Abstract
Accumulating evidence indicates that reduced fecundity associated with endometriosis reflects a failure of embryonic receptivity. Microdomains composed of endometrial gap junctions, which facilitate cell–cell communication, may be implicated. Pharmacological or genetic inhibition of connexin (Cx) 43 block human endometrial cell differentiation in vitro and conditional uterine deletion of Cx43 alleles cause implantation failure in mice. The aim of this study was to determine whether women with endometriosis have reduced eutopic endometrial Cx43. Cx26 acted as a control. Endometrial biopsies were collected from age, race and cycle phase-matched women without (15 controls) or with histologically confirmed endometriosis (15 cases). Immunohistochemistry confirmed a predominant localization of Cx43 in the endometrial stroma, whereas Cx26 was confined to the epithelium. Cx43 immunostaining was reduced in eutopic biopsies of endometriosis subjects and western blotting of tissue lysates confirmed lower Cx43 levels in endometriosis cases, with Cx43/β-actin ratios =3.4 ± 1.5 in control and =1.2 ± 0.3 in endometriosis biopsies (P < 0.01). When endometrial stromal cells (ESC) were isolated from endometriosis cases, Cx43 levels and scrape loading-dye transfer were reduced by ∼45% compared with ESC from controls. In vitro decidualization of ESC derived from endometriosis versus control subjects resulted in lesser epithelioid transformation and a significantly reduced up-regulation of Cx43 protein (1.2 ± 0.2- versus 1.7 ± 0.4-fold, P < 0.01). No changes in Cx26 were observed. While basal steady-state levels of Cx43 mRNA did not differ with respect to controls, ESC from endometriosis cases failed to manifest a response to hormone treatment in vitro. In summary, eutopic endometrial Cx43 concentrations in endometriosis cases were <50% those of controls in vivo and in vitro, functional gap junctions were reduced and hormone-induced Cx43 mRNA levels were blunted.
Keywords: endometriosis, gap junctions, immunohistochemistry, western blot, gene expression
Introduction
Endometriosis is a chronic gynecologic disorder characterized by the growth of hormone-responsive endometrial tissue outside the uterine cavity. Endometriotic implants typically are found on the pelvic peritoneal surface, within the ovarian cortex or invading the rectovaginal septum; however, many examples of more widely distributed extrapelvic lesions have been described. A recent study comparing different diagnostic methodology studies suggests that the overall prevalence of endometriosis among reproductive-age women is ∼11% (Buck Louis et al., 2011). Based on data extrapolated from the World Bank, it is estimated that >176 million reproductive-age women are affected globally (Adamson et al., 2010). Careful estimates of annual healthcare expenses for endometriosis in the USA were $22 billion in 2002 (Simoens et al., 2007); over the past decade this cost surely has grown.
The mechanisms that link endometriosis and infertility remain unclear, although the association is clinically well established (Gupta et al., 2008; de Ziegler et al., 2010). Prevailing theories support multifactorial causes by which endometriosis interferes with reproduction, with published reports of ovulatory dysfunction, reduced fertilization and impaired zygote transport in women with this disorder. The hypothesis that embryonic implantation is adversely affected in cases of endometriosis (Barnhart et al., 2002) has been supported by investigations from our own group and collaborators worldwide. Unbiased interrogations of global endometrial gene expression over the past decade revealed that many mRNA transcripts encoding proteins involved in uterine receptivity are dysregulated in endometriosis (Taylor et al., 2002; Kao et al., 2003; Giudice, 2004; Donaghay and Lessey, 2007; Eyster et al., 2007). These findings affirm the postulated progesterone (P)-resistance state of endometriosis (Bulun et al., 2010).
Recent studies have drawn attention to the critical role of endometrial decidualization for the support of healthy embryonic implantation (Brosens and Gellersen, 2006; Ramathal et al., 2010). Evidence that this differentiation process is disturbed in endometriosis has been reported (Klemmt et al., 2006; Aghajanova et al., 2009a). Our investigation of potential molecular mediators of endometrial decidualization led to the identification of the transmembrane gap junction protein, connexin (Cx)43, as necessary for endometrial decidual differentiation, including morphological, biochemical and angiogenic responses (Laws et al., 2008; Yu et al., 2011). Connexins integrate cellular coordination within a tissue by allowing diffusion of small signaling molecules through intercellular pores. While there is an emerging interest in the role of connexin gene transcription (Firestone and Kapadia, 2012), these proteins are predominantly regulated through phosphorylation at serine residues in the carboxyl terminus of the molecule. As a result, a variety of Cx43 isoforms can be generated by kinases and phosphatases and distinguished using phospho-specific antibodies (Wu et al., 2012). Post-translational modifications affect the opening and closing of central aqueous channels within gap junctions as well as the intracellular stability of the proteins (Marquez-Rosado et al., 2011).
Reduced levels of Cx43 and more epithelial distribution of the protein have been described in ectopic implants of endometriosis (Regidor et al., 1997), but to our knowledge, an analysis of Cx43 in the eutopic endometrium of such patients is lacking. The latter is important, as lower Cx43 may play a role in reduced implantation rates associated with endometriosis. In a baboon model, surgically induced endometriosis produced a dramatic decrease in eutopic endometrial Cx43 mRNA, although no obvious alteration in Cx43 immunostaining in the stroma was observed (Winterhager et al., 2009). The current study was undertaken to test the hypothesis that differences in eutopic endometrial Cx43 protein might distinguish endometriosis cases from controls without the disease.
Materials and Methods
Source of human tissues
Thirty patients undergoing laparoscopy for elective gynecological surgery, who provided written informed consent under a study protocol approved by the institutional review boards at Emory University School of Medicine and Northside Hospital in Atlanta, GA, USA and Wake Forest School of Medicine, Winston-Salem, NC, USA, were identified from an ongoing cohort. All the subjects selected were women with regular menstrual cycles, who had not received hormonal therapy for at least 3 months before surgery. The indications for surgery included evaluation of pelvic pain, evaluation of infertility and desired sterilization; the operative findings are described below. Nulligravidity per se was not an inclusion criterion and the parity of the subjects ranged from 0 to 3. Eleven of 15 endometriosis cases reported involuntary infertility persisting >12 months whereas only 5 of 15 controls were infertile. Among the endometriosis cases, 12 reported chronic pelvic pain, whereas seven controls reported this complaint. Immediately prior to laparoscopy, eutopic endometrial biopsies were collected by Pipelle aspiration under sterile conditions and transported without personal identifiers to the laboratory on ice in phosphate-buffered saline (PBS). Since the surgical diagnosis was assigned after sample collection, the investigators were blinded to the final study category. The tissue samples were apportioned for histological evaluations, lysate preparation and/or stromal cell culture establishment, based on the adequacy of the biopsy. For the majority of samples, as indicated below, representative portions were fixed in 10% formalin and embedded in paraffin for histological cycle phase dating (Noyes et al., 1975) and immunohistochemistry. At laparoscopy, a full visual inspection of the pelvic cavity was performed by senior gynecologic surgeons with extensive experience in the recognition and treatment of typical and atypical endometriotic lesions (Nezhat et al., 1991). Following surgery, women were classified as having endometriosis (n = 15) if their surgeon noted laparoscopic evidence of endometriosis lesions that were histopathologically confirmed to contain glands, stroma and hemosiderocytes. In this series, endometriosis staging ranged from I to IV (American Society for Reproductive Medicine, 1997). Women were classified as controls (n = 15) if there was no visible evidence of endometriosis. Among the controls, the primary operative findings in six were subserosal or intramural (but not submucosal) fibroids, three had pelvic adhesions, three had no evidence of pelvic pathology and three desired tubal sterilization. Controls were matched for age (±2 years), race and menstrual cycle phase (nine proliferative and six secretory) with the endometriosis cases.
