Linz et al., 2007). Thought to be specifically noteworthy about H. pylori in 1996 was the expression of Lex and Ley on the terminal ends of the lipopolysaccharides associated with 81% of all strains (Aspinall et al., 1995; Simoons-Smit et al., 1996). As described earlier, increased Ley expression had also been detected on CD4+ and CD8+ T cells following HIV infection (Adachi et al., 1988; Kashiwagi et al., 1994).
DC-SIGN binds to both Lex and Ley (Appelmelk et al., 2003; Van Die et al., 2003). H. pylori lipopolysaccharides bearing these Lewis antigens have been shown to modulate Th1/Th2 responses in favour of tolerance via their interactions with DC-SIGN (Bergman et al., 2004). H. pylori modulates the expression of these Lewis antigens on its lipopolysaccharides (i.e. phase-variable expression) depending on the level of inflammation that these bacteria encounter (Bergman et al., 2006). More recent studies indicate that lipopolysaccharides bearing Lex and Ley actively dissociate the KSR1-CNK-Raf-1 complex from the signalosome after binding to DC-SIGN (Gringhuis et al., 2009). This dissociation results in the increased secretion of IL-10 from dendritic cells, and decreased expression of IL-12 and IL-6 in a Raf-1-independent but LSP1-dependent manner. This signalling pathway is the basis for a Th1 to Th2 shift in T cell responses that favours tolerance of this bacterial pathogen (Gringhuis et al., 2009). This same skewing of the immune response could also be beneficial for blocking adaptive immune responses directed against human sperm, seminal plasma neoantigens and HIV-infected T cells that express Ley.
Schistosomes and schistosomiasis
Schistosomes are intravascular helminthic parasites that were also previously designated as a Hu-FEDS pathogen (Clark et al., 1997). Chronic infection with schistosomes (schistosomiasis) shifts the immune system from a Th1 to a Th2 response, resulting in immune suppression (Pearce et al., 1991). Mature schistosomes are highly resistant to the human immune response.
Schistosomes were initially suggested to be a Hu-FEDS pathogen because of the considerable expression of terminal fucosylated lacdiNAc and Lex on their tegumental surfaces (Ko et al., 1990; Srivatsan et al., 1992). The fucosylated lacdiNAc sequence is a major antenna associated with GdA-derived N-glycans (Fig. 5) (Dell et al., 1995). As noted previously, this sequence has been implicated as a ligand for both selectins and DC-SIGN (Grinnell et al., 1994; van Liempt et al., 2006). The glycolipids associated with the cercarial forms of Schistosoma mansoni are primarily terminated with Lex and a close structural analogue of Ley known as pseudo-Ley, another DC-SIGN ligand (Table I) (Wuhrer et al., 2000; Meyer et al., 2005).
Cancer and Hu-FEDS
Cancer was another pathological state that was originally linked to the Hu-FEDS hypothesis (Clark et al., 1997). As noted earlier, K562 human erythroleukemia cells are protected from NK cell cytotoxicity by up-regulating their surface expression of biantennary bisecting type N-glycans (Fig. 3) (el Ouagari et al., 1995; Yoshimura et al., 1996). Lectin-binding studies available in 1996 suggested that bisecting type N-glycans were expressed on human sperm and ZP (Cross and Overstreet, 1987; Patankar et al., 1997). However, definitive carbohydrate sequencing studies confirmed the presence of these glycans on human sperm but not ZP (Pang et al., 2007; 2011).
Investigators began isolating mAb that would selectively bind to tumour cells but not to progenitor cells over three decades ago (Ritz et al., 1980). A mouse mAb designated CSLEX was specifically bound to tumour cells associated with stomach, colorectal, lung, esophageal, ovarian, breast, bladder and pancreatic cancers, but not to normal cells or tissues. The carbohydrate epitope recognized by CSLEX turned out to be sLex (Fukushima et al., 1984). This study led to the designation of sLex as a tumour-associated carbohydrate antigen. Glycomic studies have confirmed that sLex-bearing N-glycans are substantially increased on serum glycoproteins in patients with breast, lung, stomach and ovarian cancer compared with normal controls (Saldova et al., 2007; Abd Hamid et al., 2008; Arnold et al., 2011; Bones et al., 2011; Julien et al., 2011).
Glycoconjugates bearing sLex could interfere with many key immune functions in cancer patients. Expression of this sequence on tumour cells has been implicated in their binding to selectins on endothelial surfaces and metastasis (Laubli and Borsig, 2010). Tumour cells expressing sLex on their plasma membrane-associated glycoconjugates could also block immune cell-mediated responses directed against them via interaction with Siglec-9 (Angata and Varki, 2000; Avril et al., 2004). Metastatic tumour cells present in lymph nodes could inhibit lymphocyte homing and antigen presentation in the lymph system by secreting glycoproteins terminated with multivalent sLex (Johnson, 1999). Studies with uromodulin indicate that glycoproteins bearing multivalent sLex could also inhibit the antigen-induced proliferation of T cells, which is required for adaptive immune responses (Muchmore and Decker, 1985; Easton et al., 2000). In summary, the multiple effects of aberrant sLex expression could completely paralyse the immune response in cancer patients.
Another mAb designated AH6 was developed against human tumour cells (Abe et al., 1983). The ligand for AH6 is Ley, but this mAb also cross-reacted with another blood group determinant (H2 antigen). A mAb with a strict specificity for Ley was bound to many different types of organ-specific tumour cells, but not to normal tissues. These findings confirmed that Ley is also a tumour-associated cancer antigen that is neoexpressed on about 70% of all tumours of epithelial origin (Hellstrom et al., 1990). The expression of ligands for DC-SIGN like Ley could evoke immune deviations that protect tumour cells from adaptive immune responses.
Ley is abundantly expressed on human sperm and seminal plasma glycoconjugates (Table I, Fig. 4) (Hanisch et al., 1986; Chalabi et al., 2002; Pang et al., 2007, 2009). It is noteworthy that three endogenous glycoprotein ligands for DC-SIGN in seminal plasma (clusterin, galectin-3 binding protein, prostatic acid phosphatase) were previously identified as tumour-associated glycoprotein markers (Huggins and Hodges, 1941; Fukaya et al., 2008; Pucci et al., 2009; Clark et al., 2012).
It is clear from this discussion that human tumour cells express sLex and Lewisx/Lewisy sequences that are associated with human gametes. However, tumour cells also become more like human gametes in other important ways. As mentioned previously, human gametes do not express HLA class I antigens (Hutter and Dohr, 1998). Tumour cells also become HLA class I negative during the progression of cancer (Algarra et al., 2004). Like the gametes, the absence of these antigens makes these tumour cells insensitive to MHC class I restricted CTL responses (Zinkernagel and Doherty, 1997). If tumour cells also up-regulate the expression of bisecting biantennary type N-glycans that are expressed on human sperm, then they can also become resistant to NK cells. The evidence indicates that aggressive tumour cells escape the human immune response by employing the same immune-deviating pathways associated with human gametes.
Summary
The evidence supporting the Hu-FEDS hypothesis was limited in 1996. However, evidence obtained since then clearly indicates that glycans act as functional groups to elicit tolerizing effects that protect human gametes and offspring in utero. Many persistent pathogens and aggressive tumour cells also either mimic or acquire these same carbohydrate sequences, enabling them to couple their survival to the human reproductive imperative. Though not reviewed here, results from several studies indicate that this system of immune subterfuge is also operating in other eutherian mammals. For this reason, this model is now referred to as the eutherian fetoembryonic defense system (Eu-FEDS) hypothesis (Clark et al., 2001).
This system of protection may not be limited to eutherians. Immune destruction of gametes in any obligate sexually reproducing metazoan will prevent that individual from contributing its genes to future generations. This powerful selection pressure ensures that these germ cells must be insulated from any type of immune response that they might normally encounter. If a pathogen or tumour cell acquires or mimics the glycans employed for the protection of gametes in lower species, they could likely also evade the host's immune system. The metazoan immune system may not be as impervious as many investigators believe. Rather, data suggest that it is tightly constrained by the reproductive imperative under normal physiological conditions.
