Abstract
Discussed herein is the development and advancement of trans-cyclooctene as a tool for facilitating bioorthogonal labeling through reactions with s-tetrazines. While a number of strained alkenes have been shown to combine with tetrazines for applications in bioorthogonal labeling, trans-cyclooctene enables fastest reactivity at low concentration with rate constants in excess of k2 = 106 M−1 s−1. In the present article, we describe advances in computation and synthesis that have enabled applications in chemical biology and nuclear medicine.
Introduction
The use of strain energy to facilitate molecular reactivity is a key concept for organic synthesis, and one of emerging importance in the design of bioorthogonal reactivity — those chemical transformations that can proceed efficiently without interference from biological functionality [1,2•,3–14]. In a seminal publication in 2004, Bertozzi illustrated that the [3+2] cycloaddition between cyclooctyne and azide derivatives proceeds without the need for Cu-catalysis [15••]. As the scope and applications of this reaction have proven robust, so too has been the approach of using ‘ring strain to promote otherwise reticent reactions with potential bioorthogonality’ [15••]. Indeed, cycloalkynes have served as highly reactive dipolarophiles in a host of new bioorthogonal reactions [8,11,16–18].
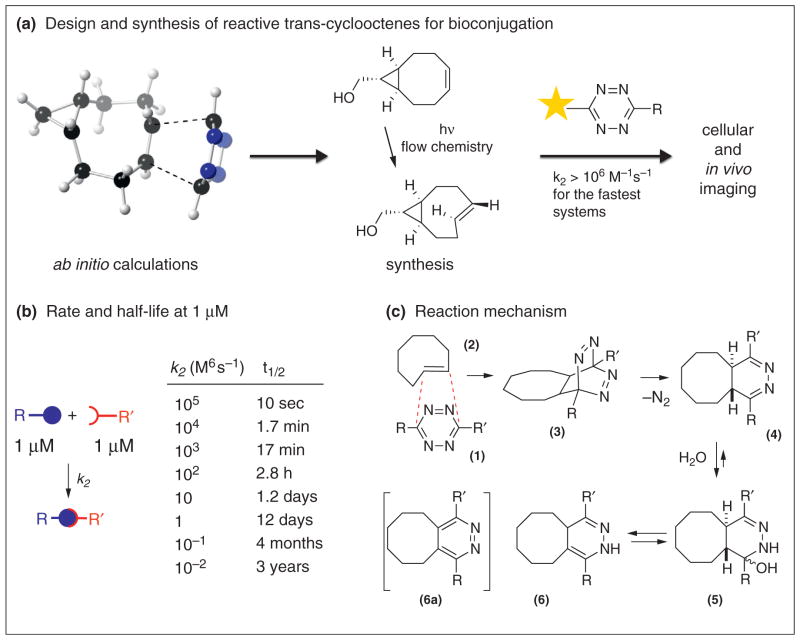
Rate is a key consideration for the development of a bioorthogonal reaction, illustrated with the simple relationship between second order rate constant and half-life at 1 μM (Scheme 1b). A goal of this opinion article is to outline the development and advancement of trans-cyclooctene (TCO) as a tool for facilitating bioorthogonal labeling reactions with s-tetrazines. This multidisciplinary effort has embodied advances by a number of research groups in computation, synthesis, chemical biology and nuclear medicine (Scheme 1a).
Scheme 1.
trans-Cyclooctene (TCO) as a tool for chemical biology and in vivo imaging. (a) The advancement of TCO as a tool is a multidisciplinary effort involving high-level computation, new synthetic methodology, chemical biology and nuclear medicine. (b) The significance of high rate constants is illustrated by simple table of rate data and corresponding half-lives. (c) Mechanism of the tetrazine–TCO ligation.
Tetrazine–TCO ligation
Diels–Alder reactions between tetrazines and alkenes have a rich history [19], exemplified by classic physical organic studies of Sauer [20,21••,22] and the striking synthetic work of Boger [19,23]. In 1990, Sauer measured rate constants for the reactions of tetrazines with more than 40 dienophiles, including norbornene, cyclopropene derivatives, cyclooctyne and TCO [21••]. Of these dienophiles, TCO was most reactive by 1–3 orders of magnitude.
Our experience with the chemistry of strained alkenes [24,25•] led us to consider developing TCO for bioorthogonal reactivity with tetrazines [26•]. That the reaction of TCO with tetrazines would be bioorthogonal was not immediately evident, given the electrophilicity of tetrazine derivatives and the potential for TCO to isomerize to cis-cyclooctene or polymerize. Moreover, there was not a general synthetic method to access functionalized derivatives of TCO derivatives. Finally, while the reaction of TCO with s-tetrazines was known to be rapid, the conjugation product was undescribed and would warrant deconvolution.
As a general method to prepare TCO derivatives, we developed a photoisomerization method that uses a closed-loop flow reactor where the reaction mixture is continuously cycled through AgNO3 on silica [25•]. Selective metal complexation of TCO perturbs an otherwise unfavorable equilibrium. This photochemical flow method can be carried out on multi-gram scale, and is robust enough to be useful in natural product synthesis [27].
With a TCO synthesis in hand, the bioorthogonality of tetrazine–TCO ligation was explored. 3,6-Diaryl-s-tetrazines offer an excellent combination of fast reaction rates and stability for both the starting material and conjugation products [28••], and protein conjugation at 15 μM was completed more quickly than could be easily assayed by mass spectrometry. While the initial product of tetrazine–TCO ligation is the expected 4,5-dihydropyridazine, this adduct reacts with water. In 1H NMR studies of reactions between TCOs and 3,6-di(2-pyridyl)-s-tetrazine, it was shown that 4,5-dihydropyridazines (4) add D2O to give aminal intermediates (5), which subsequently eliminate to give the 1,4-dihydropyridazine 6 (Scheme 1c). With more electron rich tetrazines, such as 3,6-diphenyl-s-tetrazine, dihydropyridazine 6 aromatizes to give 6a under ambient conditions.