Immunohistochemistry
Paraffin-embedded endometrial sections were subjected to immunohistochemistry as described previously (Pritts et al., 2005) with modifications. Briefly, after mounting, the slides were deparaffinized in xylene and rehydrated in graded concentrations of ethanol. The slides were then exposed to 10 min of 3% hydrogen peroxide in methanol to quench endogenous hydrogen peroxide activity. Each sample was then rinsed in water and blocked with SuperBlock buffer (Thermo Scientific, Rockford, IL, USA) overnight at 4°C. More than 20 different connexin proteins are encoded in the human genome (Firestone and Kapadia, 2012). In the human and rat endometrium, Cx43 localizes to the stroma. For comparison, similar studies were performed with Cx26, a connexin protein known to be predominantly localized in the cytoplasm of glands, particularly along the apical glycocalyx (Winterhager et al., 1991; Jahn et al., 1995; Grümmer et al., 1996). Cx43 was detected using polyclonal rabbit Cx43 antibodies (1:200 dilution, cat# 3512S, Cell Signaling Technology, Danvers, MA, USA) and monoclonal mouse Cx26 antibodies (1:250 dilution, Cat #13-8100, Life Technologies, Grand Island, NY, USA). The samples were incubated at room temperature for 1 h. Secondary antibody incubation followed by a diaminobenzidine substrate reaction was performed according to the manufacturer's protocol and the slides were counterstained with hematoxylin. Substitution of the same dilution of non-immune serum (Cat #Ab46540-1, Abcam, Cambridge, MA, USA) for the primary antibody in control sections validated the specificity of the Cx43 antibodies, which had been optimized previously (Darr et al., 2011). Non-immune mouse IgG was used as a negative control for the Cx26 antibodies, which had been validated for immunohistochemistry (Kyo et al., 2008).
Preparation of endometrial lysates
Approximately half of each fresh endometrial biopsy specimen (∼50 mg) was directly solubilized by vortexing in cell extraction buffer (cat# FNN0011, Life Technologies), followed by protein determination using a bicinchoninic acid protein assay kit (Sigma Chemical Co., St. Louis, MO, USA). These samples were stored frozen at −70°C until they were subjected to western blot analysis (see below).
Endometrial stromal cell culture conditions and in vitro decidualization
From endometrial biopsies performed in the proliferative phase (nine endometriosis and nine controls), to avoid effects of endogenous luteal phase P, tissue fragments were used to prepare cultures of endometrial stromal cells (ESC), as described (Ryan et al., 1994). Briefly, after collagenase digestion, glandular epithelial cells and debris were separated from ESC by filtration through 200 and 40 μm sieves. ESC were subcultured at least twice to eliminate contamination by macrophages or other leukocytes and were used before the sixth passage to avoid dedifferentiation. Prior studies from our laboratory confirmed that the ESC are >95% pure and retain functional estrogen and P receptors, as well as other phenotypic endometrial markers, for at least five passages in vitro (Ryan et al., 1994). The cells were grown to 60–80% confluence in 6 cm dishes with phenol red-free medium (DMEM/Ham's F-12 cat# 10-092cv, CellGro, Manassas, VA, USA) supplemented with 5% charcoal-stripped fetal calf serum. Replicate cultures from each subject were prepared. Decidualization was effected by exposing the cultures to a standard protocol of 10 nM 17β-estradiol + 100 nM P + 0.5 mM dibutyryl cAMP in 0.1% EtOH [E/P/c (estradiol, progesterone and dibutyryl cAMP)]. Basal conditions were achieved by incubation in the same medium for 7 days, but solvent alone (0.1% EtOH) was substituted for the hormones. As we and others have reported consistently, this hormone treatment induces morphological changes and biochemical decidualization [e.g. secretion of prolactin, IGFBP-1 (Gellersen et al., 2007) and VEGF (Yu et al., 2011)] that are variably observed after 4–11 days of hormone treatment, depending on the specific biomarker. In the current studies only morphology was monitored routinely during the experiments, but biochemical confirmation was obtained using a prolactin ELISA as described (Yu et al., 2011), which showed that after 7 days E/P/c exposure, endometriosis ESC secreted only ∼41% of control prolactin levels, similar to prior reports (Klemmt et al., 2006; Aghajanova et al., 2009a). Representative data are shown in the figures and the number (n) of independent observations for each experiment is indicated.
Scrape loading-dye transfer assays
To assess functional gap junction formation in cultured ESC, we used the gap junction-permeable fluorescent dye Lucifer Yellow (Molecular Probes, Life Technologies) as described previously but with minor modifications (Wu et al., 2012). ESC were grown for 7 days in the absence or presence of decidualizing hormones as described above. Confluent cell cultures were washed thoroughly with PBS and the monolayer was sliced with a scalpel blade. Lucifer Yellow dye (1 mg/ml) was added to the scraped culture and rinsed away after 5 min incubation. The ESC were washed three times with PBS, fixed with 4% paraformaldehyde, and the lateral extent of dye transfer from the scrape injury was detected by fluorescence emission under an inverted microscope. Cells that incorporated Lucifer Yellow from nearby scrape-loaded cells were considered to have gap junction communication. Lateral diffusion of Lucifer Yellow via GJIC was measured in three fields from each of 12 independent ESC cultures, derived from six cases and six controls, without or with exposure to decidualizing hormones. Total pixel area intensity was quantified using the Systat software image analysis (Systat, Inc., San Jose, CA, USA) to determine the extent of gap junction intercellular communications (GJIC).
Western blot analysis
Western blot analysis was performed on direct tissue or ESC lysates. Fifty micrograms of protein were loaded onto 12% SDS-polyacrylamide gel lanes, electrophoresced and transferred to polyvinylidene difluoride membranes. After blocking with 5% skim milk in PBS, total Cx43 was detected using rabbit polyclonal anti-Cx43 antibody (1:250 dilution, cat# 71-0700, Life Technologies). Cx43 protein bands were detected after incubation with secondary antibody linked to horseradish peroxidase and visualized by chemiluminescent detection and exposure to ECL hyper film (Amersham Pharmacia Biotech, Piscataway, NJ, USA). Blots were washed, reprobed with mouse monoclonal anti-β-actin antibodies (1:1000 dilution, cat# 31430, Sigma, St. Louis, MO, USA), and developed in an identical manner to ensure even exposure. Molecular weight standards were used to calibrate the migration of immunopositive bands.
Western blots of ESC also were evaluated using antibodies specific for phospho-serine (Ser) 368 Cx43 (1:1000, Cell Signaling Technology, cat# 3511), non-phospho-Ser 368 Cx43 (1:1000, Life Technologies, cat# 13-8300), and other connexin proteins, Cx26 (1:500, Life Technologies, cat# 13-8100) and Cx32 (1:500, Life Technologies, cat# 13-8200) were included as controls. For quantification of the Cx43 and β-actin proteins, the films were digitized on a flatbed scanner and Image J software (NIH) was used to integrate the density of each band. Data are presented as ratios of Cx43/β-actin intensity. In each set of experiments, equal numbers of endometriosis and control samples were compared on a single gel to minimize systematic errors due to subtle differences in exposure time of individual blots to film. Control connexins (26 and 32) and Cx43 phosphoform analyses were not conducted in all experiments, so these are provided as qualitative findings.
RNA isolation, end-point reverse transcription-polymerase chain reaction (RT–PCR) and real-time quantitative (q)RT–PCR
Nine control and nine endometriosis-derived ESC preparations were incubated without or with E/P/c for up to 7 days as described above. Total RNA was isolated from the cells using TRI-reagent and PureLink® RNA Mini Kit (Invitrogen, Grand Island, NY, USA, cat# 12183025) following the manufacturer's protocols and frozen at –80°C until analyzed. cDNA was synthesized from mRNA samples and subsequently used as template for conventional, end-point or qRT–PCR assays. Primers for PCR amplification were made by Integrated DNA Technology, Inc. (San Diego, CA, USA) with sequences as follows: Cx43 sense (5′-TACCATGCGACCAGTGGTGCGCT-3′), and Cx43 antisense (5′-GAATTCTGGTTATCATCGGGGAA-3′) [292-bp amplicon]); glyceraldehyde-3-phosphate dehydrogenase (GAPDH) sense (5′-CCATGGAGAAGGCTGGG-3′), GAPDH antisense (5′-CAAAGTTGTCATGGATGAC-3′ [185-bp amplicon]). Invitrogen Platinum® Blue PCR SuperMix (cat# 12580-015) and 2 µl cDNA were used for Cx43 amplification, and 1 µl GAPDH cDNA was used according to the manufacturer's specifications, which included DNase treatment of the preparations to exclude genomic (g)DNA contamination. Absence of a PCR product when reverse transcriptase was excluded from the amplification mix afforded an additional control against gDNA interference. PCR was performed in an Opticon thermocycler (Bio-Rad Laboratories, Hercules, CA, USA) under the following conditions: one denaturation cycle of 94°C for 3 min followed by 28 amplification cycles at 94°C for 30 s, 60°C for 30 s and 72°C for 1 min. cDNA amplicons were visualized on 1.0% agarose gels containing 0.2 µg/ml ethidium bromide. A 1 kb Plus DNA Ladder (Invitrogen, cat# 10787-018) was used as the molecular size standard.