There is evidence for this type of restriction, and recent data confirm the unpleasant finding that inoculation with AIDS vaccines actually increases the odds of becoming HIV infected (Cohen, 2013). Similar discouraging results have also been obtained with cancer vaccines (Goldman and DeFrancesco, 2009). Vaccines directed against H. pylori and schistosomes have been similarly unsuccessful (McWilliam et al., 2012; Sutton and Chionh, 2013). These results suggest that pathogens and tumour cells that can integrate themselves into the same immune-deviating pathways that are necessary for human reproduction are unlikely to be viable candidates for vaccination. These findings are quite demoralizing, to say the least. However, ignoring such effects will make it much more difficult if not impossible to treat these recalcitrant pathological states. In contrast, adoption of this logic and acting upon it could mean the resolution of many pathological states in diverse sexually reproducing organisms, including humans.
Dedication
Those of us who knew Robert Edwards were saddened to hear about his recent passing on 10 April after a long illness. However, we will certainly remember his razor sharp mind and keen wit, in addition to his many scientific contributions in the area of reproductive biology. The Hu-FEDS hypothesis papers were published in the ESHRE journals in the 1990s with encouragement from Bob Edwards who was then Editor-in-Chief. His fascination for the subject was clear in several telephone conversations and he predicted at that time that there would never be an AIDS vaccine. His insights continue to be relevant and this article is dedicated to him.
Funding
Studies outlined by the author have been supported by the Life Sciences Mission Enhancement Reproductive Biology Program funded by the State of Missouri and a Research Board Grant (CB000500) supported by the University of Missouri System. Funding has also been obtained from the Breeden-Adams Foundation to investigate potential linkage to tumour evasion. The author has been supported in the past by grants from the Jeffress Memorial Trust of Virginia, the American Cancer Society, and the NIH.
Conflict of interest
None declared.
Acknowledgements
The author thanks Drs Anne Dell and Danny Schust for reviewing this manuscript and making useful suggestions. The author thanks Lynn Stevenson for her editorial assistance in preparing the manuscript.
References
- Abd Hamid UM, Royle L, Saldova R, Radcliffe CM, Harvey DJ, Storr SJ, Pardo M, Antrobus R, Chapman CJ, Zitzmann N, et al. A strategy to reveal potential glycan markers from serum glycoproteins associated with breast cancer progression. Glycobiology. 2008;18:1105–1118. doi: 10.1093/glycob/cwn095. doi:10.1093/glycob/cwn095. [DOI] [PubMed] [Google Scholar]
- Abe K, McKibbin JM, Hakomori S. The monoclonal antibody directed to difucosylated type 2 chain (Fucα1-2Galβ1-4[Fucα1-3]GlcNAc; Y determinant) J Biol Chem. 1983;258:11793–11797. [PubMed] [Google Scholar]
- Adachi M, Hayami M, Kashiwagi N, Mizuta T, Ohta Y, Gill MJ, Matheson DS, Tamaoki T, Shiozawa C, Hakomori S. Expression of Ley antigen in human immunodeficiency virus-infected human T cell lines and in peripheral lymphocytes of patients with acquired immune deficiency syndrome (AIDS) and AIDS-related complex (ARC) J Exp Med. 1988;167:323–331. doi: 10.1084/jem.167.2.323. doi:10.1084/jem.167.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghokeng AF, Peeters M. Simian immunodeficiency viruses (SIVs) in Africa. J Neurovirol. 2005;11(Suppl. 1):27–32. [PubMed] [Google Scholar]
- Algarra I, Garcia-Lora A, Cabrera T, Ruiz-Cabello F, Garrido F. The selection of tumor variants with altered expression of classical and nonclassical MHC class I molecules: implications for tumor immune escape. Cancer Immunol Immunother. 2004;53:904–910. doi: 10.1007/s00262-004-0517-9. doi:10.1007/s00262-004-0517-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angata T, Varki A. Cloning, characterization, and phylogenetic analysis of siglec-9, a new member of the CD33-related group of siglecs. Evidence for co-evolution with sialic acid synthesis pathways. J Biol Chem. 2000;275:22127–22135. doi: 10.1074/jbc.M002775200. doi:10.1074/jbc.M002775200. [DOI] [PubMed] [Google Scholar]
- Appelmelk BJ, Van Die I, Van Vliet SJ, Vandenbroucke-Grauls CM, Geijtenbeek TB, Van Kooyk Y. Cutting edge: carbohydrate profiling identifies new pathogens that interact with dendritic cell-specific ICAM-3-grabbing nonintegrin on dendritic cells. J Immunol. 2003;170:1635–1639. doi: 10.4049/jimmunol.170.4.1635. [DOI] [PubMed] [Google Scholar]
- Arck PC, Hecher K. Fetomaternal immune cross-talk and its consequences for maternal and offspring's health. Nat Med. 2013;19:548–556. doi: 10.1038/nm.3160. doi:10.1038/nm.3160. [DOI] [PubMed] [Google Scholar]
- Arnold JN, Saldova R, Galligan MC, Murphy TB, Mimura-Kimura Y, Telford JE, Godwin AK, Rudd PM. Novel glycan biomarkers for the detection of lung cancer. J Proteome Res. 2011;10:1755–1764. doi: 10.1021/pr101034t. doi:10.1021/pr101034t. [DOI] [PubMed] [Google Scholar]
- Aspinall GO, Monteiro MA, Moran AP, Pang H, Penner JL, Shaver RT. Lipopolysaccharides from Helicobacter pylori. Prog Clin Biol Res. 1995;392:93–101. [PubMed] [Google Scholar]
- Avril T, Floyd H, Lopez F, Vivier E, Crocker PR. The membrane-proximal immunoreceptor tyrosine-based inhibitory motif is critical for the inhibitory signaling mediated by Siglecs-7 and -9, CD33-related Siglecs expressed on human monocytes and NK cells. J Immunol. 2004;173:6841–6849. doi: 10.4049/jimmunol.173.11.6841. [DOI] [PubMed] [Google Scholar]
- Barber LD, Patel TP, Percival L, Gumperz JE, Lanier LL, Phillips JH, Bigge JC, Wormwald MR, Parekh RB, Parham P. Unusual uniformity of the N-linked oligosaccharides of HLA-A, -B, and -C glycoproteins. J Immunol. 1996;156:3275–3284. [PubMed] [Google Scholar]
- Barondes SH, Castronovo V, Cooper DN, Cummings RD, Drickamer K, Feizi T, Gitt MA, Hirabayashi J, Hughes C, Kasai K, et al. Galectins: a family of animal β-galactoside-binding lectins. Cell. 1994;76:597–598. doi: 10.1016/0092-8674(94)90498-7. doi:10.1016/0092-8674(94)90498-7. [DOI] [PubMed] [Google Scholar]