Contemporaneous with the above work on TCO, Weissleder and Hilderbrand studied the bioorthogonal reactivity of mono-aryltetrazine derivatives (15, Scheme 2c) with norbornenes with a rate of k2 1.9 M−1 s−1 at 20 °C in PBS [29••]. Using a fluorescently labeled tetrazine, these authors demonstrated that the surfaces of live cells could be labeled. Contemporary studies were also performed by Pipkorn et al., who combined a TMZ-conjugate of tetrazine 14 with a peptide conjugate of the Reppe Anhydride [30•]. Recently, Devaraj and Presher have used cyclopropenes as dienophiles for cell surface labeling [31,32], and Wittmann has recently shown that terminal alkenes are sufficiently reactive to enable labeling by tetrazines [33]. Cyclooctynes have recently been used for reactions with tetrazines by Wang [34], and also in mutually bioorthogonal reactions [35,36]. Overall, there remains a high level of interest in TCO for achieving fastest reactivity.
Scheme 2.
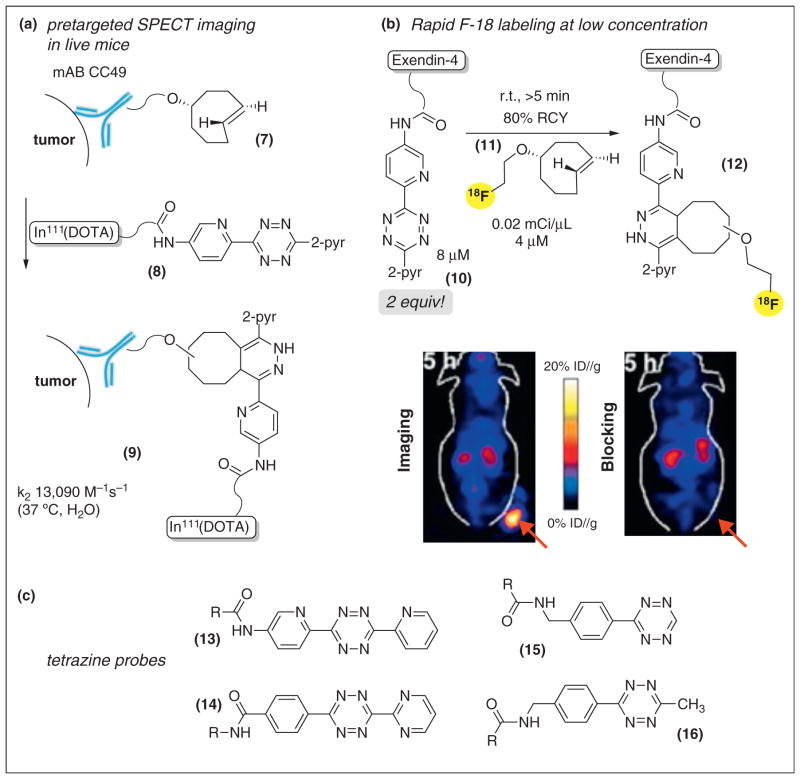
Tetrazine–TCO ligation as a tool for nuclear medicine (a) Pretargeted SPECT imaging in live mice through initial administration of a TCO-antibody conjugate with later administration of a tetrazine–111In-DOTA conjugate. (b) 18F-labeled TCO 12 enables rapid construction of PET-probes. In the shown example, the importance of efficient labeling at nearly equimolar stoichiometry is emphasized by a blocking experiment, where coinjecting with a 5-fold excess of unlabeled exendin-4 resulted in a greatly reduced signal. (c) Tetrazines commonly utilized in bioconjugation studies.
Tetrazines used frequently in bioconjugation with TCO are listed in Scheme 2c [28••,30•,37••,38], and recently Devaraj has described a catalytic method for tetrazine synthesis from alkylnitriles [39•]. The reactions of tetrazines with TCO benefit from a significant hydrophobic effect. Thus TCO combines with 3,6-di(2-pyridyl)-s-tetrazine with a rate of 1100 M−1 s−1 in MeOH, and 2000 M−1 s−1 in 9:1 MeOH:water [28••]. Derivatives of 13 have rates of 5235 M−1 s−1 at 25 °C in 45:55 water:-MeOH [37••], and 13,090 M−1 s−1 at 37° in PBS [43••] (Scheme 2c). Very recently, Robillard has shown that a 13 derivative combines with the axial diastereomer of 5-hydroxy-trans-cyclooctene with a rate of 273,000 M−1 s−1 −1 at 37 °C in PBS — approximately 10 times faster than equatorial diastereomer [61]. As has been noted [41•, 42••], the electron donating amido substituent renders 13 more stable than the parent 3,6-di(2-pyridyl)tetrazine, and derivatives of 13 have been used in a number of applications [37••,41•,43••,44,45]. Contrary to several remarks in the literature [29••,38,46], the stability of 13 in biological milieu appears comparable to other reactive tetrazines used for bioconjugation [38]. Weissleder and co-workers have reported fast rates for the reaction of monoaryltetrazines (15), where k2 is 6000 M−1 s−1 in water at 37 °C [47••]. Derivatives of this tetrazine are cell permeable and have been used in a number of applications [29••,35,38,46,47••,48••,49,50,51,52•,53•] including the conjugation of nanoparticles and quantum dots to pretargeted cells [49,50], as well as applications in cell and radiochemical imaging discussed below.