Real-time qRT–PCR was performed in a CFX Connect™ Real-Time PCR Detection System (Bio-Rad Laboratories, cat# 185-5200), using mRNA collected under basal conditions and after up to 7 days of incubation with E/P/c to determine optimal expression in a detailed kinetic analysis (see Fig. 7B). SsoAdvanced™ SYBR® Green Supermix (Bio-Rad, cat# 172-5261) was used following the vendor's guidelines with some modifications. A total reaction volume of 20 µl contained 10 µl SYBR Supermix, 1 µl 50 mM MgCl2, 2 µl primer mix for Cx43 and 1 µl primer mix for GAPDH. We also analyzed Cx26 and Cx32 transcripts as controls, using proprietary commercial primer pairs from Qiagen (cat# QT00244531 and QT00213255, respectively) under the manufacturer's recommended conditions. Amplification was carried out for 35 cycles. Normalized Cx43, Cx26 and Cx32 mRNA levels were determined from 2ΔΔct calculations using GAPDH as the housekeeping control gene, as noted above.
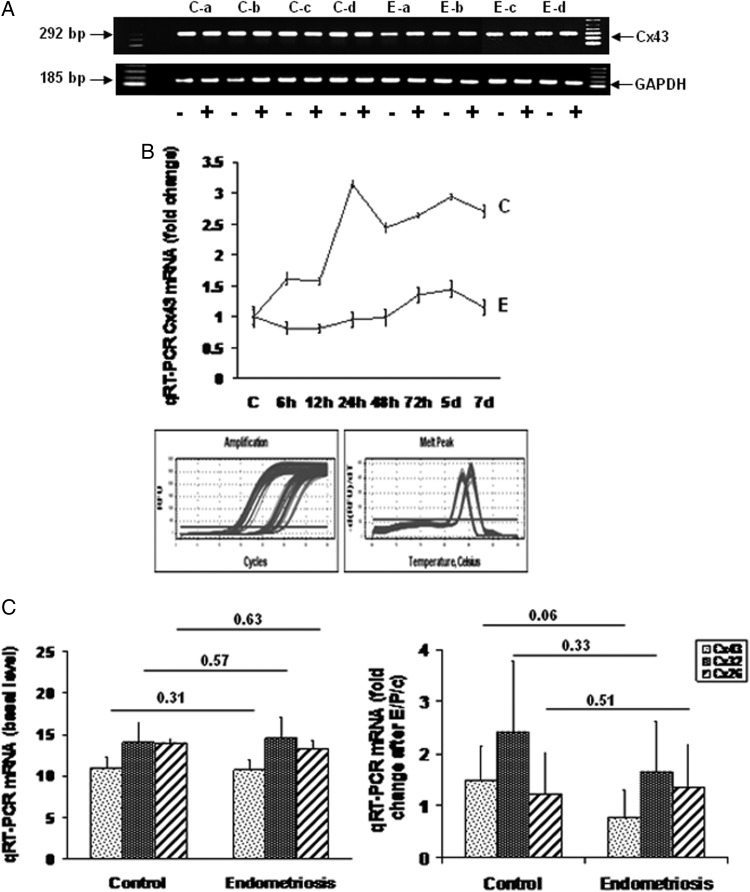
Figure 7.
(A) End-point RT–PCR for Cx43 and GAPDH mRNA in ESC from four control (Ca–d) and four endometriosis (Ea–d) subjects, incubated in the absence (−) or presence (+) of E/P/c for 7 days. The expected amplicons of 292 and 185 bp, respectively, were noted. 100-bp molecular size markers in the flanking lanes. (B) Steady-state Cx43 mRNA levels were quantified by qRT–PCR and normalized to GAPDH in ESC from a control subject (C) and from an endometriosis case (E). Accumulation over a 7-day time course of E/P/c was compared. Inset shows amplification profiles and melting curves of qRT–PCR. (C) qRT–PCR in nine control and nine endometriosis ESC samples was performed to measure steady-state Cx43 (stippled histograms), Cx32 (dark histograms) or Cx26 (striped histograms) mRNA concentrations under basal conditions (left panel) and after 24 h of E/P/c treatment (right panel).
Statistical analyses
Data are presented as mean ± SD and the number of independent samples tested in each analysis is reported. The western blot images are from representative gels and each result was replicated in a minimum of four independent experiments. The ratios of digitized band densities in the western blots were not always normally distributed, so comparisons are expressed as Z scores with corresponding P-values, determined using the conservative, nonparametric Mann–Whitney U-test. A power analysis had indicated that 12 subjects in each group would provide 1-β = 0.80 at an α = 0.05 to detect a 30% difference in Cx43 concentrations.
Results
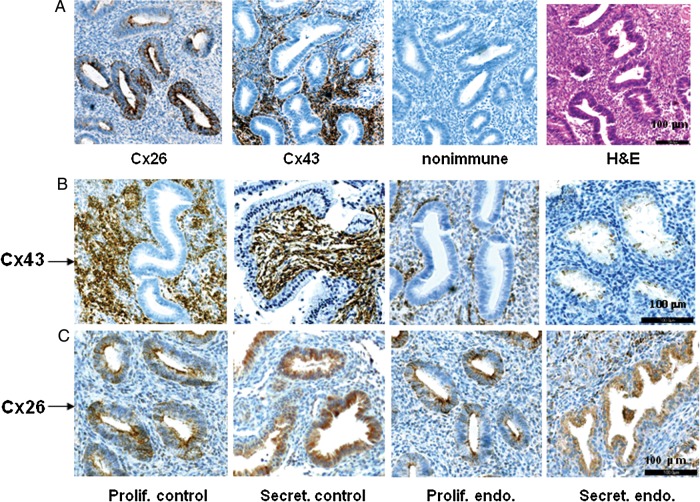
For the in vivo studies, 15 controls (35 ± 6 years) and 15 endometriosis cases (34 ± 7 years) were matched by age, race and menstrual cycle phase. Nine subjects in each clinical group represented the proliferative phase (cycle days ranged from 7 to 12) and six each in the secretory phase (cycle days ranged from 18 to 25) at the time of endometrial sampling. Immunohistochemical analysis of 10% formalin-fixed, paraffin-embedded endometrial biopsies using anti-Cx43 and anti-Cx26 antibodies was performed. To establish the histological distribution, serial sections of a proliferative phase (cycle day 10) control biopsy were stained with antibodies against Cx26, Cx43, or hematoxylin and eosin (H&E) (Fig. 1A). A negative control for the immunohistochemistry method was included (Fig. 1A). As previously reported, Cx26 was predominantly localized in the cytoplasm of glands, along the apical glycocalyx, whereas Cx43 was mostly confined to stromal cytoplasm (Winterhager et al., 1991; Jahn et al., 1995; Grümmer et al., 1996). Representative eutopic endometrial biopsies from proliferative (cycle day 11) and secretory phase (cycle day 18) controls (Fig. 1B, first and second panels) revealed strong stromal Cx43 staining, whereas samples from proliferative (cycle day 10) and secretory phase (cycle day 20) endometriosis cases (Fig. 1B, third and fourth panels) showed reduced stromal Cx43 staining with redistribution of scattered immunopositive cells within the epithelium, particularly in the secretory phase. Cx26 was largely expressed in endometrial epithelium and present in proliferative (cycle day 10) and secretory phase (cycle day 20) controls (Fig. 1C, first and second panels) as well as in proliferative (cycle day 10) and secretory phase (cycle day 19) biopsies from subjects with endometriosis (Fig. 1C, third and fourth panels). The specificity of the Cx26 and Cx43 antibody staining was confirmed in human midtrimester decidua as a positive control (data not shown). The results are representative of immunohistochemistry experiments in 12 control and 10 endometriosis cases.
Figure 1.
(A) Sections from a control, proliferative phase (cycle day 10) endometrial biopsy stained with antibodies against Cx26, Cx43, non-immune serum (control) or hematoxylin and eosin (H&E). Horizontal magnification bar at right base of the H&E panel indicates 100 μm. (B) Anti-Cx43 antibody staining of proliferative (prolif., cycle day 11) and secretory phase (secret., cycle day 18) control and proliferative (prolif., cycle day 10) and secretory phase (secret., cycle day 20) eutopic endometrium from endometriosis (endo.) cases. Horizontal magnification bar at right base of the secretory endometriosis panel indicates 100 μm. (C) Endometrial biopsies from the same subjects as in (B) immunostained using anti-Cx26 antibodies. Horizontal magnification bar at right base of the secretory endometriosis panel indicates 100 μm.
