Abstract
Circadian phase and its relation to sleep are increasingly recognized as fundamental factors influencing human physiology and behavior. Dim light melatonin onset (DLMO) is a reliable marker of the timing of the circadian clock, which has been used in experimental, clinical, and descriptive studies in the past few decades. Although DLMO and its relationship to sleep have been well documented in school-aged children, adolescents, and adults, very little is known about these processes in early childhood. The purpose of this study was 1) to describe circadian phase and phase angles of entrainment in toddlers and 2) to examine associations between DLMO and actigraphic measures of children's nighttime sleep. Participants were 45 healthy toddlers aged 30 to 36 months (33.5 ± 2.2 months; 21 females). After sleeping on a parent-selected schedule for 5 days (assessed with actigraphy and diaries), children participated in an in-home DLMO assessment involving the collection of saliva samples every 30 minutes for 6 hours. Average bedtime was 2015 ± 0036 h, average sleep onset time was 2043 ± 0043 h, average midsleep time was 0143 ± 0038 h, and average wake time was 0644 ± 0042 h. Average DLMO was 1929 ± 0051 h, with a 3.5-hour range. DLMO was normally distributed; however, the distribution of the bedtime, sleep onset time, and midsleep phase angles of entrainment were skewed. On average, DLMO occurred 47.8 ± 47.6 minutes (median = 39.4 minutes) before bedtime, 74.6 ± 48.0 minutes (median = 65.4 minutes) before sleep onset time, 6.2 ± 0.7 hours (median = 6.1 hours) before midsleep time, and 11.3 ± 0.7 hours before wake time. Toddlers with later DLMOs had later bedtimes (r = 0.46), sleep onset times (r = 0.51), midsleep times (r = 0.66), and wake times (r = 0.65) (all p < 0.001). Interindividual differences in toddlers’ circadian phase are large and associated with their sleep timing. The early DLMOs of toddlers indicate a maturational delay in the circadian timing system between early childhood and adolescence. These findings are a first step in describing the fundamental properties of the circadian system in toddlers and have important implications for understanding the emergence of sleep problems and the consequences of circadian misalignment in early childhood.
Keywords: circadian phase, melatonin, DLMO, sleep, phase angle of entrainment, early childhood, toddlers, children
The near 24-hour oscillation of the human circadian clock is essential for coordinating many physiological processes as well as complex behaviors such as the preferred timing for sleeping and eating (Vollmers et al., 2009). The suprachiasmatic nuclei (SCN) of the anterior hypothalamus comprise the circadian “master clock” that organizes mammalian circadian rhythms (Lydic et al., 1980; Rusak and Zucker, 1979; Swaab et al., 1985). The output of the SCN to the pineal gland promotes the release of the neurohormone melatonin. During entrainment of individuals maintaining a diurnal schedule, melatonin levels rise before sleep, peak during the early morning hours, and drop to low daytime levels just after morning awakening (Arendt et al., 1977; Wehr, 1991). The secretory pattern of melatonin is commonly used to quantify the timing of the circadian clock by taking samples at given intervals (e.g., 30 minutes, 60 minutes) in dimly lit conditions to avoid light-suppressing effects on melatonin production (Lewy et al., 1980). The point at which salivary melatonin levels cross a specific threshold is known as dim light melatonin onset (DLMO). DLMO is a well-established reliable marker of circadian phase (Benloucif et al., 2005; Klerman et al., 2002) that has been utilized in experimental, clinical, and descriptive studies in the past few decades.
An established literature indicates that the circadian clock driving endogenous rhythms modulates the timing, duration, and structure of sleep (Dijk and Czeisler, 1995). Sleep in turn indirectly influences entrainment of the circadian system to the 24-hour solar day through the “gating” of light/dark cycles (Kalsbeek et al., 2006). In adolescents and adults, variability in circadian phase is strongly associated with the timing of sleep such that individuals with later DLMOs are more likely to have later bedtimes, midsleep times, and morning wake times (Burgess and Eastman, 2005; Crowley et al., 2006; Wright et al., 2005). Sleep and/or wakefulness occurring at inappropriate circadian phases results in circadian misalignment (Markwald et al., 2013), which is recognized as a factor contributing to the increased risk of disease (Tenkanen et al., 1998), work-related accidents (Gold et al., 1992), and poor school performance (Wolfson and Carskadon, 1998). Such misalignment can be quantified as the phase angle of entrainment, or the time between circadian phase and a recurring behavioral event (e.g., interval between DLMO and bedtime, sleep onset time, wake time). Published data on sleep and circadian links, as well as the consequences of circadian misalignment in early childhood, are scarce (LeBourgeois et al., in press).
Although oscillation of the circadian clock is a fundamental biological process, the timing of the circadian system and its relationship to sleep are not stable across the life span. For example, Carskadon and colleagues (2002b) have demonstrated changes in the circadian system associated with pubertal development, such that the markedly later bedtimes observed across adolescence are in part due to a delay in the timing of melatonin onset (Carskadon et al., 1997; Carskadon et al., 1993). The phase angle of entrainment of DLMO to sleep onset becomes wider between adolescence and adulthood (Crowley et al., 2006; Wright et al., 2005). Furthermore, comparisons of findings from studies of mature adolescents, adults, and older individuals suggest an age-related advance in circadian phase associated with earlier bedtimes and rise times with increasing age (Crowley et al., 2006; Duffy et al., 1999). Such findings provide evidence of maturational circadian shifts, which likely occur as a result of an interaction between changing biological processes and salient social and environmental time cues.