- Barre-Sinoussi F, Chermann JC, Rey F, Nugeyre MT, Chamaret S, Gruest J, Dauguet C, Axler-Blin C, Vezinet-Brun F, Rouzioux C, et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS) Science. 1983;220:868–871. doi: 10.1126/science.6189183. doi:10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Bast RC, Jr, Feeney M, Lazarus H, Nadler LM, Colvin RB, Knapp RC. Reactivity of a monoclonal antibody with human ovarian carcinoma. J Clin Invest. 1981;68:1331–1337. doi: 10.1172/JCI110380. doi:10.1172/JCI110380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belisle JA, Horibata S, Jennifer GA, Petrie S, Kapur A, Andre S, Gabius HJ, Rancourt C, Connor J, Paulson JC, et al. Identification of Siglec-9 as the receptor for MUC16 on human NK cells, B cells, and monocytes. Mol Cancer. 2010;9:118. doi: 10.1186/1476-4598-9-118. doi:10.1186/1476-4598-9-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman MP, Engering A, Smits HH, van Vliet SJ, van Bodegraven AA, Wirth HP, Kapsenberg ML, Vandenbroucke-Grauls CM, van Kooyk Y, Appelmelk BJ. Helicobacter pylori modulates the T helper cell 1/T helper cell 2 balance through phase-variable interaction between lipopolysaccharide and DC-SIGN. J Exp Med. 2004;200:979–990. doi: 10.1084/jem.20041061. doi:10.1084/jem.20041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman M, Del Prete G, van Kooyk Y, Appelmelk B. Helicobacter pylori phase variation, immune modulation and gastric autoimmunity. Nat Rev Microbiol. 2006;4:151–159. doi: 10.1038/nrmicro1344. doi:10.1038/nrmicro1344. [DOI] [PubMed] [Google Scholar]
- Billingham RE, Brent L, Medawar PB. The antigenic stimulus in transplantation immunity. Nature. 1956;178:514–519. doi: 10.1038/178514a0. doi:10.1038/178514a0. [DOI] [PubMed] [Google Scholar]
- Blaschitz A, Hutter H, Dohr G. HLA Class I protein expression in the human placenta. Early Pregnancy. 2001;5:67–69. [PubMed] [Google Scholar]
- Blidner AG, Rabinovich GA. ‘Sweetening’ pregnancy: galectins at the fetomaternal interface. Am J Reprod Immunol. 2013;69:369–382. doi: 10.1111/aji.12090. doi:10.1111/aji.12090. [DOI] [PubMed] [Google Scholar]
- Blois SM, Ilarregui JM, Tometten M, Garcia M, Orsal AS, Cordo-Russo R, Toscano MA, Bianco GA, Kobelt P, Handjiski B, et al. A pivotal role for galectin-1 in fetomaternal tolerance. Nat Med. 2007;13:1450–1457. doi: 10.1038/nm1680. doi:10.1038/nm1680. [DOI] [PubMed] [Google Scholar]
- Bolton AE, Pockley AG, Clough KJ, Mowles EA, Stoker RJ, Westwood OM, Chapman MG. Identification of placental protein 14 as an immunosuppressive factor in human reproduction. Lancet. 1987;1:593–595. doi: 10.1016/s0140-6736(87)90235-2. doi:10.1016/S0140-6736(87)90235-2. [DOI] [PubMed] [Google Scholar]
- Bones J, Byrne JC, O'Donoghue N, McManus C, Scaife C, Boissin H, Nastase A, Rudd PM. Glycomic and glycoproteomic analysis of serum from patients with stomach cancer reveals potential markers arising from host defense response mechanisms. J Proteome Res. 2011;10:1246–1265. doi: 10.1021/pr101036b. doi:10.1021/pr101036b. [DOI] [PubMed] [Google Scholar]
- Bonomelli C, Doores KJ, Dunlop DC, Thaney V, Dwek RA, Burton DR, Crispin M, Scanlan CN. The glycan shield of HIV is predominantly oligomannose independently of production system or viral clade. PLoS One. 2011;6:e23521. doi: 10.1371/journal.pone.0023521. doi:10.1371/journal.pone.0023521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broussard SR, Staprans SI, White R, Whitehead EM, Feinberg MB, Allan JS. Simian immunodeficiency virus replicates to high levels in naturally infected African green monkeys without inducing immunologic or neurologic disease. J Virol. 2001;75:2262–2275. doi: 10.1128/JVI.75.5.2262-2275.2001. doi:10.1128/JVI.75.5.2262-2275.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton GJ, Jauniaux E. Placental oxidative stress: from miscarriage to preeclampsia. J Soc Gynecol Investig. 2004;11:342–352. doi: 10.1016/j.jsgi.2004.03.003. doi:10.1016/j.jsgi.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Carbone E, Terrazzano G, Colonna M, Tuosto L, Piccolella E, Franksson L, Palazzolo G, Perez Villar JJ, Fontana S, Karre K, et al. Natural killer clones recognize specific soluble HLA class I molecules. Eur J Immunol. 1996;26:683–689. doi: 10.1002/eji.1830260326. doi:10.1002/eji.1830260326. [DOI] [PubMed] [Google Scholar]
- Cedeno-Laurent F, Dimitroff CJ. Galectins and their ligands: negative regulators of anti-tumor immunity. Glycoconj J. 2012;29:619–625. doi: 10.1007/s10719-012-9379-0. doi:10.1007/s10719-012-9379-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahroudi A, Bosinger SE, Vanderford TH, Paiardini M, Silvestri G. Natural SIV hosts: showing AIDS the door. Science. 2012;335:1188–1193. doi: 10.1126/science.1217550. doi:10.1126/science.1217550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalabi S, Easton RL, Patankar MS, Lattanzio FA, Morrison JC, Panico M, Morris HR, Dell A, Clark GF. The expression of free oligosaccharides in human seminal plasma. J Biol Chem. 2002;277:32562–32570. doi: 10.1074/jbc.M205152200. doi:10.1074/jbc.M205152200. [DOI] [PubMed] [Google Scholar]
- Clancy KB. Reproductive ecology and the endometrium: physiology, variation, and new directions. Am J Phys Anthropol. 2009;140(Suppl. 49):137–154. doi: 10.1002/ajpa.21188. doi:10.1002/ajpa.21188. [DOI] [PubMed] [Google Scholar]
- Clark GF. The role of carbohydrate recognition during human sperm–egg binding. Hum Reprod. 2013;28:566–577. doi: 10.1093/humrep/des447. doi:10.1093/humrep/des447. [DOI] [PubMed] [Google Scholar]
- Clark GF, Dell A. Molecular models for murine sperm–egg binding. J Biol Chem. 2006;281:13853–13856. doi: 10.1074/jbc.R600001200. doi:10.1074/jbc.R600001200. [DOI] [PubMed] [Google Scholar]
- Clark GF, Patankar MS. The human fetoembryonic defence system (Hu-FEDS): in search of universal self. Mol Hum Reprod. 1997;3:985–987. doi: 10.1093/molehr/3.11.985. doi:10.1093/molehr/3.11.985. [DOI] [PubMed] [Google Scholar]
- Clark GF, Schust DJ. Manifestations of immune tolerance in the human female reproductive tract. Front Immunol. 2013;4:26. doi: 10.3389/fimmu.2013.00026. doi:10.3389/fimmu.2013.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark GF, Patankar MS, Hinsch KD, Oehninger S. New concepts in human sperm-zona pellucida interaction. Hum Reprod. 1995;10(Suppl. 1):31–37. doi: 10.1093/humrep/10.suppl_1.31. doi:10.1093/humrep/10.suppl_1.31. [DOI] [PubMed] [Google Scholar]
- Clark GF, Oehninger S, Patankar MS, Koistinen R, Dell A, Morris HR, Koistinen H, Seppala M. A role for glycoconjugates in human development: the human feto-embryonic defence system hypothesis. Hum Reprod. 1996;11:467–473. doi: 10.1093/humrep/11.3.467. doi:10.1093/HUMREP/11.3.467. [DOI] [PubMed] [Google Scholar]
- Clark GF, Dell A, Morris HR, Patankar M, Oehninger S, Seppala M. Viewing AIDS from a glycobiological perspective: potential linkages to the human fetoembryonic defence system hypothesis. Mol Hum Reprod. 1997;3:5–13. doi: 10.1093/molehr/3.1.5. doi:10.1093/molehr/3.1.5. [DOI] [PubMed] [Google Scholar]
- Clark GF, Dell A, Morris HR, Patankar MS, Easton RL. The species recognition system: a new corollary for the human fetoembryonic defense system hypothesis. Cells Tissues Organs. 2001;168:113–121. doi: 10.1159/000016812. doi:10.1159/000016812. [DOI] [PubMed] [Google Scholar]