Applications in nuclear medicine
The radiochemical labeling of macromolecules represents one of the most significant opportunities for bioorthogonal chemistry. In 2010, Robillard and coworkers demonstrated that tetrazine–TCO ligation could be applied to pretargeted SPECT/CT imaging of tumors in live mice (Scheme 2a). Pretargeting involves separate administration of tumor-targeting and radiolabeled molecules, with time allowed in-between for uptake by tumors and clearance of any unbound targeting molecule [43••]. The success of bioorthogonal chemistry in pretargeted applications is critically dependent on reaction rate. Excess probe is required for reactions with slow rates, which can adversely impact the signal-to-noise ratio if the probe does not clear quickly. As depicted in Scheme 2a, 5-hydroxy-trans-cyclooctene was conjugated to anti-TAG72 mAb CC49 via an oligoethyleneglycol linker (7), and administered to mice bearing colon cancer xenografts. One day later, these mice were injected with 3.4 equivalents of a DOTA-In-111/tetrazine conjugate 8, and tumors were successfully imaged by SPECT/CT. Impressively, the adduct 9 was formed in 52–57% yield in vivo. Recently, Robillard has found that tagging an antibody with a short linker and using an axial TCO diastereomer can improve the tumor-to-non-tumor imaging ratios in pretargeted tumor-bearing mice [61]. Also recently, Lewis and co-workers demonstrated pretargeted imaging of colorectal cancer in mice in a system using TCO-modified antibody and a Cu64-NOTA labeled tetrazine [62].
TCO has also proven to be a powerful tool for positron emission tomography (PET), which is challenged by the short half-lives of PET radionuclides (110 min for 18F). A further challenge is the ideal of achieving labeling with equimolar stoichiometry, as unlabeled precursors are generally inseparable and can decrease signal through competitive inhibition.
With Li and Conti, we developed 18F-labeled TCO 11 (Scheme 2b) [54••], which was used to prepare cyclic RGD and VEGF protein conjugates for cancer imaging [41•,55]. The cyclic RGD targets the integrin αvβ3, which is a target for cancer imaging as it is upregulated on the surface of tumor blood vessels. We recently described a tetrazine-conjugate (10) of exendin-4 a 4.8 kDa glucagon like peptide-1 receptor (GLP-1R) [56•]. With only 2 equivalents (4 μg) of 10, labeling by 0.02 mCi/mL of 11 was obtained to provide PET agent 12 in >80% yield within minutes. As shown in Scheme 2b, a GLP-1R positive tumor was successfully imaged by 12. The importance of efficient 18F incorporation was emphasized by a blocking experiment, where coinjecting 12 with a 5-fold excess of cold exendin-4 resulted in a greatly reduced signal. Compound 12 could be used to image intraportally transplanted islet cells, and it is a promising probe for monitoring the number and viability of transplanted islet cells in the liver.
Weissleder has conjugated 18F-11 to the drug AZD2281 for in vivo imaging applications, and used a TCO-functionalized resin to facilitate purification [51,52•]. Recently, 18F-11 was used for pretargeted imaging in conjunction with a dextran polymer modified by both a near-IR dye and tetrazine 15 [53•]. The dextran polymer was selected to improve pharmacokinetics. Mice were simultaneously implanted with LS174T tumors as well as A431 tumors that lack expression of the A31 glycoprotein epitope. The mice were administered a TCO-tagged anti-A33 antibody, and in second step were administered the modified polymer. The LS174T tumors were visualized whereas the control tumor showed much lower uptake [53•].
‘s-TCO’ — a (more) strained trans-cyclooctene
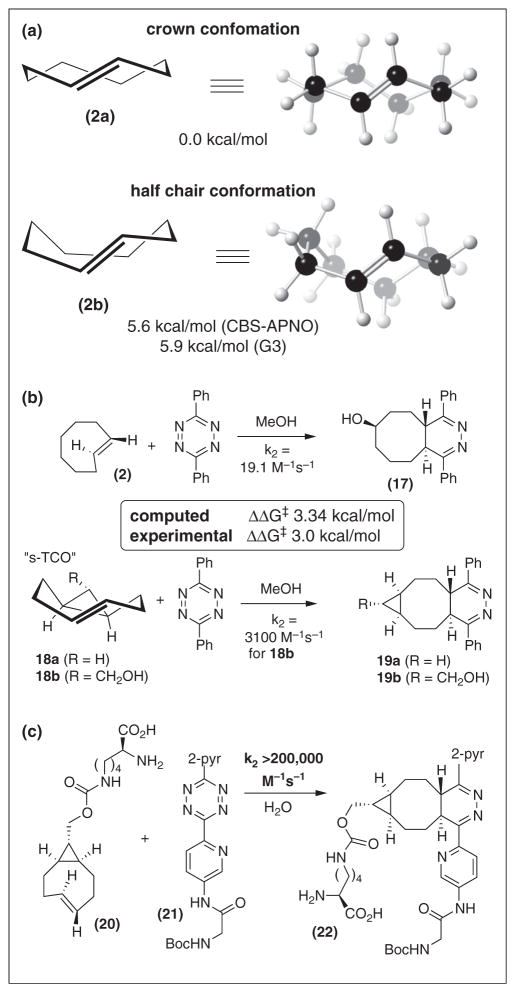
The most reactive tetrazines, such as 13–15, are imperfect when prolonged in vivo stability is required [43••]. Less electron deficient tetrazines, such as 3,6-diphenyl-s-tetra-zine, are much more resilient but are also less reactive toward TCO. van Delft has elegantly described that the strained cycloalkyne, bicyclo[6.1.0]non-4-yn-9-ylmetha-nol, displays superb reaction kinetics in bioconjugation chemistry [16]. To develop more reactive TCOs, we considered the effect that ring conformation would have on TCO reactivity. Computation was used to design a strained trans-cyclooctene (‘s-TCO’) with a cis-ring fusion, in which the eight-membered ring is forced to adopt a highly strained ‘half-chair’ conformation.