The timing of the circadian clock and its relationship to sleep have not been described in toddlers. Understanding such processes is important because early childhood is a time of significant change in the duration, timing, and quality of sleep. Findings from longitudinal and cross-sectional studies indicate that parent-reported 24-hour sleep duration declines from approximately 13.0 hours at age 2 years to approximately 11.5 hours at age 5 years (Crosby et al., 2005; Iglowstein et al., 2003). Sleep is biphasic in almost all 2-year-old children, including one long nocturnal sleep episode and a relatively short afternoon nap. Daytime napping diminishes thereafter, with most, but not all, 5-year-old children sleeping only at night (Acebo et al., 2005; Weissbluth, 1995). Coincident with these developmental sleep changes are parental reports of sleep disturbance (e.g., bedtime resistance, sleep onset delay, prolonged night awakenings), which affects approximately 25% of American children (Owens, 2008). Sleep disturbance in early childhood often persists into the school-age years (Kataria et al., 1987), is associated with internalizing, externalizing, and attentional problems (Hvolby et al., 2008; Lavigne et al., 1999), and independently predicts the onset of emotional and cognitive problems in adolescence (Friedman et al., 2009; Gregory et al., 2004; Gregory and O'Connor, 2002). Normal shifts in sleep and the onset of sleep problems are likely influenced by interactions of biologically based, maturing sleep homeostatic and circadian processes in the context of the social demands, opportunities, and stressors experienced by the child (Jenni and LeBourgeois, 2006; Jenni and O'Connor, 2005).
In this study, we examined circadian phase and its associations with sleep in a sample of napping toddlers. Children's sleep was assessed with actigraphy for 5 days prior to an in-home, child-friendly, salivary DLMO protocol. The intervals between DLMO and sleep timing measures were quantified as phase angles of entrainment. Based on previous findings in adolescents and adults, we hypothesized earlier DLMOs and more narrow bedtime phase angles in young children than older age groups. Also, because young children have relatively longer nighttime sleep intervals (Iglowstein et al., 2003), we hypothesized that midsleep and wake time phase angles would be wider than what are observed in adolescents and adults. Finally, we tested whether circadian phase was associated with the timing of sleep in toddlers, such that those with later bedtimes, sleep onset times, midsleep times, and wake times would also have later DLMOs.
MATERIALS AND METHODS
Participants
Participants were 45 healthy children (21 females; 37 white, 1 African American, 7 mixed race) aged 30 to 36 months (33.5 ± 2.2 months). Families were recruited from Providence, Rhode Island, and Boulder, Colorado, at community events and via flyers and laboratory website advertisements. Parents completed a telephone screening and questionnaires to assess inclusion/exclusion criteria. Children were included if they were 30 to 36 months of age at the time of assessment and had a daily nap opportunity, during which time they fell asleep at least 3 days per week. Children were excluded if they 1) participated in regular cosleeping; 2) had a bedtime/wake time sleep schedule that differed >2 hours between week-days and weekends; 3) had traveled beyond 2 time zones within 3 months of the study; 4) regularly used medications affecting sleep, alertness, or the circadian system; 5) had diagnosed sleep problems; 6) had developmental disabilities, neurological/metabolic disorders, chronic medical conditions, lead poisoning, or a head injury involving loss of consciousness; 7) had a conceptual age of ≤37 weeks or >42 weeks; 8) were low birth weight (<5.5 lb); or 9) had a family history (first degree) of diagnosed narcolepsy, psychosis, or bipolar disorder.
One hundred seventy-six children were screened. Of these, 83 met study inclusion criteria, 64 were enrolled, and 53 completed the study. Eleven children did not participate due to inadequate saliva production during training sessions. Three children completed the study but were excluded due to a “missed” DLMO; 5 others completed but were dropped because they did not nap during the 5 days before the DLMO assessment. Thus, this analysis includes a total of 45 children. All parents signed an informed consent form approved by the Brown University or University of Colorado Boulder institutional review board. Families were compensated with $50 cash, and children received a $50 United States savings bond following completion of the study.
Protocol
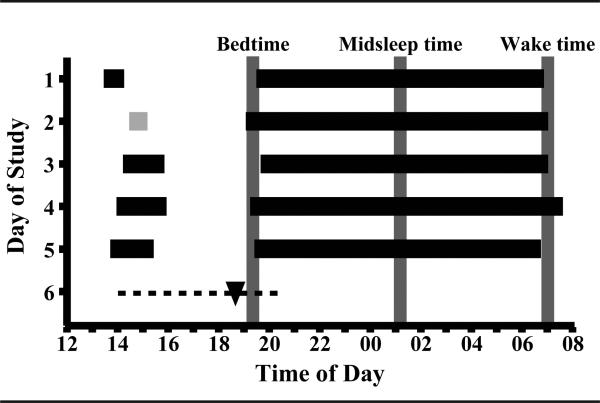
Figure 1 illustrates the study protocol in an exemplary participant. Children followed a parent-selected sleep schedule during study days 1 to 5, during which researchers made several in-home visits to train children in providing saliva samples. The DLMO assessment occurred on day 6 of the study. During the entire protocol, children neither consumed caffeine nor took medications affecting their sleep and/or circadian rhythms. Parents reported daily on their child's sleep patterns and compliance with study protocol rules. Data were neither collected during summer months (June to August) nor during the 1-week following daylight saving time changes.
Figure 1.
Study protocol for 1 child showing the parent-selected sleep schedule during the 5 days preceding the DLMO assessment. Horizontal black bars indicate actigraphically-verified time in bed, and gray bars represent a nap opportunity without sleep. The dotted line indicates the in-home saliva collection interval, and DLMO time is noted with a black triangle. Vertical gray lines show average bedtime, midsleep time, and wake time.
Measures
Sleep diary
Parents completed a daily sleep diary throughout the study. Evening diary questions inquired about the times that the actigraph was not worn, bedtime, lights-off time, and sleep onset latency. Morning questions included morning wake time and get out of bed time. Sleep diaries were used to ensure compliance with the study protocol and to aid with scoring of actigraphy data (Acebo et al., 1999).
