Abstract
Steady-state fluorescence polarization studies with the fluorescent lipid probe 1,6-diphenyl 1,3,5-hexatriene were done to determine the degree of microviscosity of cellular membrane lipids and serum lipoproteins in human normal donors and leukemic patients. The results show a marked decrease in microviscosity of cellular membrane lipids in both intact lymphocytes and isolated cellular plasma membranes obtained from leukemic patients in clinical relapse as compared to intact lymphocytes and isolated cellular plasma membranes obtained from normal donors and leukemic patients in complete clinical remission. Concomitant to these dynamic changes in cellular membrane lipids, the degree of microviscosity of lipids in the blood serum of leukemic patients in clinical relapse is markedly reduced as compared to serum obtained from normal donors and leukemic patients in complete clinical remission. Moreover, an in vitro incubation of leukemic lymphocytes with normal low density lipoproteins results in an increased microviscosity of cellular membrane lipids. In addition to the interrelation between cellular membrane lipids and serum lipoproteins, plasma membrane vesicles with a high degree of lipid microviscosity were isolated from the blood serum and pleural effusion of leukemic patients in clinical relapse. Such membrane vesicles could not be detected in normal serum. Therefore, we suggest that the two major mechanisms associated with the decreased microviscosity of membrane lipids in human leukemic cells are an abnormal exchange in lipids between the leukemic cell surface membrane and leukemic serum lipoproteins and an exfoliation of plasma membrane vesicles with a high degree of microviscosity from the cell surface of leukemic cells.
Keywords: normal and leukemic cells, plasma membranes and vesicles, serum lipids and lipoproteins
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APPELMANS F., WATTIAUX R., DE DUVE C. Tissue fractionation studies. 5. The association of acid phosphatase with a special class of cytoplasmic granules in rat liver. Biochem J. 1955 Mar;59(3):438–445. doi: 10.1042/bj0590438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AYRAULT-JARRIER M., LEVY G., WALD R., POLONOVSKI J. [Separation by ultracentrifugation of the alpha-lipoproteins of normal human serum]. Bull Soc Chim Biol (Paris) 1963;45:349–359. [PubMed] [Google Scholar]
- Aloni B., Shinitzky M., Livne A. Dynamics of erythrocyte lipids in intact cells, in ghost membranes and in liposomes. Biochim Biophys Acta. 1974 Jun 26;348(3):438–441. doi: 10.1016/0005-2760(74)90223-9. [DOI] [PubMed] [Google Scholar]
- Andrich M. P., Vanderkooi J. M. Temperature dependence of 1,6-diphenyl-1,3,5-hexatriene fluorescence in phophoslipid artificial membranes. Biochemistry. 1976 Mar 23;15(6):1257–1261. doi: 10.1021/bi00651a013. [DOI] [PubMed] [Google Scholar]
- Anner B., Mossmayer M. Rapid determination of inorganic phosphate in biological systems by a highly sensitive photometric method. Anal Biochem. 1975 May 12;65(1-2):305–309. doi: 10.1016/0003-2697(75)90514-x. [DOI] [PubMed] [Google Scholar]
- Ben-Bassat H., Polliak A., Rosenbaum S. M., Naparstek E., Shouval D., Inbar M. Fluidity of membrane lipids and lateral mobility of concanavalin A receptors in the cell surface of normal lymphocytes and lymphocytes from patients with malignant lymphomas and leukemias. Cancer Res. 1977 May;37(5):1307–1312. [PubMed] [Google Scholar]
- Collard J. G., De Wildt A., Oomen-Meulemans E. P., Smeekens J., Emmelot P. Increase in fluidity of membrane lipids in lymphocytes, fibroblasts and liver cells stimulated for growth. FEBS Lett. 1977 May 15;77(2):173–178. doi: 10.1016/0014-5793(77)80228-7. [DOI] [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmelot P., Bos C. J. Studies on plasma membranes. 3. Mg2+-ATPase,(Na+-K+-Mg2+)-ATPase and 5'-nucleotidase activity of plasma membranes isolated from rat liver. Biochim Biophys Acta. 1966 Jul 13;120(3):369–382. doi: 10.1016/0926-6585(66)90304-9. [DOI] [PubMed] [Google Scholar]
- Fuchs P., Parola A., Robbins P. W., Blout E. R. Fluorescence polarization and viscosities of membrane lipids of 3T3 cells. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3351–3354. doi: 10.1073/pnas.72.9.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
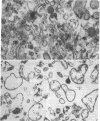
- HAGERMAN J. S., GOULD R. G. The in vitro interchange of cholesterol between plasma and red cells. Proc Soc Exp Biol Med. 1951 Oct;78(1):329–332. doi: 10.3181/00379727-78-19064. [DOI] [PubMed] [Google Scholar]