As summarized in Scheme 3a, ab initio calculations predicted the lowest energy ‘crown’ conformation (2a) of trans-cyclooctene to be 5.6–5.9 kcal/mol lower in energy than the ‘half chair’ conformation (2b) [17] and it was recognized that trans-cyclooctenes 18 with cis-ring fusions would be forced to adopt strained conformations similar to that of 2b (Scheme 3b) [17,42••]. Transition state calculations with 3,6-diphenyl-s-tetrazine predicted that s-TCO 18a would react much faster than 2a (ΔΔG‡‡= 3.34 kcal/mol at 25 °C). Experimentally, 18b was compared to 2, and found to react in MeOH at 25 °C with a rate of 3100 M−1 s−1 — 160 times faster than 2 (Scheme 3b). The experimental ΔΔG‡ (3.0 kcal/mol) was in close correlation to the predicted value.
Scheme 3.
(a) TCO conformation has a significant effect on strain energy in the ground state. (b) Computation correctly predicted that a conformationally strained TCO (‘s-TCO’) would display enhanced reactivity relative to parent TCO. (c) With more reactive tetrazine 21, s-TCO derivative reacts with a rate that is too quick to measure by stopped flow kinetics.
Of course, even faster rates are observed with more reactive tetrazines. Reactions of s-TCO with 3,6-di(2-pyridyl)-tetrazine proceed with k2 = 22,000 M−1 s−1 in MeOH at 25 °C [42••]. s-TCO also enjoys a significant hydrophobic effect. In 45:55 water:MeOH at 25° C, s-TCO 20 combines with 21 at a rate too fast to measure by stopped-flow kinetics (>200,000 M−1 s−1) [37••]. Recently, the rate of s-TCO 18b and 14 was measured to be 380,000 M−1 s−1 (7:1 water:dioxane, 25 °C) [64]. The rate of an s-TCO derivative with 8 was measured to be 2,800,000 M−1 s−1 at 37 °C in PBS [61].
Cellular imaging
Weissleder and co-workers first demonstrated that tetrazine–TCO ligation could be used to selectively label the surface (via pretargeted TCO-antibody conjugates) or interior (via TCO-taxol conjugates) of living cells with tetrazine–fluorophore conjugates [47••,48••,63]. These authors also demonstrated that tetrazines can quench the fluorescence of pendant dyes. As fluorescence is reestablished for Diels–Alder conjugates, background fluorescence is greatly reduced [47••,48••,63]. Cellular labeling has also been achieved with (E,E)-1,5-Cyclooc-tadiene via azido-tagged glycans on cell-surfaces [44].
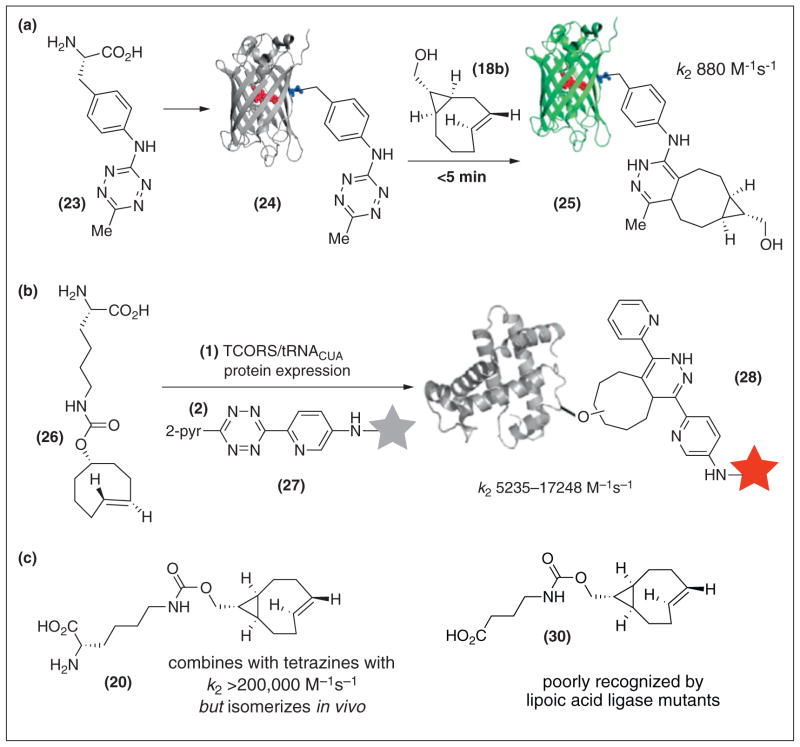
Recently, a number of in vivo methods for the selective labeling of proteins based on tetrazine ligation have been developed. With Ting and co-workers, TCO-containing lipoic acid analogs were synthesized and an E. coli lipoic acid ligase was evolved that site-specifically ligates one of these TCO derivatives onto proteins of interest. Subsequent reactivity with tetrazine–dye conjugates served for efficient fluorogenic cell-surface labeling, and for intracellular labeling of cytoskeletal proteins in live mammalian cells [57••].
It has been shown with Mehl that tetrazine-containing amino acid 23 can be genetically encoded into proteins in site-specific fashion, and subsequently be labeled by TCO derivatives (Scheme 4a). The electron donating 3-amino substituent of 23 makes this tetrazine stable enough for cellular growth conditions, but also decreases the rate of Diels–Alder reactions. Fortunately, protein labeling by s-TCO 18b and its derivatives occurs at rapid rates. Thus, with GFP derivative 24, fluorogenic labeling by 18b takes place within minutes to give conjugate 25 [58••].
Scheme 4.
Tetrazine–TCO ligation with genetically encoded proteins (a) A tetrazine-derived unnatural amino acid can be genetically encoded site-specifically into proteins of interest. s-TCO derivatives can be used to tag the unnatural amino acids with fast rates in vivo. (b) A TCO-derivatized lysine has been site specifically incorporated into proteins in E. coli and mammalian cells, and used for rapid fluorogenic labeling in live cells. (c) Current limitations of s-TCO.