Actigraphy
Children wore an actigraph (model AW64, Minimitter, Bend, OR, USA, or model AW Spectrum, Philips/Respironics, Pittsburg, PA, USA) on their nondominant wrist throughout the study to provide continuous recordings of sleep/wakefulness states via measurement of motor activity. Actiware Sleep V5.59 software (Phillips/Respironics, Pittsburg, PA, USA) was used to estimate 1-minute epochs as either sleep or wakefulness based upon activity levels in the surrounding 2-minute interval. This algorithm was applied to portions of the record identified as sleep through a combination of diary reports and actigraph event markers set by parents at “lights-off” and “lights-on.” In comparison to videosomnography in preschoolers, this algorithm shows high overall epoch-by-epoch agreement (94%) and is excellent in detecting sleep (sensitivity = 97%); however, it overestimates wake during the sleep period (specificity = 24%) (Sitnick et al., 2008). Standard scoring rules were applied to each sleep episode: sleep start was the first of 3 epochs of sleep after lights-off, and sleep end was the last of 5 epochs of sleep before lights-on (Acebo et al., 2005). Sleep episodes were excluded when 1) the actigraph was off for all/part of the sleep period, 2) the concurrent diary report was not available, or 3) the episode included external motion (e.g., sleeping in a car, stroller). Actigraphic estimates averaged across study days 1 to 5 included lights-off time (bedtime), sleep start time (sleep onset time), midsleep time (midpoint between sleep start and sleep end), and sleep end time (wake time). Phase angle of entrainment measures were calculated using these actigraphic sleep measures. We also computed 24-hour sleep duration as the interval from sleep start to sleep end for daytime and nighttime sleep episodes. Due to noncompliance (n = 2) or technical failure (n = 2), no actigraphy data were available; in these cases, we used daily diary data to compute sleep and phase angle of entrainment variables. Thus, actigraphy data were available for 41 children, with measures computed as the aggregate of 5 days/nights in 68%, 4 days/nights in 25%, and 3 days/nights in 7% of children.
Salivary DLMO assessment
Children participated in an in-home DLMO assessment on study day 6. In the early afternoon, researchers transformed the family's home into a dimly lit “cave” by blocking light from windows with black plastic attached with painter's tape and controlling light sources with dimmer switches and very low-power bulbs. Children entered dim light conditions (0.01-10.90 lux at angle of gaze) at least 1 hour before the first sample, where they remained throughout the saliva collection interval. Children provided saliva samples (~2 mL) every 30 minutes for 6 hours, ending 1 hour past their average parent-reported bedtime during study days 1 to 5 (12 samples total). With help from a researcher, children rinsed their mouths with water and, if needed, gently brushed their teeth with water for samples collected after eating (>15 minutes before obtaining each saliva sample). Children remained in a sitting posture for at least 5 minutes prior to and during collection of each saliva sample. Saliva samples were collected by having children chew on a braided dental cotton roll (Henry Schein Inc., Denver, PA, USA) for 1 to 2 minutes. Lux was measured with each saliva sample using a light meter (Extech Instruments, Spring Hill, FL, USA) held approximately 5 cm adjacent to the child's eye and directed in the angle of gaze. Samples were immediately centrifuged (LabEssentials Inc., Monroe, GA, USA) and refrigerated on site. Samples were then transported to the laboratory and frozen (−20 °C) within 12 hours. Assays were performed at the Bradley Hospital Sleep and Chronobiology Laboratory (Providence, RI, USA) or SolidPhase Inc. (Portland, ME, USA) using radioimmunoassay (ALPCO Diagnostics, Salem, NH, USA), with a minimum detection of 0.2 pg/mL. The intra-assay and interassay coefficients of variation for evening time levels of salivary melatonin were 4.1% and 6.6%, respectively.
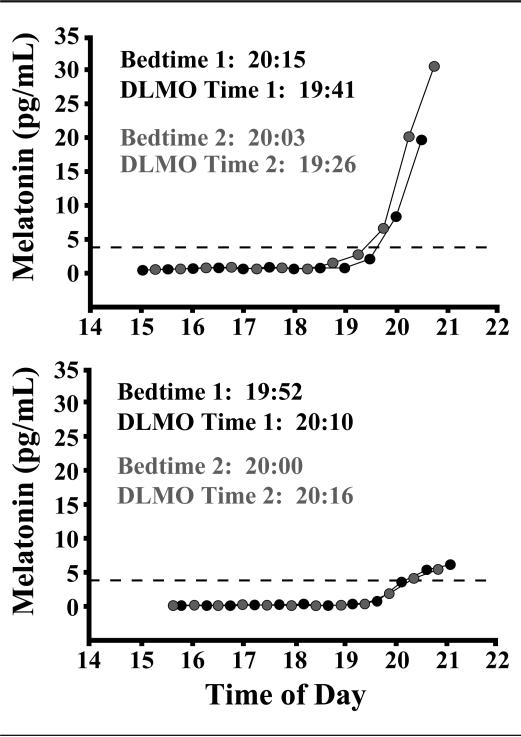
Figure 2 shows example melatonin profiles for 2 children with repeated DLMO assessments after a 1-week interval. The DLMO times were stable, with a change of 15 minutes for one child (Fig. 2A) and 6 minutes for another child (Fig. 2B); the amount and direction of change corresponded directly to differences in average bedtimes between the 2 weeks.
Figure 2.
Repeatability of melatonin profiles of 2 participants, 1 male (A) and 1 female (B), from 2 separate DLMO assessments performed 1 week apart. Dotted lines represent a DLMO threshold of 4 pg/mL.
Data Processing and Analysis
DLMO phase was defined as the clock time that evening salivary melatonin concentrations increased and remained above a 4-pg/mL threshold using linear interpolation between successive samples. This is a well-accepted standard with school-aged children and adolescents (Carskadon et al., 1997) and was derived from reports that salivary melatonin concentrations are about 40% of plasma levels in healthy young adults (10 pg/mL is the most common DLMO threshold for plasma melatonin) (Deacon and Arendt, 1994). Bedtime, sleep onset time, midsleep time, and wake time phase angles of entrainment were computed as the time interval between DLMO and averaged (study days 1-5) actigraphic estimates of the sleep variables.