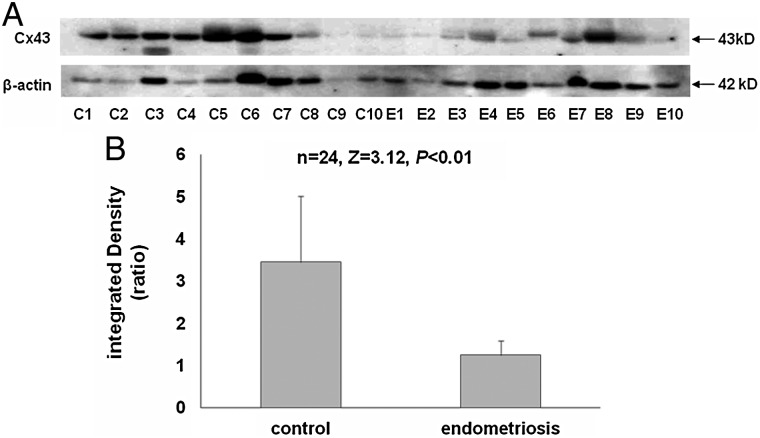
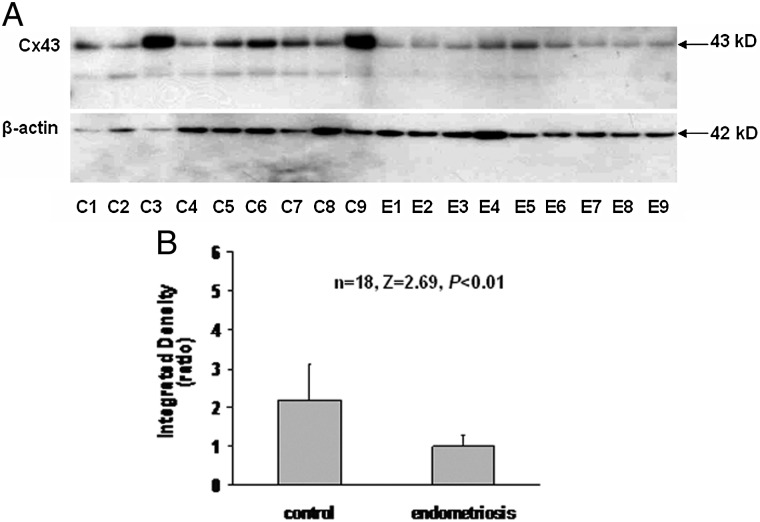
To analyze endometrial Cx43 more quantitatively and objectively, we performed western blots of eutopic endometrial sample lysates. Figure 2A shows a representative gel with 10 control and 10 endometriosis subjects' specimens (loading all lysates on a single gel was precluded by the gel comb size and need to include molecular weight markers). Cx43 levels were generally reduced in cases compared with controls (top panel). Equal amounts of total lysate protein were loaded into each gel lane, but variable amounts of plasma, mucus and secreted (extracellular) proteins are present in endometrial aspirates, potentially interfering with comparative analyses (Hannan et al., 2012). To avoid this pitfall, we used β-actin to normalize each sample for cellular protein content (lower panel). Cx43/β-actin ratios were quantified in digitized western blots, performed with a total of 12 endometriosis and 12 control samples compared on common gels to minimize errors due to exposure time of individual western blots (Fig. 2B). The data revealed that the relative concentration of endometrial Cx43 in endometriosis cases was only 35% that of controls (Cx43/β-actin ratios of 3.4 ± 1.5 in control biopsies versus 1.2 ± 0.3 in endometriosis cases [n = 24, Z = 3.12, P < 0.01]). We observed a doubling of the Cx43/β-actin ratio across the menstrual cycle in control subjects (proliferative phase, 1.4 ± 0.7 versus secretory phase, 2.8 ± 1.2; n = 12, Z = 2.40, P < 0.02), but the study was underpowered to stratify cycle phases more precisely (e.g. early versus late secretory). In our small sample, we failed to detect a significant increase in the Cx43/β-actin ratio across the menstrual cycle in endometriosis subjects (proliferative phase, 1.6 ± 1.7 versus secretory phase, 1.5 ± 1.0; n = 12, Z = 0.73, P = 0.47). Based on our cellular mRNA data (below) we suspect that this reflects a failure of endometriosis patients to up-regulate Cx43 in response to endogenous estrogen and P in the secretory phase, or it could be the result of a type II error.
Figure 2.
(A) Western blot of eutopic endometrial protein lysates from 10 control (C) and 10 endometriosis (E) subjects using anti-Cx43 (upper panel) and anti-β-actin (lower panel) antibodies. (B) Digitized Western bands from a total of 12 subjects in each group analyzed as integrated Cx43/β-actin density ratios.
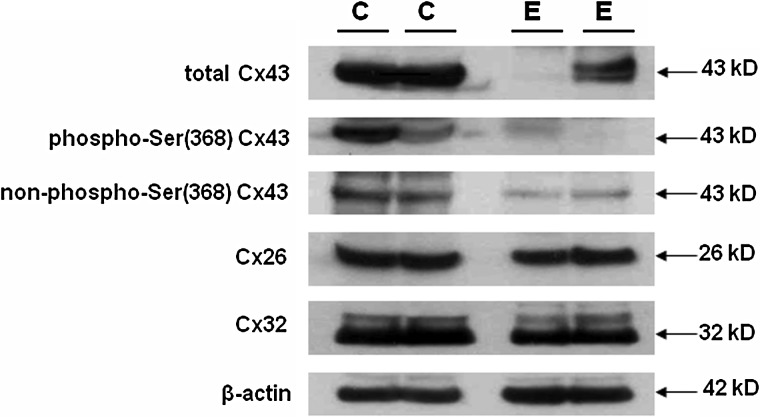
Connexin proteins are predominantly regulated through phosphorylation at serine residues in the carboxyl terminus of the molecule (Firestone and Kapadia, 2012) and these can be detected using phospho-specific Cx antisera. Experiments were undertaken to evaluate Cx43 phosphoforms in our endometrial biopsies. Results are shown for two representative controls and two representative endometriosis cases, selected for equal β-actin signal (intracellular protein concentration). In addition to reduced total Cx43 in the endometriosis tissues (Fig. 3, top panel), antibodies against the phospho- and non-phospho-Ser 368 isoforms of Cx43 indicated that these too were decreased in eutopic biopsies from endometriosis subjects, relative to controls (Fig. 3, second and third panels). By contrast, levels of the two different connexins, Cx26 and Cx32, and β-actin appeared comparable in cases and controls (Fig. 3, fourth to sixth panels). Four independent controls and four endometriosis cases examined with this set of antibodies yielded similar findings.
Figure 3.
Endometrial lysates from two representative control (C) and endometriosis (E) cases each immunoblotted with antibodies selective for total Cx43, phospho-serine (Ser) 368 and non-phospho-Ser 368 Cx43 isoforms. Cx26, Cx32 and β-actin also were quantified on the same blots. Molecular weights (in kD) are indicated.