There has also been significant activity in the genetic incorporation of TCO containing amino acids. Independent work by Chin with Deiters [40], Carroll [59], and Schultz with Lemke [60••] established that norbornene could be incorporated and used for cell labeling. Schultz and Lemke also prepared the amino acid 26, and were able to incorporate it into proteins and observe labeling in fixed cells, with a rate of 35,000 M−1 s−1 at 37 °C in vitro [60••]. Independently with Chin, the amino acid 26 was synthesized and site specifically incorporated into proteins with high efficiency [37••]. Fluorogenic labeling was demonstrated in E. coli and living mammalian cells. Also studied was incorporation of an amino acid derived from the van Delft cyclooctyne [37••]. For those amino acids that incorporated into proteins, reactivity was fastest by an order of magnitude with 26 with rates of 5235 and 17,248 M−1 s−1 (25 °C, 45:55 water:MeOH) with tetrazine derivatives of structures 13 and 14, respectively (Scheme 2b).
Conclusion and outlook
Enabled by advances in synthesis and computation, TCO has emerged as a tool for rapid labeling and imaging through bioorthogonal Diels–Alder reactions with tetrazines. Future challenges include developing the process chemistry of TCO synthesis, as needed to broaden access. Another challenge will be to engage the most stable tetrazines with fast rates across a broader spectrum of applications. While s-TCO has proven successful in labeling genetically encoded tetrazines, encoding the s-TCO amino acid 20 was less successful (it isomerized), and attempts to utilize s-TCO 30 as a substrate for lipoic acid ligase met with low incorporation (Scheme 4b). New TCO designs that optimize structure, stability and rate will be needed to address such limitations.
Acknowledgments
We gratefully acknowledge support of the National Science Foundation (NSF DMR1206310 and NSF CHE1112409) and the National Institutes of Health (NIH P20RR017716).
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Sletten EM, Bertozzi CR. Bioorthogonal chemistry: fishing for selectivity in a sea of functionality. Angew Chem Int Ed. 2009;48:6974–6998. doi: 10.1002/anie.200900942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2•.Jewett JC, Bertozzi C. Cu-free click cycloaddition reactions in chemical biology. Chem Soc Rev. 2010;39:1272–1279. doi: 10.1039/b901970g. This review describes recent advances in strain-driven cycloaddition reactions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Durek T, Alewood PF. Preformed selenoesters enable rapid native chemical ligation at intractable sites. Angew Chem Int Ed. 2011;50:12042–12045. doi: 10.1002/anie.201105512. [DOI] [PubMed] [Google Scholar]
- 4.Sletten EM, Bertozzi CR. A bioorthogonal quadricyclane ligation. J Am Chem Soc. 2011;133:17570–17573. doi: 10.1021/ja2072934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McFarland JM, Francis MB. Reductive alkylation of proteins using iridium catalyzed transfer hydrogenation. J Am Chem Soc. 2005;127:13490–13491. doi: 10.1021/ja054686c. [DOI] [PubMed] [Google Scholar]
- 6.Antos JM, Francis MB. Selective tryptophan modification with rhodium carbenoids in aqueous solution. J Am Chem Soc. 2004;126:10256–10257. doi: 10.1021/ja047272c. [DOI] [PubMed] [Google Scholar]
- 7.Lin YA, Chalker JM, Floyd N, Bernardes GJL, Davis BG. Allyl sulfides are privileged substrates in aqueous cross-metathesis: application to site-selective protein modification. J Am Chem Soc. 2008;130:9642–9643. doi: 10.1021/ja8026168. [DOI] [PubMed] [Google Scholar]
- 8.Moran J, McKay CS, Pezacki JP. Strain-promoted 1,3-dipolar cycloadditions of diazo compounds with cyclooctynes. Can J Chem. 2011;89:148–151. doi: 10.1039/b921630h. [DOI] [PubMed] [Google Scholar]
- 9.Dirksen A, Dawson PE. Rapid oxime and hydrazone ligations with aromatic aldehydes for biomolecular labeling. Bioconj Chem. 2008;19:2543–2548. doi: 10.1021/bc800310p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stockmann H, Neves AA, Stairs S, Brindle KM, Leeper FJ. Exploring isonitrile-based click chemistry for ligation with biomolecules. Org Biomol Chem. 2011;9:7303–7305. doi: 10.1039/c1ob06424j. [DOI] [PubMed] [Google Scholar]
- 11.Sanders BC, Friscourt Fdr, Ledin PA, Mbua NE, Arumugam S, Guo J, Boltje TJ, Popik VV, Boons G-J. Metal-free sequential [3+2]-dipolar cycloadditions using cyclooctynes and 1,3-dipoles of different reactivity. J Am Chem Soc. 2010;133:949–957. doi: 10.1021/ja1081519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song W, Wang Y, Qu J, Lin Q. Selective functionalization of a genetically encoded alkene-containing protein via “photoclick chemistry” in bacterial cells. J Am Chem Soc. 2008;130:9654–9655. doi: 10.1021/ja803598e. [DOI] [PubMed] [Google Scholar]
- 13.Chalker JM, Bernardes GJL, Davis BG. A ‘tag-and-modify’ approach to site-selective protein modification. Acc Chem Res. 2011;44:730–741. doi: 10.1021/ar200056q. [DOI] [PubMed] [Google Scholar]
- 14.Godinat A, Park HM, Miller SC, Cheng K, Hanahan D, Sanman LE, Bogyo M, Yu A, Nikitin GF, Stahl A, Dubikovskaya EA. A biocompatible in vivo ligation reaction and its application for noninvasive bioluminescent imaging of protease activity in living mice. ACS Chem Biol. 2013 doi: 10.1021/cb3007314. http://dx.doi.org/10.1021/cb3007314. [DOI] [PMC free article] [PubMed]