Statistical analyses were performed with PASW Statistics Package 21.0 (IBM Corp., Armonk, NY, USA). Summary measures are presented as mean ± SD. Pearson correlations were used to assess associations between continuous variables.
RESULTS
Descriptive statistics for circadian and actigraphic sleep variables are presented in Table 1. DLMO, all sleep variables, and wake time phase angle were normally distributed in this sample of healthy toddlers; however, moderate skewness was observed for bedtime phase angle (mean = −46.8 minutes; median = −39.4 minutes) and sleep onset phase angle (mean = −74.6 minutes; median = −65.4 minutes). Midsleep time phase angle also showed mild positive skewness (mean = 6.2 hours; median = 6.1 hours). We found no sex differences in DLMO or in any phase angle of entrainment measures.
Table 1.
Descriptive statistics for circadian and actigraphic sleep measures (n = 45)
| All Subjects | Mean ± SD | Range |
|---|---|---|
| DLMO, h | 1929 ± 0051 | 1735 to 2107 |
| Bedtime, h | 2015 ± 0036 | 1903 to 2200 |
| Sleep onset time, h | 2043 ± 0043 | 1912 to 2231 |
| Midsleep time, h | 0143 ± 0038 | 0000 to 0255 |
| Wake time, h | 0644 ± 0042 | 0441 to 0758 |
| Bedtime phase angle, min | −46.8 ± −47.6 | −184.8 to 24.6 |
| Sleep onset time phase angle, min | −74.6 ± −48.0 | −214.8 to 15.6 |
| Midsleep time phase angle, h | 6.2 ± 0.7 | 5.1 to 8.1 |
| Wake time phase angle, h | 11.3 ± 0.7 | 9.9 to 12.8 |
| Night sleep duration, h | 10.0 ± 0.7 | 8.8 to 11.6 |
| Nap sleep duration, min | 103.5 ± 19.4 | 59.0 to 155.5 |
| 24-hour total sleep duration, h | 11.7 ± 0.6 | 10.3 to 13.0 |
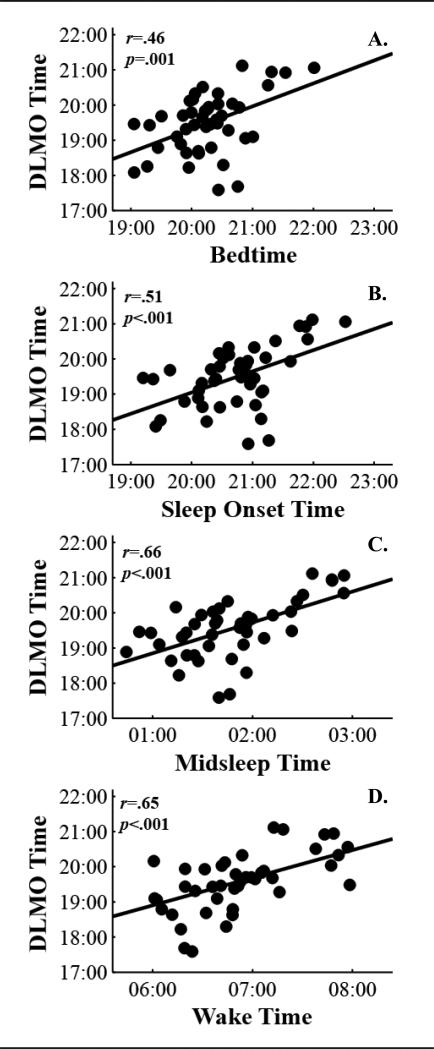
As shown in Figure 3, DLMO was moderately correlated with actigraphic sleep timing measures such that toddlers with later DLMOs were more likely to have later bedtimes (r = 0.46), sleep onset times (r = 0.51), midsleep times (r = 0.66), and wake times (r = 0.65) (all p < 0.001). DLMO was not correlated with nighttime or 24-hour sleep duration.
Figure 3.
Scatterplots of associations between DLMO time and actigraphic estimates of bedtime (A), sleep onset time (B), mid-sleep time (C), and wake time (D). Lines reflect the slope of the linear regression.
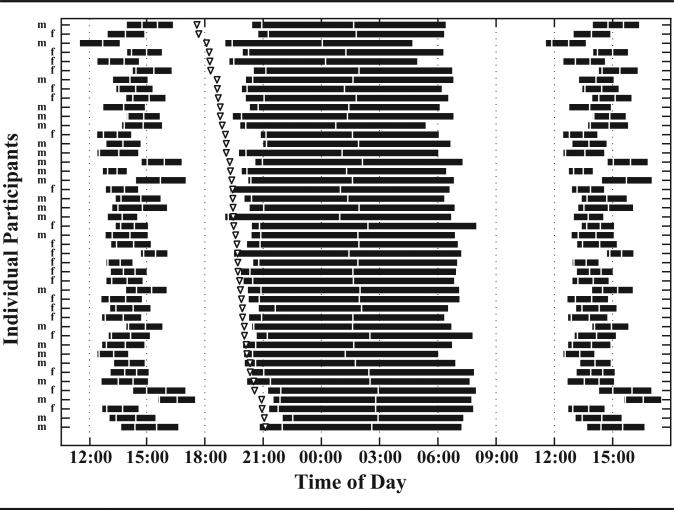
Figure 4 illustrates the wide interindividual differences we observed in not only DLMO but also its relationship to sleep timing. It is visually apparent that toddlers with early DLMOs have wider bedtime and sleep onset phase angles of entrainment, while children with later DLMOs have bedtimes very close to the onset of evening melatonin. In fact, about 20% of parents (n = 10) selected bedtimes before their children's DLMOs, thus resulting in positive bedtime phase angles of entrainment (Fig. 3A).
Figure 4.
Individual differences in DLMO and its relationship to nighttime sleep and daytime napping (n = 45). The DLMO time is noted by triangles. Black horizontal bars represent the average diary- and actigraphic-verified parent-selected sleep episodes (time in bed) during the 5 days before the corresponding DLMO assessment, ordered from earliest to latest DLMO. White vertical lines show sleep onset time and midsleep time. Naps are double plotted to permit observation of the association between daytime sleep, nighttime sleep, and wake timing. Sex is noted as male (M) and female (F).