- Clark GF, Grassi P, Pang PC, Panico M, Lafrenz D, Drobnis EZ, Baldwin MR, Morris HR, Haslam SM, Schedin-Weiss S, et al. Tumor biomarker glycoproteins in the seminal plasma of healthy human males are endogenous ligands for DC-SIGN. Mol Cell Proteomics. 2012;11:M111.008730. doi: 10.1074/mcp.M111.008730. doi:10.1074/mcp.M111.008730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. Survival of pathogenic bacteria in immunocompetent hosts. Chemioterapia. 1985;4:329–338. [PubMed] [Google Scholar]
- Cohen J. AIDS Research. More woes for struggling HIV vaccine field. Science. 2013;340:667. doi: 10.1126/science.340.6133.667. doi:10.1126/science.340.6133.667. [DOI] [PubMed] [Google Scholar]
- Cross NL, Overstreet JW. Glycoconjugates of the human sperm surface: distribution and alterations that accompany capacitation in vitro. Gamete Res. 1987;16:23–35. doi: 10.1002/mrd.1120160104. doi:10.1002/mrd.1120160104. [DOI] [PubMed] [Google Scholar]
- Cummings RD, Kornfeld S. Characterization of the structural determinants required for the high affinity interaction of asparagine-linked oligosaccharides with immobilized Phaseolus vulgaris leukoagglutinating and erythroagglutinating lectins. J Biol Chem. 1982;257:11230–11234. [PubMed] [Google Scholar]
- Cummings RD, Liu FT. Galectins: Chapter 33. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Essentials of Glycobiology. 2nd edn. NY: Cold Spring Harbor Laboratory Press, Cold Spring Harbor; 2009. [PubMed] [Google Scholar]
- Dalgleish AG, Wilson S, Gompels M, Ludlam C, Gazzard B, Coates AM, Habeshaw J. T-cell receptor variable gene products and early HIV-1 infection. Lancet. 1992;339:824–828. doi: 10.1016/0140-6736(92)90277-a. doi:10.1016/0140-6736(92)90277-A. [DOI] [PubMed] [Google Scholar]
- Dalton CF, Laird SM, Serle E, Saravelos H, Warren MA, Li TC, Bolton AE. The measurement of CA125 and placental protein 14 in uterine flushings in women with recurrent miscarriage; relation to endometrial morphology. Hum Reprod. 1995;10:2680–2684. doi: 10.1093/oxfordjournals.humrep.a135767. [DOI] [PubMed] [Google Scholar]
- Daniel MD, Letvin NL, Sehgal PK, Hunsmann G, Schmidt DK, King NW, Desrosiers RC. Long-term persistent infection of macaque monkeys with the simian immunodeficiency virus. J Gen Virol. 1987;68:3183–3189. doi: 10.1099/0022-1317-68-12-3183. doi:10.1099/0022-1317-68-12-3183. [DOI] [PubMed] [Google Scholar]
- Dell A, Morris HR, Easton RL, Panico M, Patankar M, Oehniger S, Koistinen R, Koistinen H, Seppala M, Clark GF. Structural analysis of the oligosaccharides derived from glycodelin, a human glycoprotein with potent immunosuppressive and contraceptive activities. J Biol Chem. 1995;270:24116–24126. doi: 10.1074/jbc.270.41.24116. doi:10.1074/jbc.270.21.12380. [DOI] [PubMed] [Google Scholar]
- Demetriou M, Granovsky M, Quaggin S, Dennis JW. Negative regulation of T-cell activation and autoimmunity by Mgat5 N-glycosylation. Nature. 2001;409:733–739. doi: 10.1038/35055582. doi:10.1038/35055582. [DOI] [PubMed] [Google Scholar]
- Drake PM, Gunn MD, Charo IF, Tsou CL, Zhou Y, Huang L, Fisher SJ. Human placental cytotrophoblasts attract monocytes and CD56(bright) natural killer cells via the actions of monocyte inflammatory protein 1α. J Exp Med. 2001;193:1199–1212. doi: 10.1084/jem.193.10.1199. doi:10.1084/jem.193.10.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duc-Goiran P, Mignot TM, Bourgeois C, Ferre F. Embryo-maternal interactions at the implantation site: a delicate equilibrium. Eur J Obstet Gynecol Reprod Biol. 1999;83:85–100. doi: 10.1016/s0301-2115(98)00310-8. doi:10.1016/S0301-2115(98)00310-8. [DOI] [PubMed] [Google Scholar]
- Dym M. The fine structure of the monkey (Macaca) Sertoli cell and its role in maintaining the blood-testis barrier. Anat Rec. 1973;175:639–656. doi: 10.1002/ar.1091750402. doi:10.1002/ar.1091750402. [DOI] [PubMed] [Google Scholar]
- Easton RL, Patankar MS, Clark GF, Morris HR, Dell A. Pregnancy-associated changes in the glycosylation of Tamm–Horsfall glycoprotein. Expression of sialyl Lewisx sequence on core 2 type O-glycans derived from uromodulin. J Biol Chem. 2000;275:21928–21938. doi: 10.1074/jbc.M001534200. [DOI] [PubMed] [Google Scholar]
- el Ouagari K, Teissie J, Benoist H. Glycophorin A protects K562 cells from natural killer cell attack. Role of oligosaccharides. J Biol Chem. 1995;270:26970–26975. doi: 10.1074/jbc.270.45.26970. [DOI] [PubMed] [Google Scholar]
- Erlebacher A. Mechanisms of T cell tolerance towards the allogeneic fetus. Nat Rev Immunol. 2013;13:23–33. doi: 10.1038/nri3361. doi:10.1038/nri3361. [DOI] [PubMed] [Google Scholar]
- Fauci AS. Multifactorial nature of human immunodeficiency virus disease: implications for therapy. Science. 1993;262:1011–1018. doi: 10.1126/science.8235617. doi:10.1126/science.8235617. [DOI] [PubMed] [Google Scholar]
- Feinberg H, Mitchell DA, Drickamer K, Weis W. Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science. 2001;294:2163–2166. doi: 10.1126/science.1066371. doi:10.1126/science.1066371. [DOI] [PubMed] [Google Scholar]
- Fijak M, Meinhardt A. The testis in immune privilege. Immunol Rev. 2006;213:66–81. doi: 10.1111/j.1600-065X.2006.00438.x. doi:10.1111/j.1600-065X.2006.00438.x. [DOI] [PubMed] [Google Scholar]
- Founds SA, Conley YP, Lyons-Weiler JF, Jeyabalan A, Hogge WA, Conrad KP. Altered global gene expression in first trimester placentas of women destined to develop preeclampsia. Placenta. 2009;30:15–24. doi: 10.1016/j.placenta.2008.09.015. doi:10.1016/j.placenta.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxall C, Watson SR, Dowbenko D, Fennie C, Lasky LA, Kiso M, Hasegawa A, Asa D, Brandley BK. The three members of the selectin receptor family recognize a common carbohydrate epitope, the sialyl Lewisx oligosaccharide. J Cell Biol. 1992;117:895–902. doi: 10.1083/jcb.117.4.895. doi:10.1083/jcb.117.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukaya Y, Shimada H, Wang LC, Zandi E, DeClerck YA. Identification of galectin-3-binding protein as a factor secreted by tumor cells that stimulates interleukin-6 expression in the bone marrow stroma. J Biol Chem. 2008;283:18573–18581. doi: 10.1074/jbc.M803115200. doi:10.1074/jbc.M803115200. [DOI] [PubMed] [Google Scholar]
- Fukushima K, Hirota M, Terasaki PI, Wakisaka A, Togashi H, Chia D, Suyama N, Fukushi Y, Nudelman E, Hakomori S. Characterization of sialosylated Lewisx as a new tumor-associated antigen. Cancer Res. 1984;44:5279–5285. [PubMed] [Google Scholar]
- Garcia-Vallejo JJ, van Kooyk Y. Endogenous ligands for C-type lectin receptors: the true regulators of immune homeostasis. Immunol Rev. 2009;230:22–37. doi: 10.1111/j.1600-065X.2009.00786.x. doi:10.1111/j.1600-065X.2009.00786.x. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek TB, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol. 2009;9:465–479. doi: 10.1038/nri2569. doi:10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geijtenbeek TB, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GC, Middel J, Cornelissen IL, Nottet HS, KewalRamani VN, Littman DR, et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. doi:10.1016/S0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- Geyer H, Holschbach C, Hunsmann G, Schneider J. Carbohydrates of human immunodeficiency virus. Structures of oligosaccharides linked to the envelope glycoprotein 120. J Biol Chem. 1988;263:11760–11767. [PubMed] [Google Scholar]