- 15••.Agard NJ, Prescher JA, Bertozzi CR. A strain-promoted [3+2] azide-alkyne cycloaddition for covalent modification of biomolecules in living systems. J Am Chem Soc. 2004;126:15046–15047. doi: 10.1021/ja044996f. Seminal report on the development of strain-driven cycloaddition reactions for bioorthogonal labeling. [DOI] [PubMed] [Google Scholar]
- 16.Dommerholt J, Schmidt S, Temming R, Hendriks LJA, Rutjes FPJT, van Hest JCM, Lefeber DJ, Friedl P, van Delft FL. Readily accessible bicyclononynes for bioorthogonal labeling and three-dimensional imaging of living cells. Angew Chem Int Ed. 2010;49:9422–9425. doi: 10.1002/anie.201003761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bach RD. Ring strain energy in the cyclooctyl system. The effect of strain energy on [3+2] cycloaddition reactions with azides. J Am Chem Soc. 2009;131:5233–5243. doi: 10.1021/ja8094137. [DOI] [PubMed] [Google Scholar]
- 18.Baskin JM, Prescher JA, Laughlin ST, Agard NJ, Chang PV, Miller IA, Lo A, Codelli JA, Bertozzi CR. Copper-free click chemistry for dynamic in vivo imaging. Proc Natl Acad Sci. 2007;104:16793–16797. doi: 10.1073/pnas.0707090104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boger DL. Diels–Alder reactions of heterocyclic aza dienes. Scope and applications. Chem Rev. 1986;86:781–793. [Google Scholar]
- 20.Sauer J, Heldmann DK, Hetzenegger J, Krauthan J, Sichert H, Schuster J. 1,2,4,5-Tetrazine: synthesis and reactivity in [4+2] cycloadditions. Eur J Org Chem. 1998;1998:2885–2896. [Google Scholar]
- 21••.Thalhammer F, Wallfahrer U, Sauer J. Reaktivität einfacher offenkettiger und cyclischer dienophile bei Diels–Alder-reaktionen mit inversem elektronenbedarf. Tetrahedron Lett. 1990;31:6851–6854. Seminal study in which rate constants for reactions between tetrazines and a range of strained alkenes are reported. [Google Scholar]
- 22.Balcar J, Chrisam G, Huber FX, Sauer J. Reaktivität von stickstoff-heterocyclen genenüber cyclooctin als dienophil. Tetrahedron Lett. 1983;24:1481–1484. [Google Scholar]
- 23.Hamasaki A, Zimpleman JM, Hwang I, Boger DL. Total synthesis of Ningalin D. J Am Chem Soc. 2005;127:10767–10770. doi: 10.1021/ja0526416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liao L-a, Fox JM. A copper-catalyzed method for the facially selective addition of Grignard reagents to cyclopropenes. J Am Chem Soc. 2002;124:14322–14323. doi: 10.1021/ja0278234. [DOI] [PubMed] [Google Scholar]
- 25•.Royzen M, Yap GPA, Fox JM. A photochemical synthesis of functionalized trans-cyclooctenes driven by metal complexation. J Am Chem Soc. 2008;130:3760–3761. doi: 10.1021/ja8001919. Report on the first general method of preparing trans-cyclooctene derivatives. [DOI] [PubMed] [Google Scholar]
- 26•.Knall A-C, Slugovc C. Inverse electron demand Diels–Alder (IEDDA)-initiated conjugation: a (high) potential click chemistry scheme. Chem Soc Rev. 2013 doi: 10.1039/c3cs60049a. http://dx.doi.org/10.1039/C1033CS60049ARecent review on Diels–Alder reactions involving tetrazines for bioconjugation. [DOI] [PubMed]
- 27.Royzen M, Taylor MT, DeAngelis A, Fox JM. Total synthesis of hyacinthacine a2: stereocontrolled 5-aza-cyclooctene photoisomerization and transannular hydroamination with planar-to-point chirality transfer. Chem Sci. 2011;2:2162–2165. doi: 10.1039/C1SC00442E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28••.Blackman ML, Royzen M, Fox JM. Tetrazine ligation: fast bioconjugation based on inverse-electron-demand Diels– Alder reactivity. J Am Chem Soc. 2008;130:13518–13519. doi: 10.1021/ja8053805. Initial report on bioconjugation based on trans-cyclooctene derivatives with tetrazines in which unprecedented rates (k2 > 103 M−1 s−1) are reported for bioorthogonal reactivity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29••.Devaraj NK, Weissleder R, Hilderbrand SA. Tetrazine-based cycloadditions: application to pretargeted live cell imaging. Bioconj Chem. 2008;19:2297–2299. doi: 10.1021/bc8004446. Initial report on bioconjugation based on norbornene derivatives with tetrazines. Cell surface labeling is demonstrated for bioconjugations with rates of k2 ~ 2 M−1 s−1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30•.Pipkorn R, Waldeck W, Didinger B, Koch M, Mueller G, Wiessler M, Braun K. Inverse-electron-demand Diels–Alder reaction as a highly efficient chemoselective ligation procedure: synthesis and function of a bioshuttle for temozolomide transport into prostate cancer cells. J Peptide Sci. 2009;15:235–241. doi: 10.1002/psc.1108. Initial report of bioconjugation based on the Reppe Anhydride with tetrazines. Rates were not reported. [DOI] [PubMed] [Google Scholar]
- 31.Yang J, Šečkutė J, Cole CM, Devaraj NK. Live-cell imaging of cyclopropene tags with fluorogenic tetrazine cycloadditions. Angew Chem Int Ed. 2012;51:7476–7479. doi: 10.1002/anie.201202122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patterson DM, Nazarova LA, Xie B, Kamber DN, Prescher JA. Functionalized cyclopropenes as bioorthogonal chemical reporters. J Am Chem Soc. 2012;134:18638–18643. doi: 10.1021/ja3060436. [DOI] [PubMed] [Google Scholar]