DISCUSSION
To our knowledge, this is the first study to describe the timing of the endogenous circadian clock and its relationship to nighttime sleep in toddlers. We developed child- and family-friendly in-home procedures simulating standard DLMO assessments used with adolescents and adults in laboratory and field settings. We also obtained multimodal assessments of sleep, including daily parent reports, sleep diaries, and actigraphy. Several main findings emerged from this study using such well-controlled procedures. First, the average melatonin onset in healthy, good-sleeping 30- to 36-month-old children occurred at 1929 h, with times ranging from 1735 h to 2107 h. Second, substantial interindividual differences in circadian phase and associated phase angles of entrainment are apparent in early childhood. Third, the midsleep phase angle of entrainment was approximately 6 hours, whereas the wake time phase angle of entrainment was approximately 11 hours. Finally, DLMO was moderately associated with bedtime, sleep onset time, midsleep time, and wake time.
To date, published data on the timing of the circadian system in individuals younger than 9 years of age are scarce. Our findings mark one important step in addressing this gap and suggest a nonlinear, inverted U-shaped relationship between circadian phase and age across the life span. An advance in circadian phase from adulthood to old age has been supported by cross-sectional findings of an average DLMO in young adults aged 22 to 39 years of 2251 h, as compared to a DLMO of 2132 h in middle-aged adults of 40 to 58 years (Kawinska et al., 2005) and 2104 h in adults older than 65 years of age (Gooneratne et al., 2003). In this study, toddlers exhibited an average DLMO of 1929 h, which is approximately 60 minutes earlier than that in 9- to 12-year-old children (2028 h) and approximately 75 minutes earlier than that in 13- to 16-year-old adolescents (2041 h) studied during the academic year (Crowley et al., 2006). Our findings indicate a delay in circadian phase between early and late childhood; however, whether such a change occurs gradually or in a more abrupt step-wise fashion related to intrinsic processes and/or extrinsic demands is an unanswered question best addressed with the longitudinal assessment of children in the first decade of life.
Because toddlers have relatively long sleep episodes and early bedtimes, we hypothesized that their phase angles of entrainment would differ from older age groups, which was supported by our findings. Results from several laboratory studies indicate that the interval between DLMO and wake time ranges from 10.3 to 10.7 hours in adolescents and young adults (Burgess and Eastman, 2005a; Crowley et al., 2006), which is over 1 hour shorter than what we observed in young children (11.2 hours). Furthermore, Wright and colleagues (2005) found that the bedtime phase angle of adults was 129 minutes, while Crowley and colleagues (2006) reported an average bedtime phase angle of 116 minutes in older adolescents and 71 minutes in young adolescents. The median interval between bedtime and DLMO in this sample of toddlers was approximately 40 minutes, which was approximately 30 minutes shorter than the sleep onset DLMO interval. Thus, while adults typically choose bedtimes about 2 hours after the onset of melatonin in the evening (Burgess and Eastman, 2005; Kawinska et al., 2005; Wright et al., 2005), parents appear to select bedtimes for their young children that are much closer to their DLMO. In some cases (20%), children in this study were even put to bed before their DLMO, leading to a positive phase angle. Indeed, in a subset of napping toddlers from the current sample, narrower bedtime phase angles of entrainment, including those of a positive nature, were associated with longer sleep onset latencies and increased bedtime resistance (LeBourgeois et al., in press). Taken together, these findings have implications for understanding circadian misalignment, as well as identifying optimal bedtimes in early childhood, which are a common concern of parents and a topic of interest in the media.
Similar to previous studies of adolescents and adults (Burgess and Eastman, 2005; Crowley et al., 2006; Sletten et al., 2010; Wright et al., 2005), we found a large variability in the timing of DLMO and in all phase angle of entrainment measures. Interactions between intrinsic and extrinsic factors may account for the observed variation in circadian parameters. For example, individual differences in circadian period (tau) predict the observed variability in bedtime phase angles of entrainment (Duffy et al., 2011; Duffy et al., 2001; Gronfier et al., 2007; Wright et al., 2005), and exposure to a strong zeitgeber such as natural sunlight reduces individual differences in circadian phase (Wright et al., 2013). Genetic differences in circadian clock genes may also contribute to variability in circadian phase, as there is a growing body of evidence linking several polymorphisms to circadian preference but not to circadian phase per se (Archer et al., 2003; Gau et al., 2007; Katzenberg et al., 1998). Changes in light exposure patterns may also influence differences in circadian phase, and variability in sleep schedules may gate the light/dark cycle to elicit either a phase delay or phase advance depending on the timing, intensity, and duration of light exposure (Czeisler et al., 1989; Dewan et al., 2011; Zeitzer et al., 2005). Finally, a developmental change in the diurnal sensitivity to light has been suggested; however, further research is needed to support this hypothesis (Carskadon et al., 2002a; Crowley et al., 2007).
Our current findings add to an ongoing dialogue of the bidirectional relationship between the sleep and circadian systems. In this study, DLMO was positively associated with bedtime, sleep onset time, midsleep time, and wake time. As the timing of the circadian clock may “gate” sleep, our results suggest that individual differences in DLMO contribute to the timing of sleep in young children and help explain the striking variability in parental reports of sleep in the early years of life (Iglowstein et al., 2003). Our findings also extend those of prior studies aimed at determining what sleep parameters most strongly influence the circadian clock. The close correspondence between bedtime and circadian phase suggests that the “lights-off” time selected by parents plays an important role in determining DLMO timing. Midsleep time, which is derived from both bedtime and wake time, has consistently been associated with DLMO (Burgess and Eastman, 2005a; Crowley et al., 2006; Martin and Eastman, 2002; Wright et al., 2013). It has been postulated that circadian phase is most dependent on wake time because light exposure in the morning is more intense (Burgess and Eastman, 2005a). This assumption was supported by our findings, as we observed a moderate (r = 0.65) association between wake time and DLMO in young children. However, we also found moderate associations between melatonin onset and bedtime (r = 0.46) and sleep onset time (r = 0.51). Further understanding of our results would require a phase response curve to light in children, which has yet to be established. Additionally, future research should examine associations between light exposure at different times of the day and the timing of the circadian clock in young children.