- Golding H, Robey FA, Gates FT, III, Linder W, Beining PR, Hoffman T, Golding B. Identification of homologous regions in human immunodeficiency virus I gp41 and human MHC class II β1 domain. I. Monoclonal antibodies against the gp41-derived peptide and patients’ sera react with native HLA class II antigens, suggesting a role for autoimmunity in the pathogenesis of acquired immune deficiency syndrome. J Exp Med. 1988;167:914–923. doi: 10.1084/jem.167.3.914. doi:10.1084/jem.167.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding H, Shearer GM, Hillman K, Lucas P, Manischewitz J, Zajac RA, Clerici M, Gress RE, Boswell RN, Golding B. Common epitope in human immunodeficiency virus (HIV) I-GP41 and HLA class II elicits immunosuppressive autoantibodies capable of contributing to immune dysfunction in HIV I-infected individuals. J Clin Invest. 1989;83:1430–1435. doi: 10.1172/JCI114034. doi:10.1172/JCI114034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman B, DeFrancesco L. The cancer vaccine roller coaster. Nat Biotechnol. 2009;27:129–139. doi: 10.1038/nbt0209-129. doi:10.1038/nbt0209-129. [DOI] [PubMed] [Google Scholar]
- Gringhuis SI, den Dunnen J, Litjens M, van der Vlist M, Geijtenbeek TB. Carbohydrate-specific signaling through the DC-SIGN signalosome tailors immunity to Mycobacterium tuberculosis, HIV-1 and Helicobacter pylori. Nat Immunol. 2009;10:1081–1088. doi: 10.1038/ni.1778. doi:10.1038/ni.1778. [DOI] [PubMed] [Google Scholar]
- Grinnell BW, Hermann RB, Yan SB. Human protein C inhibits selectin-mediated cell adhesion: role of unique fucosylated oligosaccharide. Glycobiology. 1994;4:221–225. doi: 10.1093/glycob/4.2.221. doi:10.1093/glycob/4.2.221. [DOI] [PubMed] [Google Scholar]
- Hanisch FG, Egge H, Peter-Katalinic J, Uhlenbruck G. Structure of neutral oligosaccharides derived from mucus glycoproteins of human seminal plasma. Eur J Biochem. 1986;155:239–247. doi: 10.1111/j.1432-1033.1986.tb09482.x. doi:10.1111/j.1432-1033.1986.tb09482.x. [DOI] [PubMed] [Google Scholar]
- Head JR, Billingham RE. Immunologically privileged sites in transplantation immunology and oncology. Perspect Biol Med. 1985;29:115–131. doi: 10.1353/pbm.1985.0038. [DOI] [PubMed] [Google Scholar]
- Head JR, Billingham RE. Concerning the immunology of the uterus. Am J Reprod Immunol Microbiol. 1986;10:76–81. [PubMed] [Google Scholar]
- Hellstrom I, Garrigues HJ, Garrigues U, Hellstrom KE. Highly tumor-reactive, internalizing, mouse monoclonal antibodies to Ley-related cell surface antigens. Cancer Res. 1990;50:2183–2190. [PubMed] [Google Scholar]
- Hession C, Decker JM, Sherblom AP, Kumar S, Yue CC, Mattaliano RJ, Tizard R, Kawashima E, Schmeissner U, Heletky S, et al. Uromodulin (Tamm–Horsfall glycoprotein): a renal ligand for lymphokines. Science. 1987;237:1479–1484. doi: 10.1126/science.3498215. doi:10.1126/science.3498215. [DOI] [PubMed] [Google Scholar]
- Hirabayashi J, Hashidate T, Arata Y, Nishi N, Nakamura T, Hirashima M, Urashima T, Oka T, Futai M, Muller W, et al. Oligosaccharide specificity of galectins: a search by frontal affinity chromatography. Biochim Biophys Acta. 2002;1572:232–254. doi: 10.1016/s0304-4165(02)00311-2. doi:10.1016/S0304-4165(02)00311-2. [DOI] [PubMed] [Google Scholar]
- Hirsch VM, Dapolito G, Johnson PR, Elkins WR, London WT, Montali RJ, Goldstein S, Brown C. Induction of AIDS by simian immunodeficiency virus from an African green monkey: species-specific variation in pathogenicity correlates with the extent of in vivo replication. J Virol. 1995;69:955–967. doi: 10.1128/jvi.69.2.955-967.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holschbach C, Schneider J, Geyer H. Glycosylation of the envelope glycoprotein gp130 of simian immunodeficiency virus from sooty mangabey (Cercocebus atys) Biochem J. 1990;267:759–766. doi: 10.1042/bj2670759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TTF, Ohzu E, Yanagimachi R. Evidence suggesting that L-fucose is part of a recognition signal for sperm–zona pellucida attachment in mammals. Gamete Res. 1982;5:355–361. doi:10.1002/mrd.1120050406. [Google Scholar]
- Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293–297. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- Hutter H, Dohr G. HLA Expression on immature and mature human germ cells. J Reprod Immunol. 1998;38:101–122. doi: 10.1016/s0165-0378(98)00032-1. doi:10.1016/S0165-0378(98)00032-1. [DOI] [PubMed] [Google Scholar]
- Imai Y, Lasky LA, Rosen SD. Sulphation requirement for GlyCAM-1, an endothelial ligand for L-selectin. Nature. 1993;361:555–557. doi: 10.1038/361555a0. doi:10.1038/361555a0. [DOI] [PubMed] [Google Scholar]
- Imberti L, Sottini A, Bettinardi A, Puoti M, Primi D. Selective depletion in HIV infection of T cells that bear specific T cell receptor V β sequences. Science. 1991;254:860–862. doi: 10.1126/science.1948066. doi:10.1126/science.1948066. [DOI] [PubMed] [Google Scholar]
- Jeschke U, Wang X, Briese V, Friese K, Stahn R. Glycodelin and amniotic fluid transferrin as inhibitors of E-selectin-mediated cell adhesion. Histochem Cell Biol. 2003;119:345–354. doi: 10.1007/s00418-003-0529-0. [DOI] [PubMed] [Google Scholar]
- Johnson JP. Cell adhesion molecules in the development and progression of malignant melanoma. Cancer Metastasis Rev. 1999;18:345–357. doi: 10.1023/a:1006304806799. doi:10.1023/A:1006304806799. [DOI] [PubMed] [Google Scholar]
- Jones JL, Saraswati S, Block AS, Lichti CF, Mahadevan M, Diekman AB. Galectin-3 is associated with prostasomes in human semen. Glycoconj J. 2010;27:227–236. doi: 10.1007/s10719-009-9262-9. doi:10.1007/s10719-009-9262-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien S, Ivetic A, Grigoriadis A, Qize D, Burford B, Sproviero D, Picco G, Gillett C, Papp SL, Schaffer L, et al. Selectin ligand sialyl-Lewis × antigen drives metastasis of hormone-dependent breast cancers. Cancer Res. 2011;71:7683–7693. doi: 10.1158/0008-5472.CAN-11-1139. doi:10.1158/0008-5472.CAN-11-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julkunen M, Wahlstrom T, Seppala M, Koistinen R, Koskimies A, Stenman UH, Bohn H. Detection and localization of placental protein 14-like protein in human seminal plasma and in the male genital tract. Arch Androl. 1984;12(Suppl.):59–67. [PubMed] [Google Scholar]
- Julkunen M, Rutanen EM, Koskimies A, Ranta T, Bohn H, Seppala M. Distribution of placental protein 14 in tissues and body fluids during pregnancy. Br J Obstet Gynaecol. 1985;92:1145–1151. doi: 10.1111/j.1471-0528.1985.tb03027.x. doi:10.1111/j.1471-0528.1985.tb03027.x. [DOI] [PubMed] [Google Scholar]