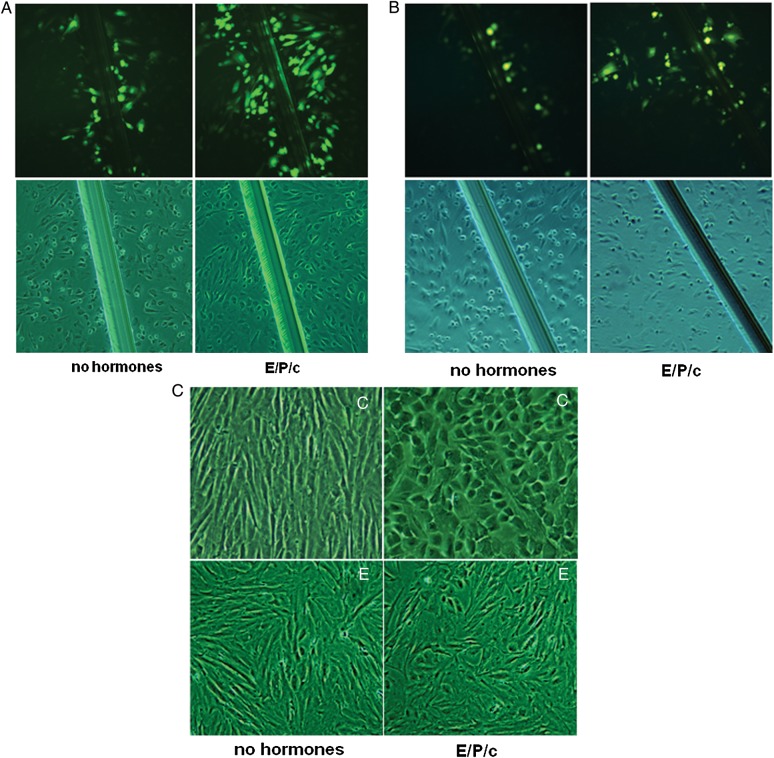
In a prior publication (Yu et al., 2011), we reported that exposure of proliferative phase, control ESC cultures to decidualizing hormones (E/P/c) induced an up-regulation of Cx43, concomitant with the classical biochemical marker of stromal differentiation, prolactin. In the current study, using a new series of study participants and specimens, we desired to test whether this response was due to an increase in functional GJIC and whether it might be different in cells derived from women without or with endometriosis. Thus, primary ESC prepared from biopsied, proliferative phase eutopic endometrial tissues from controls and endometriosis cases were compared. Scrape loading-dye transfer assays in cells derived from controls demonstrated that 7 days of incubation in the presence of decidualizing hormones (E/P/c) increased functional GJIC relative to those exposed to vehicle alone for 7 days (Fig. 4A). Image analysis of fluorescent signal along the scrape injury revealed a 6.1 ± 3.9-fold increase with E/P/c treatment in control ESC from six independent cultures. In ESC preparations derived from endometriosis subjects, basal GJIC was reduced (Fig. 4B) and hormone treatment resulted in smaller increase in fluorescence density (3.4 ± 1.8-fold; n = 6, Z = 2.74, P < 0.01) relative to untreated cells. This finding was confirmed when the cells were examined for morphological changes associated with decidualization. An experiment representative of six cultures is shown in Fig. 4C, where ESC derived from a proliferative phase control biopsy manifested typical fibroblastic stromal shape in the absence of hormones, but underwent transformation to polygonal, epithelioid cells after 7 days of E/P/c (right upper panel). By contrast, these effects were attenuated in cells derived from subjects with endometriosis, with few cells showing prominent decidual morphology (Fig. 4C, right lower panel), consistent with prior studies (Klemmt et al., 2006; Aghajanova et al., 2009a).
Figure 4.
(A) Primary ESC established from proliferative phase control biopsies and cultured for 7 days without (left panel) or with (right panel) estradiol, progesterone and dibutyryl cAMP (E/P/c) were scrape-loaded after a scalpel scratch. Gap junctions were revealed by diffusion of fluorescent Lucifer Yellow dye (upper panels). Phase contrast views (lower panels) show scrape injury. (B) Scrape-loading dye transfer in ESC monolayers derived from endometriosis cases under basal (left panel) and hormone-induced (right panel) conditions for 7 days. Phase contrast views (lower panels) show scrape injury. (C) ESC monolayers under phase contrast microscopy with fibroblastic morphology in the absence of hormones (left panels) and after 7 days of hormone exposure (E/P/c). Control (C, upper panels) and endometriosis-derived (E, lower panels) ESC.
Figure 5A shows a western blot of Cx43 levels (top panel) in ESC lysates prepared from nine control and nine endometriosis specimens, collected in the proliferative phase. As observed in the intact tissues, Cx43 levels were reduced in the endometriosis-derived cells when normalized for β-actin (bottom panel). Using digitized results, the calculated mean Cx43/β-actin ratios were 2.2 ± 0.9 in control ESC and 1.0 ± 0.3 in endometriosis ESC (n = 18, Z = 2.96, P < 0.01, Fig. 5B). In contrast, Cx26/β-actin ratios in control and endometriosis-derived ESC were 0.6 ± 0.1 and 0.6 ± 0.1, respectively (n = 6, Z = 0.67, P = 0.51, data not shown).
Figure 5.
(A) Western blotting for Cx43 (top panel) and β-actin (bottom panel) in ESC lysates prepared from nine control (C) and nine endometriosis (E) specimens. (B) Digitized western blots analyzed as integrated Cx43/β-actin density ratios in ESC.
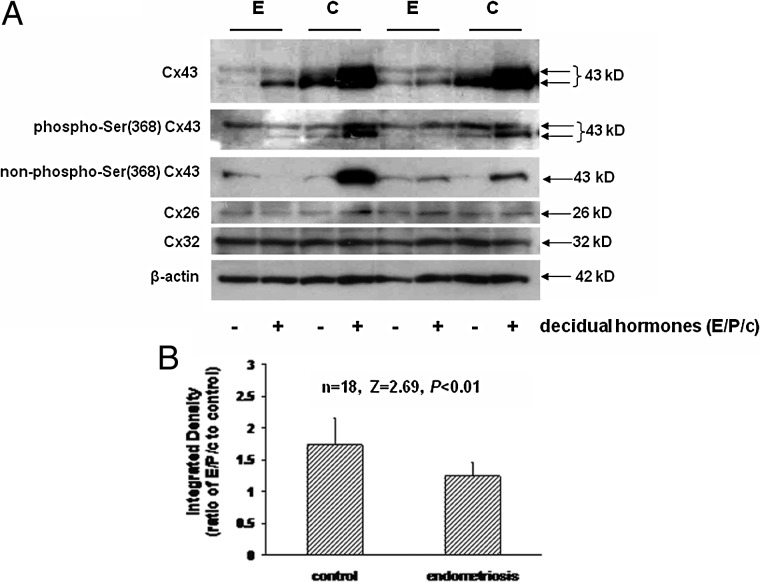
Further characterization of ESC responses to decidualization is shown in examples of cells derived from two endometriosis cases and two sets of control cells. Total Cx43 levels were lower under basal (−) and hormone-treated (+) conditions in samples derived from subjects with endometriosis (E) compared with controls (C) (Fig. 6A, top panel). Two major isoforms of Cx43 can be resolved in the ESC Westerns using antibodies against total Cx43 and Cx43 phosphorylated at serine (Ser) 368. The intensity of most Cx43 bands appear to increase in response to E/P/c, particularly in control ESC. In other experiments we have demonstrated that alkaline phosphatase treatment converts the upper band to the lower species in these cells (Wu et al., 2012), a finding consistent with the observation that antibodies specific for Cx43 not phosphorylated at Ser 368 selectively recognized the lower band (Fig. 6A, third panel). By contrast, Cx26 and Cx32 levels do not differ between endometriosis and control cells, nor are they affected much by decidualization (Fig. 6A, third panel). The lower level of expression of Cx26 in all the isolated ESC preparations is consistent with its predominant epithelial derivation (Fig. 1). Levels of β-actin also were neither affected by clinical group nor hormone treatment (Fig. 6A, lower panel). These findings are representative of four independent samples from each clinical group.
Figure 6.
(A) Total Cx43 levels under basal (−) and decidual hormone (E/P/c)-treated (+) conditions in ESC derived from subjects with endometriosis (E) and controls (C) (top panel). Two isoforms (arrows) correspond to putative phosphorylated (upper) and unphosphorylated (lower) species. Antisera selective for phospho-Ser 368 Cx43 (second panel), non-phospho-Ser 368 Cx43 (third panel), Cx26 (fourth panel), Cx32 (fifth panel) and β-actin (sixth panel). (B) Digitized western blots analyzed as integrated Cx43/β-actin density ratios without versus with 7 days of decidualizing hormones in control versus endometriosis ESC.
In nine control and nine endometriosis ESC lysates, prepared from proliferative phase specimens and treated for 7 days with E/P/c, the Cx43 response quantified on western blots was noted to be blunted in ESC derived from endometriosis cases. Whereas in vitro decidualization induced a mean increase in the Cx43/β-actin ratio of 1.7 ± 0.4-fold in control ESC, the same hormone treatment afforded only a 1.2 ± 0.2-fold increase of Cx43/β-actin ratio in endometriosis ESC (n = 18, Z = 2.69, P < 0.01, Fig. 6B).