- 33.Niederwieser A, Späte A-K, Nguyen LD, Jüngst C, Reutter W, Wittmann V. Two-color glycan labeling of live cells by a combination of Diels–Alder and click chemistry. Angew Chem Int Ed. 2013;52:4265–4268. doi: 10.1002/anie.201208991. [DOI] [PubMed] [Google Scholar]
- 34.Chen W, Wang D, Dai C, Hamelberg D, Wang B. Clicking 1,2,4,5-tetrazine and cyclooctynes with tunable reaction rates. Chem Commun. 2012;48:1736–1738. doi: 10.1039/c2cc16716f. [DOI] [PubMed] [Google Scholar]
- 35.Karver MR, Weissleder R, Hilderbrand SA. Bioorthogonal reaction pairs enable simultaneous, selective, multi-target imaging. Angew Chem Int Ed. 2012;51:920–922. doi: 10.1002/anie.201104389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang Y, Mackey JL, Lopez SA, Liu F, Houk KN. Control and design of mutual orthogonality in bioorthogonal cycloadditions. J Am Chem Soc. 2012;134:17904–17907. doi: 10.1021/ja309241e. [DOI] [PubMed] [Google Scholar]
- 37••.Lang K, Davis L, Wallace S, Mahesh M, Cox DJ, Blackman ML, Fox JM, Chin JW. Genetic encoding of bicyclononynes and trans-cyclooctenes for site-specific protein labeling in vitro and in live mammalian cells via rapid fluorogenic Diels–Alder reactions. J Am Chem Soc. 2012;134:10317–10320. doi: 10.1021/ja302832g. A method for genetically encoding a TCO-derivatized amino acid into proteins of interest is described, and a series of tetrazine and TCO derivatives are evaluated by stopped flow kinetics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karver MR, Weissleder R, Hilderbrand SA. Synthesis and evaluation of a series of 1,2,4,5-tetrazines for bioorthogonal conjugation. Bioconj Chem. 2011;22:2263–2270. doi: 10.1021/bc200295y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39•.Yang J, Karver MR, Li W, Sahu S, Devaraj NK. Metal-catalyzed one-pot synthesis of tetrazines directly from aliphatic nitriles and hydrazine. Angew Chem Int Ed. 2012;51:5222–5225. doi: 10.1002/anie.201201117. A useful catalytic method for preparing unsymmetrical tetrazines is described. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lang K, Davis L, Torres-Kolbus J, Chou C, Deiters A, Chin JW. Genetically encoded norbornene directs site-specific cellular protein labelling via a rapid bioorthogonal reaction. Nat Chem. 2012;4:298–304. doi: 10.1038/nchem.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41•.Selvaraj R, Liu S, Hassink M, Huang C-w, Yap L-p, Park R, Fox JM, Li Z, Conti PS. Tetrazine–trans-cyclooctene ligation for the rapid construction of integrin αvβ3 targeted PET tracer based on a cyclic RGD peptide. Biorg Med Chem Lett. 2011;21:5011–5014. doi: 10.1016/j.bmcl.2011.04.116. Description of F-18 PET probe construction and tumor imaging in mice using tetrazine–TCO ligation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42••.Taylor MT, Blackman ML, Dmitrenko O, Fox JM. Design and synthesis of highly reactive dienophiles for the tetrazine–trans-cyclooctene ligation. J Am Chem Soc. 2011;133:9646–9649. doi: 10.1021/ja201844c. Strained trans-cyclooctenes are designed using conformational analysis and ab initio transition state calculations. This s-TCO is the most reactive dienophile to-date toward tetrazines in Diels–Alder reactivity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43••.Rossin R, Renart Verkerk P, van den Bosch SM, Vulders RCM, Verel I, Lub J, Robillard MS. In vivo chemistry for pretargeted tumor imaging in live mice. Angew Chem Int Ed. 2010;49:3375–3378. doi: 10.1002/anie.200906294. Initial description of the use of TCO–tetrazine ligation for pretargeted radiochemical imaging in live mice. [DOI] [PubMed] [Google Scholar]
- 44.Stockmann H, Neves AA, Day HA, Stairs S, Brindle KM, Leeper FJ. (E,E)-1,5-Cyclooctadiene: a small and fast click-chemistry multitalent. Chem Commun. 2011;47:7203–7205. doi: 10.1039/c1cc12161h. [DOI] [PubMed] [Google Scholar]
- 45.Herth MM, Andersen VL, Lehel S, Madsen J, Knudsen GM, Kristensen JL. Development of a 11C-labeled tetrazine for rapid tetrazine–trans-cyclooctene ligation. Chem Commun. 2013;49:3805–3807. doi: 10.1039/c3cc41027g. [DOI] [PubMed] [Google Scholar]
- 46.Devaraj NK, Weissleder R. Biomedical applications of tetrazine cycloadditions. Acc Chem Res. 2011;44:816–827. doi: 10.1021/ar200037t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47••.Devaraj NK, Upadhyay R, Haun JB, Hilderbrand SA, Weissleder R. Fast and sensitive pretargeted labeling of cancer cells through a tetrazine/trans-cyclooctene cycloaddition. Angew Chem Int Ed. 2009;48:7013–7016. doi: 10.1002/anie.200903233. Initial demonstration of live cell labeling using tetrazine–TCO ligation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48••.Devaraj NK, Hilderbrand S, Upadhyay R, Mazitschek R, Weissleder R. Bioorthogonal turn-on probes for imaging small molecules inside living cells. Angew Chem Int Ed. 2010;49:2869–2872. doi: 10.1002/anie.200906120. Initial description of fluorogenicity for tetrazine–fluorophore conjugates and intracellular labeling using tetrazine–TCO ligation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han H-S, Devaraj NK, Lee J, Hilderbrand SA, Weissleder R, Bawendi MG. Development of a bioorthogonal and highly efficient conjugation method for quantum dots using tetrazine–norbornene cycloaddition. J Am Chem Soc. 2010;132:7838–7839. doi: 10.1021/ja101677r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haun JB, Devaraj NK, Hilderbrand SA, Lee H, Weissleder R. Bioorthogonal chemistry amplifies nanoparticle binding and enhances the sensitivity of cell detection. Nat Nano. 2010;5:660–665. doi: 10.1038/nnano.2010.