- Inbar M., Ben-Bassat H. Fluidity difference in the surface membrane lipid core of human lymphoblastoid and lymphoma cell lines. Int J Cancer. 1976 Sep 15;18(3):293–297. doi: 10.1002/ijc.2910180305. [DOI] [PubMed] [Google Scholar]
- Inbar M. Fluidity of membrane lipids: a single cell analysis of mouse normal lymphocytes and malignant lymphoma cells. FEBS Lett. 1976 Aug 15;67(2):180–185. doi: 10.1016/0014-5793(76)80361-4. [DOI] [PubMed] [Google Scholar]
- Inbar M., Goldman R., Inbar L., Bursuker I., Goldman B., Akstein E., Segal P., Ipp E., Ben-Bassat I. Fluidity difference of membrane lipids in human normal and leukemic lymphocytes as controlled by serum components. Cancer Res. 1977 Sep;37(9):3037–3041. [PubMed] [Google Scholar]
- Inbar M., Larnicol N., Jasmin C., Mishal Z., Augery Y., Rosenfeld C., Mathé G. A method for the quantitative detection of human acute lymphatic leukemia. Eur J Cancer. 1977 Nov;13(11):1231–1236. doi: 10.1016/0014-2964(77)90029-9. [DOI] [PubMed] [Google Scholar]
- Inbar M., Shinitzky M. Cholesterol as a bioregulator in the development and inhibition of leukemia. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4229–4231. doi: 10.1073/pnas.71.10.4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inbar M., Shinitzky M. Decrease in microviscosity of lymphocyte surface membrane associated with stimulation induced by concanavalin A. Eur J Immunol. 1975 Mar;5(3):166–170. doi: 10.1002/eji.1830050303. [DOI] [PubMed] [Google Scholar]
- Inbar M., Shinitzky M. Increase of cholesterol level in the surface membrane of lymphoma cells and its inhibitory effect on ascites tumor development. Proc Natl Acad Sci U S A. 1974 May;71(5):2128–2130. doi: 10.1073/pnas.71.5.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inbar M., Shinitzky M., Sachs L. Microviscosity in the surface membrane lipid layer of intact normal lymphocytes and leukemic cells. FEBS Lett. 1974 Jan 15;38(3):268–270. doi: 10.1016/0014-5793(74)80069-4. [DOI] [PubMed] [Google Scholar]
- Inbar M., Yuli I., Raz A. Contact-mediated changes in the fluidity of membrane lipids in normal and malignant transformed mammalian fibroblasts. Exp Cell Res. 1977 Mar 15;105(2):325–335. doi: 10.1016/0014-4827(77)90131-8. [DOI] [PubMed] [Google Scholar]
- Kramers M. T., Catovsky D., Foa R., Cherchi M., Galton D. A. 5' nucleotidase activity in leukaemic lymphocytes. Biomedicine. 1976 Dec 30;25(10):363–365. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lelievre L. Plasma membranes from fibroblastic cells in culture. Isolation, morphological and enzymatic identification. Biochim Biophys Acta. 1973 Feb 16;291(3):662–670. doi: 10.1016/0005-2736(73)90471-9. [DOI] [PubMed] [Google Scholar]
- Lentz B. R., Barenholz Y., Thompson T. E. Fluorescence depolarization studies of phase transitions and fluidity in phospholipid bilayers. 1. Single component phosphatidylcholine liposomes. Biochemistry. 1976 Oct 5;15(20):4521–4528. doi: 10.1021/bi00665a029. [DOI] [PubMed] [Google Scholar]
- Lopes J., Zucker-Franklin D., Silber R. Heterogeneity of 5'-nucleotidase activity in lymphocytes in chronic lymphocytic leukemia. J Clin Invest. 1973 May;52(5):1297–1300. doi: 10.1172/JCI107298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORRISON W. R. A FAST, SIMPLE AND RELIABLE METHOD FOR THE MICRODETERMINATION OF PHOSPHORUS IN BIOLOGICAL MATERIALS. Anal Biochem. 1964 Feb;7:218–224. doi: 10.1016/0003-2697(64)90231-3. [DOI] [PubMed] [Google Scholar]
- Shinitzky M., Inbar M. Microviscosity parameters and protein mobility in biological membranes. Biochim Biophys Acta. 1976 Apr 16;433(1):133–149. doi: 10.1016/0005-2736(76)90183-8. [DOI] [PubMed] [Google Scholar]
- Stein O., Vanderhoek J., Stein Y. Cholesterol content and sterol synthesis in human skin fibroblasts and rat aortic smooth muscle cells exposed to lipoprotein-depleted serum and high density apolipoprotein/phospholipid mixtures. Biochim Biophys Acta. 1976 May 27;431(2):347–358. doi: 10.1016/0005-2760(76)90155-7. [DOI] [PubMed] [Google Scholar]
- Van Blitterswijk W. J., Emmelot P., Hilgers J., Kamlag D., Nusse R., Feltkamp C. A. Quantitation of virus-induced (MLr) and normal (Thy.1.2) cell surface antigens in isolated plasma membranes and the extracellular ascites fluid of mouse leukemia cells. Cancer Res. 1975 Oct;35(10):2743–2751. [PubMed] [Google Scholar]
- van Blitterswijk W. J., Emmelot P., Hilkmann H. A., Oomenmeulemans E. P., Inbar M. Differences in lipid fluidity among isolated plasma membranes of normal and leukemic lympocytes and membranes exfoliated from their cell surface. Biochim Biophys Acta. 1977 Jun 16;467(3):309–320. doi: 10.1016/0005-2736(77)90308-x. [DOI] [PubMed] [Google Scholar]