These findings led us to consider the mechanisms by which Cx43 production was specifically regulated in ESC. To validate the detection of Cx43 mRNA in the cell culture model, end-point RT–PCR was undertaken using four samples each of ESC from control and endometriosis cases. As documented in Fig. 7A, amplicons of the expected base pair lengths for Cx43 and GAPDH transcripts were observed after 7 days without (−) or with (+) E/P/c exposure (Fig. 7A). To rigourously quantify Cx43 mRNA concentrations, qRT–PCR experiments were performed. An example of time-course analyses of ESC mRNA from a representative control and an endometriosis subject is provided in Fig. 7B. Cx43 transcript levels were determined at intervals over the 7-day E/P/c exposure using the 2ΔΔct method normalized to GAPDH mRNA. Although basal levels of Cx43 mRNA did not differ between control and endometriosis cells, the response to E/P/c treatment was greater in control cells with a maximal effect 24 h after hormone exposure. This finding is summarized in Fig. 7C, where nine control and nine endometriosis cultures revealed no differences in Cx43 mRNA under basal conditions (2ΔΔct = 10.9 ± 1.3 versus 10.7 ± 1.2; n = 18, Z = 0.31, P = 0.76). However, after 24 h of E/P/c, Cx43 transcripts increased 1.5 ± 0.7-fold in control cells, whereas no increase was noted in endometriosis cells (0.8 ± 0.5-fold; n = 18, Z = 1.90, P = 0.06). In contrast, neither Cx26 nor Cx32 mRNA levels differed between control versus endometriosis ESC under basal (P = 0.63 and 0.57, respectively) or decidualized conditions (P = 0.51 and 0.33, respectively; Fig. 7C).
Discussion
There is convincing evidence to support the association of endometriosis with reduced fecundity (D'Hooghe et al., 2003); however, establishing causation has been challenging. Several plausible, hypothetical mechanisms have been offered over the past decades: ovulatory dysfunction with inappropriate gonadotrophin secretion and luteal function (Cheesman et al., 1983); luteinized unruptured follicle syndrome (Ory, 1987); phagocytosis of spermatozoa by activated pelvic fluid macrophages (Martínez-Román et al., 1997); abnormal tubal anatomy (Fakih and Marshall, 1994) and dysfunctional gamete transport (Kissler et al., 2007). More recently, findings have converged from a few research groups that attribute endometriosis-induced subfertility to defective embryonic implantation (Klemmt et al., 2006; Aghajanova et al., 2009a, b; Weiss et al., 2009). In support of the latter hypothesis, we and others have documented that physiologically expressed mRNAs associated with endometrial receptivity are repressed in eutopic endometrial biopsies from women with surgically diagnosed endometriosis (Taylor et al., 2002; Kao et al., 2003; Giudice, 2004; Burney et al., 2007; Donaghay and Lessey, 2007; Eyster et al., 2007).
We observed nearly exclusive Cx43 immunostaining in the stromal compartment of control biopsies, but the signal was reduced and redistributed to scattered epithelial cells of the eutopic endometrium in endometriosis (Fig. 2A). Similar findings in human ectopic lesions (Regidor et al., 1997) and in the eutopic endometrium of baboons with experimental endometriosis (Winterhager et al., 2009) have been reported. The current studies are descriptive and only document an association between endometriosis and reduced endometrial Cx43 concentrations. However, through a combination of translational human cell and tissue-specific murine knock-out studies, we previously established a determinative role for Cx43 function on decidual differentiation and embryonic implantation (Laws et al., 2008; Yu et al., 2011). In both models, pharmacological or genetic ablation of Cx43 gap junctions prevented morphological and biochemical decidualization. The clinical significance of these observations is supported by findings that Cx43 levels are reduced in the decidua of women with recurrent early pregnancy loss (Nair et al., 2011) and the fact that the anti-malarial medication mefloquine, which blocks Cx43 gap junctions, is associated with an increased risk of spontaneous abortion (Nevin, 2011). It was of interest that we observed a selective reduction in Cx43 protein, but in neither of the related Cx26 or Cx32 proteins in endometrial biopsies from endometriosis cases (Figs 1 and 3). Moreover, we confirmed that isolated stromal cells obtained from women with endometriosis demonstrated ∼45% reduction in GJIC as manifested by Lucifer Yellow diffusion and had reduced basal and hormone-induced Cx43 protein production (Figs 5A and B and 6A and B), and an attenuated Cx43 mRNA response to 24 h of E/P/c exposure (Fig. 7C). ESC from endometriosis subjects also showed morphological evidence of impaired decidual differentiation in vitro (Fig. 4C). In agreement with these results, prior studies indicated that prolactin production was reduced by >50% in decidualized ESC derived from endometriosis subjects, compared with cells from endometriosis-free controls (Klemmt et al., 2006; Aghajanova et al., 2009a). Although Aghajanova et al. (2009a) observed impaired decidual responses in endometriosis-derived samples independent of the cycle phase at collection, we elected to compare only proliferative phase-derived cultures, as the growth characteristics of cells collected in this phase are most consistent and reproducible in our hands (Yu, Ryan and Taylor, unpublished results).
The endocrine regulation of endometrial Cx43 is complex and somewhat controversial. In the mouse and rat, Cx43 is up-regulated by estrogen and expressed in the decidua prior to placental invasion (Pauken and Lo, 1995; Grümmer et al., 1996). The temporospatial expression of Cx43 in cycling human endometrium was reported to be maximal in the periovulatory period with a predominantly stromal localization (Jahn et al., 1995). In contrast, Granot et al. (2000) observed an increase in Cx43 during the mid-late secretory phase, when P levels are greatest. According to our quantification of Cx43 in control endometrial tissue lysates, protein concentrations were 2-fold higher in secretory than in proliferative phase samples, consistent with our observation that in vitro decidualization of endometrial stromal cells afforded a 1.7-fold increase in Cx43 protein (Fig. 6B) and a 1.5-fold increase in Cx43 mRNA (Fig. 7C). Elevated human endometrial Cx43 in the secretory phase, when a combination of estrogen and P effects are present, also is in keeping with our murine studies. In both the delayed implantation and induced decidualization models in this species, P priming of the endometrium is required before estrogen can activate Cx43 expression in the uterine stroma (Laws et al., 2008).
A number of studies implicate a state of P insensitivity in subjects with endometriosis (Burney et al., 2007; Bulun et al., 2010). More recent research has uncovered several novel signaling molecules downstream of the P receptor, including the transcription factor CCAAT/enhancer-binding protein (C/EBP)-β, interleukin-11, signal transducer and activator of transcription 3 (STAT3), bone morphogenetic protein (BMP)-2, wingless-related murine mammary tumor virus integration site 4 (WNT4) and β-catenin that appear to function in a rather linear pathway to induce the ‘progestational’ phenotype of decidualized ESC (Wang et al., 2012; Li et al., 2013). Moreover, we have shown that pharmacological or genetic ablation of Cx43 gap junctions prevents morphological and biochemical decidualization in transgenic mice and human ESC (Laws et al., 2008; Yu et al., 2011). Thus, we postulate that the attenuated levels of Cx43 protein in cases of endometriosis cause failure of both ectopic and eutopic endometrium to undergo physiological differentiation during the secretory phase, presumably by limiting the intercellular transport of small signaling molecules that mediate the pathway implicated above. While there is an emerging interest in the role of connexin gene transcription (Firestone and Kapadia, 2012), these proteins are predominantly regulated through phosphorylation at serine residues in the carboxyl terminus of the molecule. Our qRT–PCR experiments revealed that basal concentrations of ESC mRNAs encoding Cx43, Cx26 and Cx32 did not differ based on their source. However, we did note that control ESC showed a 1.5-fold increase in Cx43 following 24 h of in vitro decidualization (Fig. 7C), whereas ESC derived from cycle phase-matched endometriosis cases failed to up-regulate Cx43 mRNA in response to hormones. This differential effect approached statistical significance (P = 0.06) based on a stringent, nonparametric test and was not observed with Cx26 and Cx32 transcripts, consistent with their stable protein responses. It should be noted that the kinetics of optimal Cx43 mRNA accumulation (∼24 h) differ substantially from those of Cx43 protein expression (∼7 days) following E/P/c treatment. This is not surprising given the extensive translational and post-translational modifications that Cx proteins undergo (Kjenseth et al., 2012). Studies by others, using RT–PCR arrays, reported that mRNAs encoding human gap junction proteins Cx30 and Cx31.9 were down-regulated in ectopic endometriosis lesions relative to autologous eutopic tissue in 11 subjects (Eyster et al., 2007), but comparisons to normal endometrial tissue were not performed. Further studies into the regulation of Cx43 gene expression in this condition are warranted.