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Keliher EJ, Reiner T, Turetsky A, Hilderbrand SA, Weissleder R. High-yielding, two-step 18F labeling strategy for 18F-PARP1 inhibitors. ChemMedChem. 2011;6:424–427. doi: 10.1002/cmdc.201000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52•.Reiner T, Keliher EJ, Earley S, Marinelli B, Weissleder R. Synthesis and in vivo imaging of a 18F-labeled PARP1 inhibitor using a chemically orthogonal scavenger-assisted high-performance method. Angew Chem Int Ed. 2011;50:1922–1925. doi: 10.1002/anie.201006579. Use of F-18 labeled TCO for PET probe construction and in vivo imaging of a PARP1 inhibitor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53•.Devaraj NK, Thurber GM, Keliher EJ, Marinelli B, Weissleder R. Reactive polymer enables efficient in vivo bioorthogonal chemistry. Proc Natl Acad Sci. 2012;109:4762–4767. doi: 10.1073/pnas.1113466109. Pretargeted in vivo imaging of tumors in mice using an 18F-TCO and a tetrazine-modified dextran polymer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54••.Li Z, Cai H, Hassink M, Blackman ML, Brown RCD, Conti PS, Fox JM. Tetrazine–trans-cyclooctene ligation for the rapid construction of F-18 labeled probes. Chem Commun. 2010;46:8043–8045. doi: 10.1039/c0cc03078c. Initial description of an F-18 labeled trans-cyclooctene for PET imaging applications. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu S, Hassink M, Selvaraj R, Yap LP, Park R, Wang H, Chen X, Fox JM, Li Z, Conti PS. Efficient 18F labeling of cysteine-containing peptides and proteins using tetrazine–trans-cyclooctene ligation. Mol Imaging. 2013;12:121–128. [PMC free article] [PubMed] [Google Scholar]
- 56•.Wu Z, Liu S, Hassink M, Nair I, Park R, Li L, Todorov I, Fox JM, Li Z, Shively JE, Conti PS, et al. Development and evaluation of 18F-TTCO-cys40-exendin-4: a novel PET probe for transplanted islets imaging. J Nucl Med. 2013;54:244–251. doi: 10.2967/jnumed.112.109694. Use of F-18 labeled TCO for PET probe construction and in vivo imaging of GLP-1R positive tumors in mice, and imaging of intraportally transplanted islet cells. [DOI] [PubMed] [Google Scholar]
- 57••.Liu DS, Tangpeerachaikul A, Selvaraj R, Taylor MT, Fox JM, Ting AY. Diels–Alder cycloaddition for fluorophore targeting to specific proteins inside living cells. J Am Chem Soc. 2011;134:792–795. doi: 10.1021/ja209325n. A lipoic acid ligase mutant is engineered to specifically recognize TCO analogs, which can be tagged by fluorophore–tetrazine conjugates with high efficiency. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58••.Seitchik JL, Peeler JC, Taylor MT, Blackman ML, Rhoads TW, Cooley RB, Refakis C, Fox JM, Mehl RA. Genetically encoded tetrazine amino acid directs rapid site-specific in vivo bioorthogonal ligation with trans-cyclooctenes. J Am Chem Soc. 2012;134:2898–2901. doi: 10.1021/ja2109745. A tetrazine-functionalized amino acid can be site specifically incorporated into proteins and labeled with high efficiency by derivatives of s-TCO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kaya E, Vrabel M, Deiml C, Prill S, Fluxa VS, Carell T. A genetically encoded norbornene amino acid for the mild and selective modification of proteins in a copper-free click reaction. Angew Chem Int Ed. 2012;51:4466–4469. doi: 10.1002/anie.201109252. [DOI] [PubMed] [Google Scholar]
- 60••.Plass T, Milles S, Koehler C, Szymański J, Mueller R, Wießler M, Schultz C, Lemke EA. Amino acids for Diels–Alder reactions in living cells. Angew Chem Int Ed. 2012;51:4166–4170. doi: 10.1002/anie.201108231. A method for genetically encoding a TCO-derivatized amino acid into proteins of interest is described. [DOI] [PubMed] [Google Scholar]
- 61.Rossin R, van den Bosch SM, ten Hoeve W, Carvelli M, Versteegen RM, Lub J, Robillard MS. Highly reactive trans-cyclooctene tags with improved stability for Diels–Alder chemistry in living systems. Bioconj Chem. 2013;24:1210–1217. doi: 10.1021/bc400153y. [DOI] [PubMed] [Google Scholar]
- 62.Zeglis BM, Sevak KK, Reiner T, Mohindra P, Carlin SD, Zanzonico P, Weissleder R, Lewis JS. A pretargeted PET imaging strategy based on bioorthogonal Diels–Alder click chemistry. J Nucl Med. 2013 doi: 10.2967/jnumed.112.115840. http://dx.doi.org/10.2967/jnumed.112.115840. [DOI] [PMC free article] [PubMed]
- 63.Carlson JCT, Meimetis LG, Hilderbrand SA, Weissleder R. BODIPY–tetrazine derivatives as superbright bioorthogonal turn-on probes. Angew Chem Int Ed. 2013;52:6917–6920. doi: 10.1002/anie.201301100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schoch J, Staudt M, Samanta A, Wiessler M, Jäschke A. Site-specific one-pot dual labeling of DNA by orthogonal cycloaddition chemistry. Bioconj Chem. 2012;23:1382–1386. doi: 10.1021/bc300181n. [DOI] [PubMed] [Google Scholar]