Although this study provides some unknown pieces of the circadian puzzle in early childhood, it has some limitations that support the need for additional studies. Our findings are correlational, and thus, we cannot imply causation. Future research should test the effects of altered sleep schedules on circadian phase. We enrolled only children who were good sleepers with relatively stable sleep schedules, which limits the generalizability of our findings. Also, the current analysis focused on the relationship specifically between circadian phase and nighttime sleep in only napping children. Given that early childhood is characterized by a biphasic sleep pattern that varies across individuals, further analyses are needed to understand links between napping and the timing of the circadian clock. Circadian parameters in nonnappers may differ as a function of experiencing greater sleep pressures in the evening. Finally, it is unclear whether the tight association between DLMO and sleep onset time is driven primarily by the strength of the circadian signal, a more intense homeostatic sleep drive, or a combination of both. Clearly, additional research in children with more variable sleep schedules and/or with sleep problems is warranted.
In summary, these findings are an important first step in understanding the fundamental properties of the circadian timing system in the early years of life, which may be clinically relevant. Parents report sleep problems in about 25% of young children, which often persist across childhood (Kataria et al., 1987; Owens, 2008). Understanding the timing of the circadian clock in young children may help improve the diagnosis and treatment of sleep and circadian disorders in the general population. Our findings also provide a foundation for future studies examining circadian rhythms in clinical populations, such as children with autism spectrum disorders, where sleep problems may be attributed to circadian rhythm disturbances and decreased melatonin production (Melke et al., 2008). Therefore, knowledge of circadian rhythms in normally developing children may help clinicians appropriately time treatment with melatonin as a therapeutic intervention. Additionally, determining whether young children with emerging emotional problems have circadian misalignment in the form of a phase delay similar to that of adults with depression is important because childhood depression is a serious psychiatric disorder with a high risk for relapse (Cole et al., 2008; Lewy et al., 2006). Taken together, with standard procedures for assessing circadian melatonin phase in young children now established, we can begin to answer key questions linking circadian rhythms, sleep, and young children's health and development.
ACKNOWLEDGMENTS
The authors are most grateful to the children and families for their generosity, time, and effort in making this study possible. They also thank all Brown University and University of Colorado Boulder undergraduate students and research assistants who collected these data. Hannah LeBourgeois assisted in the scoring and management of actigraphy data. This research was made possible with funds from the following grants: K01-MH074643, R01-MH08656, Sepracor Inc. ESRC026, HHMI to the Biological Sciences Initiative at the University of Colorado Boulder, and NIH/NCRR Colorado CTSI UL1-TR000154.
Footnotes
CONFLICT OF INTEREST STATEMENT
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
REFERENCES
- Acebo C, Sadeh A, Seifer R, Tzischinsky O, Hafer A, Carskadon MA. Sleep/wake patterns derived from activity monitoring and maternal report for healthy 1- to 5-year-old children. Sleep. 2005;28:1568–1577. doi: 10.1093/sleep/28.12.1568. [DOI] [PubMed] [Google Scholar]
- Acebo C, Sadeh A, Seifer R, Tzischinsky O, Wolfson AR, Hafer A, Carskadon MA. Estimating sleep patterns with activity monitoring in children and adolescents: how many nights are necessary for reliable measures? Sleep. 1999;22:95–103. doi: 10.1093/sleep/22.1.95. [DOI] [PubMed] [Google Scholar]
- Archer SN, Robilliard DL, Skene DJ, Smits M, Williams A, Arendt J, von Schantz M. A length polymorphism in the circadian clock gene Per3 is linked to delayed sleep phase syndrome and extreme diurnal preference. Sleep. 2003;26:413–415. doi: 10.1093/sleep/26.4.413. [DOI] [PubMed] [Google Scholar]
- Arendt J, Wetterberg L, Heyden T, Sizonenko PC, Paunier L. Radioimmunoassay of melatonin: human serum and cerebrospinal fluid. Horm Res. 1977;8:65–75. doi: 10.1159/000178782. [DOI] [PubMed] [Google Scholar]
- Benloucif S, Guico MJ, Reid KJ, Wolfe LF, L'hermite-Baleriaux M, Zee PC. Stability of melatonin and temperature as circadian phase markers and their relation to sleep times in humans. J Biol Rhythms. 2005;20:178–188. doi: 10.1177/0748730404273983. [DOI] [PubMed] [Google Scholar]
- Burgess HJ, Eastman CI. The dim light melatonin onset following fixed and free sleep schedules. J Sleep Res. 2005;14:229–237. doi: 10.1111/j.1365-2869.2005.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA, Acebo C, Arnedt JT. Failure to identify pubertally-mediated melatonin sensitivity to light in adolescents. Sleep. 2002a;25:A191. [Google Scholar]
- Carskadon MA, Acebo C, Richardson GS, Tate BA, Seifer R. An approach to studying circadian rhythms of adolescent humans. J Biol Rhythms. 1997;12:278–289. doi: 10.1177/074873049701200309. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Harvey K, Duke P, Anders TF, Litt IF, Dement WC. Pubertal changes in daytime sleepiness: 1980. Sleep. 2002b;25:453–460. [PubMed] [Google Scholar]