Our study establishes that Cx43, a protein with well-characterized properties as a facilitator of cell–cell communication (Ramathal et al., 2010), can be added to the growing list of endometrial products whose production is impaired in cases of endometriosis. Unfortunately, many of the previously identified mRNAs and proteins [e.g. glycodelin (Taylor et al., 2002), dickkopf homolog-1 (Burney et al., 2007), prolactin, IGFBP-1 and calcitonin (Brosens and Gellersen, 2006; Aghajanova et al., 2009b)] have mostly unknown functions within the endometrium. Our new Cx43 findings prompt us to postulate that the intercellular transport of small (<1 kD) metabolites capable of traversing gap junctions is needed to promote the distinct morphological, biochemical and functional characteristics of endometrial receptivity, including angiogenesis (Laws et al., 2008; Yu et al., 2011), that optimize embryonic implantation. These actions appear to be compromised by dysfunctional GJIC in cases of endometriosis. Identification of the specific critical metabolites that traffic via gap junctions is likely to suggest novel interventions for endometriosis-associated subfertility.
Authors' roles
J.Y., I.C.B., M.K.B., N.S. and R.N.T.: study concept and design; J.Y., A.B., K.L.B., C.O.J., C.N. and R.N.T.: acquisition of data; J.Y., I.C.B., M.K.B., N.S. and R.N.T.: data analysis and interpretation; A.B., K.L.B. and C.N.: provision of clinical samples; J.Y., I.C.B., M.K.B., N.S. and R.N.T.: writing the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human DevelopmentNational Institutes of Health (USA)as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research (U54 HD55787 to J.Y., I.C.B., M.K.B. and R.N.T.) and the Cooperative Research Partnerships to Promote Workforce Diversity in the Reproductive Sciences (U01 HD66439 to N.S. and R.N.T.).
Conflict of interest
None declared.
Acknowledgements
The authors thank the operating room and nursing staffs who contributed to the study at Emory Midtown and Northside Hospitals, Atlanta, GA, USA and at Wake Forest Baptist Hospital, Winston-Salem, NC, USA. The helpful guidance and equipment of Dr Jorge Figueroa, Department of Obstetrics and Gynecology, allowed us to perform fluorescence quantification and Dr Dana Greene-Schloesser, Department of Radiation Oncology, Wake Forest School of Medicine, provided expert assistance with photomicroscopy. Dr Yihua Che, Bio-Rad Laboratories, provided valuable advice regarding qRT–PCR optimization and data analysis. The generous participation of the study subjects is greatly appreciated.
References
- Adamson GD, Kennedy S, Hummelshoj L. Creating solutions in endometriosis: global collaboration through the World Endometriosis Foundation. J Endometr. 2010;2:3–6. [Google Scholar]
- Aghajanova L, Hamilton A, Kwintkiewicz J, Vo KC, Giudice LC. Steroidogenic enzyme and key decidualization marker dysregulation in endometrial stromal cells from women with versus without endometriosis. Biol Reprod. 2009a;80:105–114. doi: 10.1095/biolreprod.108.070300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghajanova L, Velarde MC, Giudice LC. The progesterone receptor coactivator Hic-5 is involved in the pathophysiology of endometriosis. Endocrinology. 2009b;150:3863–3870. doi: 10.1210/en.2009-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Society for Reproductive Medicine. Revised ASRM classification for endometriosis: 1996. Fertil Steril. 1997;67:817–821. doi: 10.1016/s0015-0282(97)81391-x. [DOI] [PubMed] [Google Scholar]
- Barnhart K, Dunsmoor-Su R, Coutifaris C. Effect of endometriosis on in vitro fertilization. Fertil Steril. 2002;77:1148–1155. doi: 10.1016/s0015-0282(02)03112-6. [DOI] [PubMed] [Google Scholar]
- Brosens JJ, Gellersen B. Death or survival—progesterone-dependent cell fate decisions in the human endometrial stroma. J Mol Endocrinol. 2006;36:389–398. doi: 10.1677/jme.1.02060. [DOI] [PubMed] [Google Scholar]
- Buck Louis GM, Hediger ML, Peterson CM, Croughan M, Sundaram R, Stanford J, Chen Z, Fujimoto VY, Varner MW, Trumble A, et al. Incidence of endometriosis by study population and diagnostic method: the ENDO study. Fertil Steril. 2011;96:360–365. doi: 10.1016/j.fertnstert.2011.05.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulun SE, Cheng YH, Pavone ME, Xue Q, Attar E, Trukhacheva E, Tokunaga H, Utsunomiya H, Yin P, Luo X, et al. Estrogen receptor-beta, estrogen receptor-alpha, and progesterone resistance in endometriosis. Semin Reprod Med. 2010;28:36–43. doi: 10.1055/s-0029-1242991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, Lessey BA, Giudice LC. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;148:3814–3826. doi: 10.1210/en.2006-1692. [DOI] [PubMed] [Google Scholar]
- Cheesman KL, Cheesman SD, Chatterton RT, Jr, Cohen MR. Alterations in progesterone metabolism and luteal function in infertile women with endometriosis. Fertil Steril. 1983;40:590–595. [PubMed] [Google Scholar]
- Darr EA, Patel AD, Yu G, Komorowski Z, McCormick S, Tiwari R, Schantz SP, Geliebter J. Reduced Cx43 gap junction plaque expression differentiates thyroid carcinomas from benign disease. Arch Otolaryngol Head Neck Surg. 2011;137:1161–1165. doi: 10.1001/archoto.2011.186. [DOI] [PubMed] [Google Scholar]
- de Ziegler D, Borghese B, Chapron C. Endometriosis and infertility: pathophysiology and management. Lancet. 2010;376:730–738. doi: 10.1016/S0140-6736(10)60490-4. [DOI] [PubMed] [Google Scholar]
- D'Hooghe TM, Debrock S, Hill JA, Meuleman C. Endometriosis and subfertility: is the relationship resolved? Semin Reprod Med. 2003;21:243–254. doi: 10.1055/s-2003-41330. [DOI] [PubMed] [Google Scholar]
- Donaghay M, Lessey BA. Uterine receptivity: alterations associated with benign gynecological disease. Semin Reprod Med. 2007;25:461–475. doi: 10.1055/s-2007-991044. [DOI] [PubMed] [Google Scholar]
- Eyster KM, Klinkova O, Kennedy V, Hansen KA. Whole genome deoxyribonucleic acid microarray analysis of gene expression in ectopic versus eutopic endometrium. Fertil Steril. 2007;88:1505–1533. doi: 10.1016/j.fertnstert.2007.01.056. [DOI] [PubMed] [Google Scholar]
- Fakih H, Marshall J. Subtle tubal abnormalities adversely affect gamete intrafallopian transfer outcome in women with endometriosis. Fertil Steril. 1994;62:799–801. doi: 10.1016/s0015-0282(16)57007-1. [DOI] [PubMed] [Google Scholar]
- Firestone GL, Kapadia BJ. Minireview: regulation of gap junction dynamics by nuclear hormone receptors and their ligands. Mol Endocrinol. 2012;26:1798–1807. doi: 10.1210/me.2012-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellersen B, Brosens IA, Brosens JJ. Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin Reprod Med. 2007;25:445–453. doi: 10.1055/s-2007-991042. [DOI] [PubMed] [Google Scholar]
- Giudice LC. Microarray expression profiling reveals candidate genes for human uterine receptivity. Am J Pharmacogenomics. 2004;4:299–312. doi: 10.2165/00129785-200404050-00003. [DOI] [PubMed] [Google Scholar]
- Granot I, Dekel N, Bechor E, Segal I, Fieldust S, Barash A. Temporal analysis of connexin43 protein and gene expression throughout the menstrual cycle in human endometrium. Fertil Steril. 2000;73:381–386. doi: 10.1016/s0015-0282(99)00531-2. [DOI] [PubMed] [Google Scholar]
- Grümmer R, Reuss B, Winterhager E. Expression pattern of different gap junction connexins is related to embryo implantation. Int J Dev Biol. 1996;40:361–367. [PubMed] [Google Scholar]