- Julkunen M, Koistinen R, Sjoberg J, Rutanen EM, Wahlstrom T, Seppala M. Secretory endometrium synthesizes placental protein 14. Endocrinology. 1986;118:1782–1786. doi: 10.1210/endo-118-5-1782. doi:10.1210/endo-118-5-1782. [DOI] [PubMed] [Google Scholar]
- Julkunen M, Seppala M, Janne OA. Molecular cloning of complementary DNAs for two human endometrial proteins and cellular localization of their messenger RNAs. Ann N Y Acad Sci. 1991;626:284–294. doi: 10.1111/j.1749-6632.1991.tb37923.x. doi:10.1111/j.1749-6632.1991.tb37923.x. [DOI] [PubMed] [Google Scholar]
- Karre K. NK Cells, MHC class I molecules and the missing self. Scand J Immunol. 2002;55:221–228. doi: 10.1046/j.1365-3083.2002.01053.x. doi:10.1046/j.1365-3083.2002.01053.x. [DOI] [PubMed] [Google Scholar]
- Kashiwagi N, Gill MJ, Adachi M, Church D, Wong SJ, Poon MC, Hakomori S, Tamaoki T, Shiozawa C. Lymphocyte membrane modifications induced by HIV infection. Tohoku J Exp Med. 1994;173:115–131. doi: 10.1620/tjem.173.115. doi:10.1620/tjem.173.115. [DOI] [PubMed] [Google Scholar]
- Kelly RW. Immunomodulators in human seminal plasma: a vital protection for spermatozoa in the presence of infection? Int J Androl. 1999;22:2–12. doi: 10.1046/j.1365-2605.1999.00142.x. doi:10.1046/j.1365-2605.1999.00142.x. [DOI] [PubMed] [Google Scholar]
- Kelly RW, Critchley HO. Immunomodulation by human seminal plasma: a benefit for spermatozoon and pathogen? Hum Reprod. 1997;12:2200–2207. doi: 10.1093/oxfordjournals.humrep.a019559. doi:10.1093/oxfordjournals.humrep.a019559. [DOI] [PubMed] [Google Scholar]
- King A, Loke YW. Human trophoblast and JEG choriocarcinoma cells are sensitive to lysis by IL-2-stimulated decidual NK cells. Cell Immunol. 1990;129:435–448. doi: 10.1016/0008-8749(90)90219-h. doi:10.1016/0008-8749(90)90219-H. [DOI] [PubMed] [Google Scholar]
- King A, Balendran N, Wooding P, Carter NP, Loke YW. CD3-leukocytes present in the human uterus during early placentation: phenotypic and morphologic characterization of the CD56++ population. Dev Immunol. 1991;1:169–190. doi: 10.1155/1991/83493. doi:10.1155/1991/83493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko AI, Drager UC, Harn DA. A Schistosoma mansoni epitope recognized by a protective monoclonal antibody is identical to the stage-specific embryonic antigen 1. Proc Natl Acad Sci USA. 1990;87:4159–4163. doi: 10.1073/pnas.87.11.4159. doi:10.1073/pnas.87.11.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopcow HD, Allan DS, Chen X, Rybalov B, Andzelm MM, Ge B, Strominger JL. Human decidual NK cells form immature activating synapses and are not cytotoxic. Proc Natl Acad Sci USA. 2005;102:15563–15568. doi: 10.1073/pnas.0507835102. doi:10.1073/pnas.0507835102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopcow HD, Rosetti F, Leung Y, Allan DS, Kutok JL, Strominger JL. T cell apoptosis at the maternal-fetal interface in early human pregnancy, involvement of galectin-1. Proc Natl Acad Sci USA. 2008;105:18472–18477. doi: 10.1073/pnas.0809233105. doi:10.1073/pnas.0809233105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus G, Werner A, Baier M, Binniger D, Ferdinand FJ, Norley S, Kurth R. Isolation of human immunodeficiency virus-related simian immunodeficiency viruses from African green monkeys. Proc Natl Acad Sci USA. 1989;86:2892–2896. doi: 10.1073/pnas.86.8.2892. doi:10.1073/pnas.86.8.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kui Wong N, Easton RL, Panico M, Sutton-Smith M, Morrison JC, Lattanzio FA, Morris HR, Clark GF, Dell A, Patankar MS. Characterization of the oligosaccharides associated with the human ovarian tumor marker CA125. J Biol Chem. 2003;278:28619–28634. doi: 10.1074/jbc.M302741200. doi:10.1074/jbc.M302741200. [DOI] [PubMed] [Google Scholar]
- Lam KK, Chiu PC, Lee CL, Pang RT, Leung CO, Koistinen H, Seppala M, Ho PC, Yeung WS. Glycodelin-A protein interacts with Siglec-6 protein to suppress trophoblast invasiveness by down-regulating extracellular signal-regulated kinase (ERK)/c-Jun signaling pathway. J Biol Chem. 2011;286:37118–37127. doi: 10.1074/jbc.M111.233841. doi:10.1074/jbc.M111.233841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laubli H, Borsig L. Selectins promote tumor metastasis. Semin Cancer Biol. 2010;20:169–177. doi: 10.1016/j.semcancer.2010.04.005. doi:10.1016/j.semcancer.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Lee MC, Damjanov I. Lectin binding sites on human sperm and spermatogenic cells. Anat Rec. 1985;212:282–287. doi: 10.1002/ar.1092120310. doi:10.1002/ar.1092120310. [DOI] [PubMed] [Google Scholar]
- Lee CL, Pang PC, Yeung WS, Tissot B, Panico M, Lao TT, Chu IK, Lee KF, Chung MK, Lam KK, et al. Effects of differential glycosylation of glycodelins on lymphocyte survival. J Biol Chem. 2009;284:15084–15096. doi: 10.1074/jbc.M807960200. doi:10.1074/jbc.M807960200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy JA. Pathogenesis of human immunodeficiency virus infection. Microbiol Rev. 1993;57:183–289. doi: 10.1128/mr.57.1.183-289.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linz B, Balloux F, Moodley Y, Manica A, Liu H, Roumagnac P, Falush D, Stamer C, Prugnolle F, van der Merwe SW, et al. African origin for the intimate association between humans and Helicobacter pylori. Nature. 2007;445:915–918. doi: 10.1038/nature05562. doi:10.1038/nature05562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo F, Gandini L, Dondero F, Lenzi A. Antisperm immunity in natural and assisted reproduction. Hum Reprod Update. 2001;7:450–456. doi: 10.1093/humupd/7.5.450. doi:10.1093/humupd/7.5.450. [DOI] [PubMed] [Google Scholar]
- Marshall BJ. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1:1273–1275. [PubMed] [Google Scholar]
- Marshall BJ. Helicobacter pylori: a primer for 1994. Gastroenterologist. 1993;1:241–247. [PubMed] [Google Scholar]
- Matsuura M. Structural modifications of bacterial lipopolysaccharide that facilitate Gram-negative bacterial evasion of host innate immunity. Front Immunol. 2013;4:109. doi: 10.3389/fimmu.2013.00109. doi:10.3389/fimmu.2013.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWilliam HE, Driguez P, Piedrafita D, McManus DP, Meeusen EN. Novel immunomic technologies for schistosome vaccine development. Parasite Immunol. 2012;34:276–284. doi: 10.1111/j.1365-3024.2011.01330.x. doi:10.1111/j.1365-3024.2011.01330.x. [DOI] [PubMed] [Google Scholar]
- Medawar P. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. Symp Soc Exp Biol. 1953;VII:320–338. [Google Scholar]
- Meyer S, van Liempt E, Imberty A, van Kooyk Y, Geyer H, Geyer R, van Die I. DC-SIGN mediates binding of dendritic cells to authentic pseudo-LewisY glycolipids of Schistosoma mansoni cercariae, the first parasite-specific ligand of DC-SIGN. J Biol Chem. 2005;280:37349–37359. doi: 10.1074/jbc.M507100200. doi:10.1074/jbc.M507100200. [DOI] [PubMed] [Google Scholar]