- Carskadon MA, Vieira C, Acebo C. Association between puberty and delayed phase preference. Sleep. 1993;16:258–262. doi: 10.1093/sleep/16.3.258. [DOI] [PubMed] [Google Scholar]
- Cole PM, Luby J, Sullivan MW. Emotions and the development of childhood depression: bridging the gap. Child Dev Perspect. 2008;2:141–148. doi: 10.1111/j.1750-8606.2008.00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby B, LeBourgeois MK, Harsh J. Racial differences in reported napping and nocturnal sleep in 2-to 8-year-old children. Pediatrics. 2005;115:225–232. doi: 10.1542/peds.2004-0815D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley SJ, Acebo C, Carskadon MA. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Med. 2007;8:602–612. doi: 10.1016/j.sleep.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Crowley SJ, Acebo C, Fallone G, Carskadon MA. Estimating dim light melatonin onset (DLMO) phase in adolescents using summer or school-year sleep/wake schedules. Sleep. 2006;29:1632–1641. doi: 10.1093/sleep/29.12.1632. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Kronauer RE, Allan JS, Duffy JF, Jewett ME, Brown EN, Ronda JM. Bright light induction of strong (type 0) resetting of the human circadian pacemaker. Science. 1989;244:1328–1333. doi: 10.1126/science.2734611. [DOI] [PubMed] [Google Scholar]
- Deacon S, Arendt J. Posture influences melatonin concentrations in plasma and saliva in humans. Neurosci Lett. 1994;167:191–194. doi: 10.1016/0304-3940(94)91059-6. [DOI] [PubMed] [Google Scholar]
- Dewan K, Benloucif S, Reid K, Wolfe LF, Zee PC. Light-induced changes of the circadian clock of humans: increasing duration is more effective than increasing light intensity. Sleep. 2011;34:593–599. doi: 10.1093/sleep/34.5.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci. 1995;15:3526–3538. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JF, Cain SW, Chang AM, Phillips AJ, Munch MY, Gronfier C, Wyatt JK, Dijk DJ, Wright KP, Jr., Czeisler CA. Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc Natl Acad Sci U S A 108 Suppl. 2011;3:15602–15608. doi: 10.1073/pnas.1010666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JF, Dijk DJ, Hall EF, Czeisler CA. Relationship of endogenous circadian melatonin and temperature rhythms to self-reported preference for morning or evening activity in young and older people. J Investig Med. 1999;47:141–150. [PMC free article] [PubMed] [Google Scholar]
- Duffy JF, Rimmer DW, Czeisler CA. Association of intrinsic circadian period with morningness-eveningness, usual wake time, and circadian phase. Behav Neurosci. 2001;115:895–899. doi: 10.1037//0735-7044.115.4.895. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Corley RP, Hewitt JK, Wright KP. Individual differences in childhood sleep problems predict later cognitive executive control. Sleep. 2009;32:323–333. doi: 10.1093/sleep/32.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gau SS, Shang CY, Merikangas KR, Chiu YN, Soong WT, Cheng AT. Association between morningness-eveningness and behavioral/emotional problems among adolescents. J Biol Rhythms. 2007;22:268–274. doi: 10.1177/0748730406298447. [DOI] [PubMed] [Google Scholar]
- Gold DR, Rogacz S, Bock N, Tosteson TD, Baum TM, Speizer FE, Czeisler CA. Rotating shift work, sleep, and accidents related to sleepiness in hospital nurses. Am J Public Health. 1992;82:1011–1014. doi: 10.2105/ajph.82.7.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooneratne NS, Metlay JP, Guo W, Pack FM, Kapoor S, Pack AI. The validity and feasibility of saliva melatonin assessment in the elderly. J Pineal Res. 2003;34:88–94. doi: 10.1034/j.1600-079x.2003.02945.x. [DOI] [PubMed] [Google Scholar]
- Gregory AM, Eley TC, O'Connor TG, Plomin R. Etiologies of associations between childhood sleep and behavioral problems in a large twin sample. J Am Acad Child Adolesc Psychiatry. 2004;43:744–751. doi: 10.1097/01.chi/0000122798.47863.a5. [DOI] [PubMed] [Google Scholar]
- Gregory AM, O'Connor TG. Sleep problems in childhood: a longitudinal study of developmental change and association with behavioral problems. J Am Acad Child Adolesc Psychiatry. 2002;41:964–971. doi: 10.1097/00004583-200208000-00015. [DOI] [PubMed] [Google Scholar]
- Gronfier C, Wright KP, Jr., Kronauer RE, Czeisler CA. Entrainment of the human circadian pacemaker to longer-than-24-h days. Proc Natl Acad Sci U S A. 2007;104:9081–9086. doi: 10.1073/pnas.0702835104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hvolby A, Jorgensen J, Bilenberg N. Actigraphic and parental reports of sleep difficulties in children with attention-deficit/hyperactivity disorder. Arch Pediatr Adolesc Med. 2008;162:323–329. doi: 10.1001/archpedi.162.4.323. [DOI] [PubMed] [Google Scholar]
- Iglowstein I, Jenni OG, Molinari L, Largo RH. Sleep duration from infancy to adolescence: reference values and generational trends. Pediatrics. 2003;111:302–307. doi: 10.1542/peds.111.2.302. [DOI] [PubMed] [Google Scholar]
- Jenni OG, LeBourgeois MK. Understanding sleep-wake behavior and sleep disorders in children: the value of a model. Curr Opin Psychiatry. 2006;19:282–287. doi: 10.1097/01.yco.0000218599.32969.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenni OG, O'Connor BB. Children's sleep: an interplay between culture and biology. Pediatrics. 2005;115:204–216. doi: 10.1542/peds.2004-0815B. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Palm IF, La Fleur SE, Scheer FAJL, Perreau-Lenz S, Ruiter M, Kreier F, Cailotto C, Buijs RM. SCN outputs and the hypothalamic balance of life. J Biol Rhythms. 2006;21:458–469. doi: 10.1177/0748730406293854. [DOI] [PubMed] [Google Scholar]