- Gupta S, Goldberg JM, Aziz N, Goldberg E, Krajcir N, Agarwal A. Pathogenic mechanisms in endometriosis-associated infertility. Fertil Steril. 2008;90:247–257. doi: 10.1016/j.fertnstert.2008.02.093. [DOI] [PubMed] [Google Scholar]
- Hannan NJ, Nie G, Rainzcuk A, Rombauts LJ, Salamonsen LA. Uterine lavage or aspirate: which view of the intrauterine environment? Reprod Sci. 2012;19:1125–1132. doi: 10.1177/1933719112443879. [DOI] [PubMed] [Google Scholar]
- Jahn E, Classen-Linke I, Kusche M, Beier HM, Traub O, Grümmer R, Winterhager E. Expression of gap junction connexins in the human endometrium throughout the menstrual cycle. Hum Reprod. 1995;10:2666–2670. doi: 10.1093/oxfordjournals.humrep.a135764. [DOI] [PubMed] [Google Scholar]
- Kao LC, Germeyer A, Tulac S, Lobo S, Yang JP, Taylor RN, Osteen K, Lessey BA, Giudice LC. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology. 2003;144:2870–2881. doi: 10.1210/en.2003-0043. [DOI] [PubMed] [Google Scholar]
- Kissler S, Zangos S, Wiegratz I, Kohl J, Rody A, Gaetje R, Doebert N, Wildt L, Kunz G, Leyendecker G, et al. Utero-tubal sperm transport and its impairment in endometriosis and adenomyosis. Ann N Y Acad Sci. 2007;1101:38–48. doi: 10.1196/annals.1389.036. [DOI] [PubMed] [Google Scholar]
- Kjenseth A, Fykerud TA, Sirnes S, Bruun J, Yohannes Z, Kolberg M, Omori Y, Rivedal E, Leithe E. The gap junction channel protein connexin 43 is covalently modified and regulated by SUMOylation. J Biol Chem. 2012;287:15851–15861. doi: 10.1074/jbc.M111.281832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemmt PA, Carver JG, Kennedy SH, Koninckx PR, Mardon HJ. Stromal cells from endometriotic lesions and endometrium from women with endometriosis have reduced decidualization capacity. Fertil Steril. 2006;85:564–572. doi: 10.1016/j.fertnstert.2005.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyo N, Yamamoto H, Takeda Y, Ezumi K, Ngan CY, Terayama M, Miyake M, Takemasa I, Ikeda M, Doki Y, et al. Overexpression of connexin 26 in carcinoma of the pancreas. Oncol Rep. 2008;19:627–631. [PubMed] [Google Scholar]
- Laws MJ, Taylor RN, Sidell N, DeMayo FJ, Lydon JP, Gutstein DE, Bagchi MK, Bagchi IC. Gap junction communication between uterine stromal cells plays a critical role in pregnancy-associated neovascularization and embryo survival. Development. 2008;135:2659–2668. doi: 10.1242/dev.019810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Kannan A, Das A, DeMayo FJ, Hornsby PJ, Young SL, Taylor RN, Bagchi MK, Bagchi IC. WNT4 acts downstream of BMP2 and functions via β-catenin signaling pathway to regulate human endometrial stromal cell differentiation. Endocrinology. 2013;154:446–457. doi: 10.1210/en.2012-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez-Rosado L, Solan JL, Dunn CA, Norris RP, Lampe PD. Connexin43 phosphorylation in brain, cardiac, endothelial and epithelial tissues. Biochim Biophys Acta. 2012;1818:1985–1992. doi: 10.1016/j.bbamem.2011.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Román S, Balasch J, Creus M, Fábregues F, Carmona F, Vilella R, Vanrell JA. Immunological factors in endometriosis-associated reproductive failure: studies in fertile and infertile women with and without endometriosis. Hum Reprod. 1997;12:1794–1799. doi: 10.1093/humrep/12.8.1794. [DOI] [PubMed] [Google Scholar]
- Nair RR, Jain M, Singh K. Reduced expression of gap junction gene connexin 43 in recurrent early pregnancy loss patients. Placenta. 2011;32:619–621. doi: 10.1016/j.placenta.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Nevin RL. Mefloquine blockade of connexin 43 (Cx43) and risk of pregnancy loss. Placenta. 2011;32:712. doi: 10.1016/j.placenta.2011.06.016. [DOI] [PubMed] [Google Scholar]
- Nezhat C, Silfen S, Nezhat F, Martin D. Surgery for endometriosis. Curr Opin Obstet Gynecol. 1991;3:385–393. [PubMed] [Google Scholar]
- Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Am J Obstet Gynecol. 1975;122:262–263. doi: 10.1016/s0002-9378(16)33500-1. [DOI] [PubMed] [Google Scholar]
- Ory SJ. Pelvic endometriosis. Obstet Gynecol Clin North Am. 1987;14:999–1014. [PubMed] [Google Scholar]
- Pauken CM, Lo CW. Nonoverlapping expression of Cx43 and Cx26 in the mouse placenta and decidua: a pattern of gap junction gene expression differing from that in the rat. Mol Reprod Dev. 1995;41:195–203. doi: 10.1002/mrd.1080410210. [DOI] [PubMed] [Google Scholar]
- Pritts EA, Ryan IP, Mueller MD, Lebovic DI, Shifren JL, Zaloudek CJ, Korn AP, Darney PD, Taylor RN. Angiogenic effects of norplant contraception on endometrial histology and uterine bleeding. J Clin Endocrinol Metab. 2005;90:2142–2147. doi: 10.1210/jc.2004-1692. [DOI] [PubMed] [Google Scholar]
- Ramathal CY, Bagchi IC, Taylor RN, Bagchi MK. Endometrial decidualization: of mice and men. Semin Reprod Med. 2010;28:17–26. doi: 10.1055/s-0029-1242989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regidor PA, Regidor M, Schindler AE, Winterhager E. Aberrant expression pattern of gap junction connexins in endometriotic tissues. Mol Hum Reprod. 1997;3:375–381. doi: 10.1093/molehr/3.5.375. [DOI] [PubMed] [Google Scholar]
- Ryan IP, Schriock ED, Taylor RN. Isolation, characterization, and comparison of human endometrial and endometriosis cells in vitro. J Clin Endocrinol Metab. 1994;78:642–649. doi: 10.1210/jcem.78.3.8126136. [DOI] [PubMed] [Google Scholar]
- Simoens S, Hummelshoj L, D'Hooghe T. Endometriosis: cost estimates and methodological perspective. Hum Reprod Update. 2007;13:395–404. doi: 10.1093/humupd/dmm010. [DOI] [PubMed] [Google Scholar]
- Taylor RN, Lundeen SG, Giudice LC. Emerging role of genomics in endometriosis research. Fertil Steril. 2002;78:694–698. doi: 10.1016/s0015-0282(02)03325-3. [DOI] [PubMed] [Google Scholar]
- Wang W, Taylor RN, Bagchi IC, Bagchi MK. Regulation of human endometrial stromal proliferation and differentiation by C/EBPβ involves cyclin E-cdk2 and STAT3. Mol Endocrinol. 2012;26:2016–2030. doi: 10.1210/me.2012-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss G, Goldsmith LT, Taylor RN, Bellet D, Taylor HS. Inflammation in reproductive disorders. Reprod Sci. 2009;16:216–229. doi: 10.1177/1933719108330087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterhager E, Stutenkemper R, Traub O, Beyer E, Willecke K. Expression of different connexin genes in rat uterus during decidualization and at term. Eur J Cell Biol. 1991;55:133–142. [PubMed] [Google Scholar]
- Winterhager E, Grümmer R, Mavrogianis PA, Jones CJ, Hastings JM, Fazleabas AT. Connexin expression pattern in the endometrium of baboons is influenced by hormonal changes and the presence of endometriotic lesions. Mol Hum Reprod. 2009;15:645–652. doi: 10.1093/molehr/gap060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Taylor RN, Sidell N. Retinoic acid regulates gap junction intercellular communication in human endometrial stromal cells through modulation of the phosphorylation status of connexin 43. J Cell Physiol. 2012;228:903–910. doi: 10.1002/jcp.24241. [DOI] [PubMed] [Google Scholar]
- Yu J, Wu J, Bagchi IC, Bagchi MK, Sidell N, Taylor RN. Disruption of gap junctions reduces biomarkers of decidualization and angiogenesis and increases inflammatory mediators in human endometrial stromal cell cultures. Mol Cell Endocrinol. 2011;344:25–34. doi: 10.1016/j.mce.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]