- Mizuochi T, Matthews TJ, Kato M, Hamako J, Titani K, Solomon J, Feizi T. Diversity of the oligosaccharide structures on the envelope glycoprotein gp120 of human immunodeficiency virus 1 from the lymphoblastoid cell line H9. J Biol Chem. 1990;265:8519–8524. [PubMed] [Google Scholar]
- Moffett A, Loke C. Immunology of placentation in eutherian mammals. Nat Rev Immunol. 2006;6:584–594. doi: 10.1038/nri1897. doi:10.1038/nri1897. [DOI] [PubMed] [Google Scholar]
- Morris HR, Dell A, Easton RL, Panico M, Koistinen H, Koistinen R, Oehninger S, Patankar MS, Seppala M, Clark GF. Gender-specific glycosylation of human glycodelin affects its contraceptive activity. J Biol Chem. 1996;271:32159–32167. doi: 10.1074/jbc.271.50.32159. doi:10.1074/jbc.271.50.32159. [DOI] [PubMed] [Google Scholar]
- Muchmore AV, Decker JM. Uromodulin: a unique 85-kilodalton immunosuppressive glycoprotein isolated from urine of pregnant women. Science. 1985;229:479–481. doi: 10.1126/science.2409603. doi:10.1126/science.2409603. [DOI] [PubMed] [Google Scholar]
- Muchmore AV, Shifrin S, Decker JM. In vitro evidence that carbohydrate moieties derived from uromodulin, an 85,000 dalton immunosuppressive glycoprotein isolated from human pregnancy urine, are immunosuppressive in the absence of intact protein. J Immunol. 1987;138:2547–2553. [PubMed] [Google Scholar]
- Nagler A, Lanier L, Cwirla S, Phillips JH. Comparative studies of human FcRIII-positive and negative natural killer cells. J Immunol. 1989;143:3183–3191. [PubMed] [Google Scholar]
- Oehninger S, Coddington CC, Hodgen GD, Seppala M. Factors affecting fertilization: endometrial placental protein 14 reduces the capacity of human spermatozoa to bind to the human zona pellucida. Fertil Steril. 1995;63:377–383. doi: 10.1016/s0015-0282(16)57372-5. [DOI] [PubMed] [Google Scholar]
- Pang PC, Tissot B, Drobnis EZ, Sutovsky P, Morris HR, Clark GF, Dell A. Expression of bisecting type and Lewisx/Lewisy terminated N-glycans on human sperm. J Biol Chem. 2007;282:36593–36602. doi: 10.1074/jbc.M705134200. doi:10.1074/jbc.M705134200. [DOI] [PubMed] [Google Scholar]
- Pang PC, Tissot B, Drobnis EZ, Morris HR, Dell A, Clark GF. Analysis of the human seminal plasma glycome reveals the presence of immunomodulatory carbohydrate functional groups. J Proteome Res. 2009;8:4906–4915. doi: 10.1021/pr9001756. doi:10.1021/pr9001756. [DOI] [PubMed] [Google Scholar]
- Pang PC, Chiu PC, Lee CL, Chang LY, Panico M, Morris HR, Haslam SM, Khoo KH, Clark GF, Yeung WS, et al. Human sperm binding is mediated by the sialyl-Lewisx oligosaccharide on the zona pellucida. Science. 2011;333:1761–1764. doi: 10.1126/science.1207438. doi:10.1126/science.1207438. [DOI] [PubMed] [Google Scholar]
- Patankar MS, Oehninger S, Barnett T, Williams RL, Clark GF. A revised structure for fucoidan may explain some of its biological activities. J Biol Chem. 1993;268:21770–21776. [PubMed] [Google Scholar]
- Patankar MS, Ozgur K, Oehninger S, Dell A, Morris H, Seppala M, Clark GF. Expression of glycans linked to natural killer cell inhibition on the human zona pellucida. Mol Hum Reprod. 1997;3:501–505. doi: 10.1093/molehr/3.6.501. doi:10.1093/molehr/3.6.501. [DOI] [PubMed] [Google Scholar]
- Patankar MS, Jing Y, Morrison JC, Belisle JA, Lattanzio FA, Deng Y, Wong NK, Morris HR, Dell A, Clark GF. Potent suppression of natural killer cell response mediated by the ovarian tumor marker CA125. Gynecol Oncol. 2005;99:704–713. doi: 10.1016/j.ygyno.2005.07.030. doi:10.1016/j.ygyno.2005.07.030. [DOI] [PubMed] [Google Scholar]
- Pearce EJ, Caspar P, Grzych JM, Lewis FA, Sher A. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J Exp Med. 1991;173:159–166. doi: 10.1084/jem.173.1.159. doi:10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington KA, Schlitt JM, Jackson DL, Schulz LC, Schust DJ. Preeclampsia: multiple approaches for a multifactorial disease. Dis Model Mech. 2012;5:9–18. doi: 10.1242/dmm.008516. doi:10.1242/dmm.008516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijnenborg R, Vercruysse L, Carter AM. Deep trophoblast invasion and spiral artery remodelling in the placental bed of the chimpanzee. Placenta. 2011;32:400–408. doi: 10.1016/j.placenta.2011.02.009. doi:10.1016/j.placenta.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Prins JR, Gomez-Lopez N, Robertson SA. Interleukin-6 in pregnancy and gestational disorders. J Reprod Immunol. 2012;95:1–14. doi: 10.1016/j.jri.2012.05.004. doi:10.1016/j.jri.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Pucci S, Mazzarelli P, Nucci C, Ricci F, Spagnoli LG. CLU ‘in and out’: looking for a link. Adv Cancer Res. 2009;105:93–113. doi: 10.1016/S0065-230X(09)05006-4. [DOI] [PubMed] [Google Scholar]
- Rabinovich GA, Croci DO. Regulatory circuits mediated by lectin-glycan interactions in autoimmunity and cancer. Immunity. 2012;36:322–335. doi: 10.1016/j.immuni.2012.03.004. doi:10.1016/j.immuni.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Ritz J, Pesando JM, Notis-McConarty J, Lazarus H, Schlossman SF. A monoclonal antibody to human acute lymphoblastic leukaemia antigen. Nature. 1980;283:583–585. doi: 10.1038/283583a0. doi:10.1038/283583a0. [DOI] [PubMed] [Google Scholar]
- Robertson SA, Guerin LR, Moldenhauer LM, Hayball JD. Activating T regulatory cells for tolerance in early pregnancy—the contribution of seminal fluid. J Reprod Immunol. 2009;83:109–116. doi: 10.1016/j.jri.2009.08.003. doi:10.1016/j.jri.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Rumke P, Hellinga G. Autoantibodies against spermatozoa in sterile men. Am J Clin Pathol. 1959;32:357–363. doi: 10.1093/ajcp/32.4.357. [DOI] [PubMed] [Google Scholar]
- Saari T, Jahnukainen K, Pollanen P. Autoantigenicity of the basal compartment of seminiferous tubules in the rat. J Reprod Immunol. 1996;31:65–79. doi: 10.1016/0165-0378(96)00962-x. doi:10.1016/0165-0378(96)00962-X. [DOI] [PubMed] [Google Scholar]
- Saldova R, Royle L, Radcliffe CM, Abd Hamid UM, Evans R, Arnold JN, Banks RE, Hutson R, Harvey DJ, Antrobus R, et al. Ovarian cancer is associated with changes in glycosylation in both acute-phase proteins and IgG. Glycobiology. 2007;17:1344–1356. doi: 10.1093/glycob/cwm100. doi:10.1093/glycob/cwm100. [DOI] [PubMed] [Google Scholar]
- Setchell BP. The blood-testicular fluid barrier in sheep. J Physiol. 1967;189:63P–65P. [PubMed] [Google Scholar]
- Sharp PM, Hahn BH. Origins of HIV and the AIDS pandemic. Cold Spring Harb Perspect Med. 2011;1:a006841. doi: 10.1101/cshperspect.a006841. doi:10.1101/cshperspect.a006841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestri G, Sodora DL, Koup RA, Paiardini M, O'Neil SP, McClure HM, Staprans SI, Feinberg MB. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity. 2003;18:441–452. doi: 10.1016/s1074-7613(03)00060-8. doi:10.1016/S1074-7613(03)00060-8. [DOI] [PubMed] [Google Scholar]