- Kataria S, Swanson MS, Trevathan GE. Persistence of sleep disturbances in preschool children. J Pediatr. 1987;110:642–646. doi: 10.1016/s0022-3476(87)80571-1. [DOI] [PubMed] [Google Scholar]
- Katzenberg D, Young T, Finn L, Lin L, King DP, Takahashi JS, Mignot E. A CLOCK polymorphism associated with human diurnal preference. Sleep. 1998;21:569–576. doi: 10.1093/sleep/21.6.569. [DOI] [PubMed] [Google Scholar]
- Kawinska A, Dumont M, Selmaoui B, Paquet J, Carrier J. Are modifications of melatonin circadian rhythm in the middle years of life related to habitual patterns of light exposure? J Biol Rhythms. 2005;20:451–460. doi: 10.1177/0748730405280248. [DOI] [PubMed] [Google Scholar]
- Klerman EB, Gershengorn HB, Duffy JF, Kronauer RE. Comparisons of the variablity of three markers of the human circadian pacemaker. J Biol Rhythms. 2002;17:181–193. doi: 10.1177/074873002129002474. [DOI] [PubMed] [Google Scholar]
- Lavigne JV, Arend R, Rosenbaum D, Smith A, Weissbluth M, Binns HJ, Christoffel KK. Sleep and behavior problems among preschoolers. J Dev Behav Pediatr. 1999;20:164–169. doi: 10.1097/00004703-199906000-00005. [DOI] [PubMed] [Google Scholar]
- LeBourgeois MK, Wright KP, Jr., LeBourgeois HB, Jenni OG. Dissonance between parent-selected bedtimes and young children's circadian physiology influences nighttime settling difficulties. Mind Brain Educ. doi: 10.1111/mbe.12032. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewy AJ, Lefler BJ, Emens JS, Bauer VK. The circadian basis of winter depression. Proc Natl Acad Sci U S A. 2006;103:7414–7419. doi: 10.1073/pnas.0602425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewy AJ, Wehr TA, Goodwin FK, Newsome DA, Markey SP. Light suppresses melatonin secretion in humans. Science. 1980;210:1267–1269. doi: 10.1126/science.7434030. [DOI] [PubMed] [Google Scholar]
- Lydic R, Schoene WC, Czeisler CA, Moore-Ede MC. Suprachiasmatic region of the human hypothalamus: homolog to the primate circadian pacemaker? Sleep. 1980;2:355–361. doi: 10.1093/sleep/2.3.355. [DOI] [PubMed] [Google Scholar]
- Markwald RR, Melanson EL, Smith MR, Higgins J, Perreault L, Eckel RH, Wright KP., Jr Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc Natl Acad Sci U S A. 2013;110:5695–5700. doi: 10.1073/pnas.1216951110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SK, Eastman CI. Sleep logs of young adults with self-selected sleep times predict the dim light melatonin onset. Chronobiol Int. 2002;19:695–707. doi: 10.1081/cbi-120006080. [DOI] [PubMed] [Google Scholar]
- Melke J, Goubran Botros H, Chaste P, Betancur C, Nygren G, Anckarsater H, Rastam M, Stahlberg O, Gillberg IC, Delorme R, et al. Abnormal melatonin synthesis in autism spectrum disorders. Mol Psychiatry. 2008;13:90–98. doi: 10.1038/sj.mp.4002016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens J. Classification and epidemiology of childhood sleep disorders. Prim Care. 2008;35:533–546. doi: 10.1016/j.pop.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Rusak B, Zucker I. Neural regulation of circadian rhythms. Physiol Rev. 1979;59:449–526. doi: 10.1152/physrev.1979.59.3.449. [DOI] [PubMed] [Google Scholar]
- Sitnick SL, Goodlin-Jones BL, Anders TF. The use of actigraphy to study sleep disorders in preschoolers: some concerns about detection of nighttime awakenings. Sleep. 2008;31:395–401. doi: 10.1093/sleep/31.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sletten TL, Vincenzi S, Redman JR, Lockley SW, Rajaratnam SM. Timing of sleep and its relationship with the endogenous melatonin rhythm. Front Neurol. 2010;1:137. doi: 10.3389/fneur.2010.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaab DF, Fliers E, Partiman TS. The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Res. 1985;342:37–44. doi: 10.1016/0006-8993(85)91350-2. [DOI] [PubMed] [Google Scholar]
- Tenkanen L, Sjoblom T, Harma M. Joint effect of shift work and adverse life-style factors on the risk of coronary heart disease. Scand J Work Envrion Health. 1998;24:351–357. doi: 10.5271/sjweh.355. [DOI] [PubMed] [Google Scholar]
- Vollmers C, Gill S, DiTacchio L, Pulivarthy S, Le HD, Panda S. Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc Natl Acad Sci U S A. 2009;106:21453–21458. doi: 10.1073/pnas.0909591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehr TA. The durations of human melatonin secretion and sleep respond to changes in daylength (photo-period). J Clin Endocrinol Metab. 1991;73:1276–1280. doi: 10.1210/jcem-73-6-1276. [DOI] [PubMed] [Google Scholar]
- Weissbluth M. Naps in children: 6 months-7 years. Sleep. 1995;18:82–87. doi: 10.1093/sleep/18.2.82. [DOI] [PubMed] [Google Scholar]
- Wolfson AR, Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child Dev. 1998;69:875–887. [PubMed] [Google Scholar]
- Wright KP, Gronfier C, Duffy JF, Czeisler CA. Intrinsic period and light intensity determine the phase relationship between melatonin and sleep in humans. J Biol Rhythms. 2005;20:168–177. doi: 10.1177/0748730404274265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KP, Jr., McHill AW, Birks BR, Griffin BR, Rusterholz T, Chinoy ED. Entrainment of the human circadian clock to the natural light-dark cycle. Curr Biol. 2013;23:1554–1558. doi: 10.1016/j.cub.2013.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitzer JM, Khalsa SB, Boivin DB, Duffy JF, Shanahan TL, Kronauer RE, Czeisler CA. Temporal dynamics of late-night photic stimulation of the human circadian timing system. Am J Physiol Regul Integr Comp Physiol. 2005;289:R839–R844. doi: 10.1152/ajpregu.00232.2005. [DOI] [PubMed] [Google Scholar]