- Simoons-Smit IM, Appelmelk BJ, Verboom T, Negrini R, Penner JL, Aspinall GO, Moran AP, Fei SF, Shi BS, Rudnica W, et al. Typing of Helicobacter pylori with monoclonal antibodies against Lewis antigens in lipopolysaccharide. J Clin Microbiol. 1996;34:2196–2200. doi: 10.1128/jcm.34.9.2196-2200.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Xia B, Stowell SR, Lasanajak Y, Smith DF, Cummings RD. Novel fluorescent glycan microarray strategy reveals ligands for galectins. Chem Biol. 2009;16:36–47. doi: 10.1016/j.chembiol.2008.11.004. doi:10.1016/j.chembiol.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivatsan J, Smith DF, Cummings RD. Schistosoma mansoni synthesizes novel biantennary Asn-linked oligosaccharides containing terminal β-linked N-acetylgalactosamine. Glycobiology. 1992;2:445–452. doi: 10.1093/glycob/2.5.445. doi:10.1093/glycob/2.5.445. [DOI] [PubMed] [Google Scholar]
- Stowell SR, Arthur CM, Mehta P, Slanina KA, Blixt O, Leffler H, Smith DF, Cummings RD. Galectin-1, -2, and -3 exhibit differential recognition of sialylated glycans and blood group antigens. J Biol Chem. 2008a;283:10109–10123. doi: 10.1074/jbc.M709545200. doi:10.1074/jbc.M709545200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowell SR, Qian Y, Karmakar S, Koyama NS, Dias-Baruffi M, Leffler H, McEver RP, Cummings RD. Differential roles of galectin-1 and galectin-3 in regulating leukocyte viability and cytokine secretion. J Immunol. 2008b;180:3091–3102. doi: 10.4049/jimmunol.180.5.3091. [DOI] [PubMed] [Google Scholar]
- Sublett JW, Bernstein JA. Seminal plasma hypersensitivity reactions: an updated review. Mt Sinai J Med. 2011;78:803–809. doi: 10.1002/msj.20283. doi:10.1002/msj.20283. [DOI] [PubMed] [Google Scholar]
- Sutton P, Chionh YT. Why can't we make an effective vaccine against Helicobacter pylori? Expert Rev Vaccines. 2013;12:433–441. doi: 10.1586/erv.13.20. doi:10.1586/erv.13.20. [DOI] [PubMed] [Google Scholar]
- Tamm I, Horsfall FL. A mucoprotein derived from human urine which reacts with influenza, mumps and Newcastle disease viruses. J Exp Med. 1952;95:71–97. doi: 10.1084/jem.95.1.71. doi:10.1084/jem.95.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Than NG, Sumegi B, Than GN, Berente Z, Bohn H. Isolation and sequence analysis of a cDNA encoding human placental tissue protein 13 (PP13), a new lysophospholipase, homologue of human eosinophil Charcot-Leyden Crystal protein. Placenta. 1999;20:703–710. doi: 10.1053/plac.1999.0436. doi:10.1053/plac.1999.0436. [DOI] [PubMed] [Google Scholar]
- Than NG, Romero R, Goodman M, Weckle A, Xing J, Dong Z, Xu Y, Tarquini F, Szilagyi A, Gal P, et al. A primate subfamily of galectins expressed at the maternal-fetal interface that promote immune cell death. Proc Natl Acad Sci USA. 2009;106:9731–9736. doi: 10.1073/pnas.0903568106. doi:10.1073/pnas.0903568106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Than NG, Romero R, Kim CJ, McGowen MR, Papp Z, Wildman DE. Galectins: guardians of eutherian pregnancy at the maternal-fetal interface. Trends Endocrinol Metab. 2012;23:23–31. doi: 10.1016/j.tem.2011.09.003. doi:10.1016/j.tem.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirado-Gonzalez I, Freitag N, Barrientos G, Shaikly V, Nagaeva O, Strand M, Kjellberg L, Klapp BF, Mincheva-Nilsson L, Cohen M, et al. Galectin-1 influences trophoblast immune evasion and emerges as a predictive factor for the outcome of pregnancy. Mol Hum Reprod. 2013;19:43–53. doi: 10.1093/molehr/gas043. doi:10.1093/molehr/gas043. [DOI] [PubMed] [Google Scholar]
- Trowsdale J, Betz AG. Mother's little helpers: mechanisms of maternal-fetal tolerance. Nat Immunol. 2006;7:241–246. doi: 10.1038/ni1317. doi:10.1038/ni1317. [DOI] [PubMed] [Google Scholar]
- Tung KS, Teuscher C, Meng AL. Autoimmunity to spermatozoa and the testis. Immunol Rev. 1981;55:217–255. doi: 10.1111/j.1600-065x.1981.tb00344.x. doi:10.1111/j.1600-065X.1981.tb00344.x. [DOI] [PubMed] [Google Scholar]
- Van Die I, Van Vliet SJ, Kwame Nyame A, Cummings RD, Bank CM, Appelmelk B, Geijtenbeek TB, Van Kooyk Y. The dendritic cell specific C-type lectin DC-SIGN is a receptor for Schistosoma mansoni egg antigens and recognizes the glycan antigen Lewisx. Glycobiology. 2003;13:471–478. doi: 10.1093/glycob/cwg052. doi:10.1093/glycob/cwg052. [DOI] [PubMed] [Google Scholar]
- van Liempt E, Bank CM, Mehta P, Garcia-Vallejo JJ, Kawar ZS, Geyer R, Alvarez RA, Cummings RD, Kooyk Y, van Die I. Specificity of DC-SIGN for mannose- and fucose-containing glycans. FEBS Lett. 2006;580:6123–6131. doi: 10.1016/j.febslet.2006.10.009. doi:10.1016/j.febslet.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Vasta GR. Roles of galectins in infection. Nature Rev Microbiol. 2009;7:424–438. doi: 10.1038/nrmicro2146. doi:10.1038/nrmicro2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasta GR, Ahmed H, Bianchet MA, Fernandez-Robledo JA, Amzel LM. Diversity in recognition of glycans by F-type lectins and galectins: molecular, structural, and biophysical aspects. Ann N Y Acad Sci. 2012;1253:14–26. doi: 10.1111/j.1749-6632.2012.06698.x. doi:10.1111/j.1749-6632.2012.06698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villinger F, Folks TM, Lauro S, Powell JD, Sundstrom JB, Mayne A, Ansari AA. Immunological and virological studies of natural SIV infection of disease-resistant nonhuman primates. Immunol Lett. 1996;51:59–68. doi: 10.1016/0165-2478(96)02556-4. doi:10.1016/0165-2478(96)02556-4. [DOI] [PubMed] [Google Scholar]
- Whiteside TL. Tricks tumors use to escape from immune control. Oral Oncol. 2009;45:e119–e123. doi: 10.1016/j.oraloncology.2009.03.006. doi:10.1016/j.oraloncology.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Wilson RA. Virulence factors of schistosomes. Microbes Infect. 2012;14:1442–1450. doi: 10.1016/j.micinf.2012.09.001. doi:10.1016/j.micinf.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Wuhrer M, Dennis RD, Doenhaff MJ, Lochnit G, Geyer R. Schistosoma mansoni cercarial glycolipids are dominated by Lewisx and pseudo-Lewisy structures. Glycobiology. 2000;10:89–101. doi: 10.1093/glycob/10.1.89. doi:10.1093/glycob/10.1.89. [DOI] [PubMed] [Google Scholar]
- Yamashita K, Hitoi A, Kobata A. Structural determinants of Phaseolus vulgaris erythroagglutinating lectin for oligosaccharides. J Biol Chem. 1983;258:14753–14755. [PubMed] [Google Scholar]
- Yoshimura M, Ihara Y, Ohnishi A, Ijuhin N, Nishiura T, Kanakura Y, Matsuzawa Y, Taniguchi N. Bisecting N-acetylglucosamine on K562 cells suppresses natural killer cytotoxicity and promotes spleen colonization. Cancer Res. 1996;56:412–418. [PubMed] [Google Scholar]
- Yule TD, Montoya GD, Russell LD, Williams TM, Tung KS. Autoantigenic germ cells exist outside the blood testis barrier. J Immunol. 1988;141:1161–1167. [PubMed] [Google Scholar]
- Zinkernagel RM, Doherty PC. The discovery of MHC restriction. Immunol Today. 1997;18:14–17. doi: 10.1016/s0167-5699(97)80008-4. doi:10.1016/S0167-5699(97)80008-4. [DOI] [PubMed] [Google Scholar]