Abstract
Background
Rapid diagnostic tests (RDTs) are immune chromatographic tests targeting antigens of one or more Plasmodium species and offer the potential to extend accurate malaria diagnosis in endemic areas. In this study, the performance of Plasmodium falciparum-specific histidine-rich protein-2 (PfHRP-2) RDT in the detection of asymptomatic carriers from a hyperendemic region of Burkina Faso was compared with microscopy to gain further insight on its relevance in community-based interventions.
Methods
The performance of HRP-2 test was evaluated in terms of sensitivity, specificity, positive and negative predictive values, discordant values, likelihood ratios, accuracy, and precision using microscopy as the 'gold standard’. This analysis was carried out in a controlled, parallel, cluster-randomized (18 clusters; 1:1) study in children and adults. The effect of systematic treatment of P. falciparum asymptomatic carriers during three consecutive monthly community screening campaigns on the incidence of symptomatic malaria episodes over a 12-month period was compared with no treatment of asymptomatic carriers.
Results
Sensitivity of HRP-2 test in asymptomatic carriers was higher in campaign 1 (92.4%) when compared to campaign 2 (84.0%) and campaign 3 (77.8%). The sensitivity of HRP-2 test increased as parasite density increased across all the age groups. Highest sensitivity (≥97.0%) was recorded at parasite densities of 1,000-4,999/μl, except for children aged 10 to 14 years. The specificity of HRP-2 test was comparable across age groups and highest in campaign 3 (95.9%). The negative predictive values were high across the three campaigns (≥92.7%) while the positive predictive values ranged from 23.2 to 73.8%. False-positive and false-negative rates were high in campaign 1 and campaign 3, respectively.
Conclusion
The performance of HRP-2 test in detecting asymptomatic carriers of P. falciparum varied by age and parasite density. Although the use of HRP-2 test is beneficial for the diagnosis of acute malaria, its low sensitivity in screening asymptomatic carriers may limit its utility in pre-elimination interventional settings. The use of a practical and more sensitive test such as loop-mediated isothermal amplification in combination with a cost effective HRP-2 test may be worth exploring in such settings.
Keywords: Malaria, Plasmodium falciparum, Asymptomatic carriers, Community screening, Rapid diagnostic tests
Background
Both microscopy and rapid diagnostic tests (RDTs) have been recommended by the World Health Organization (WHO) for diagnosis of malaria. The choice between the two methods varies according to available skills, patient-case load and local setting. Microscopy is the reference method for malaria diagnosis and can be used to identify species, parasite density and response to anti-malarial therapy. However, a major drawback of this tool is its requirement of well-trained, skilled staff and an energy source to power the microscope [1].
There are currently over 200 RDTs produced by 60 manufacturers, which detect Plasmodium falciparum, Plasmodium vivax or all human Plasmodium species [2]. Target antigens for P. falciparum detection are P. falciparum-specific histidine-rich protein-2 (PfHRP-2) and P. falciparum-specific parasite lactate dehydrogenase (Pf-pLDH). For P. vivax detection, the target antigen is P. vivax-specific parasite lactate dehydrogenase (Pv-pLDH). For all human Plasmodium species, pan-pLDH and aldolase can be detected by RDTs [3]. In field trials, malaria RDTs have demonstrated ≥90% sensitivity and specificity for P. falciparum infection with parasite densities of ≥200 parasites/μl [4]. HRP-2 tests are highly sensitive for P. falciparum infections at parasite densities above 100–200 parasites/μl, but have limited reliability at lower parasite densities [4]. Detection of P. vivax varies at densities of 100–200 parasites/μl and higher, depending on the RDT product and target antigen [5]. The specificity of HRP-2 tests raises concerns in areas of intense malaria transmission due to slow clearance and persistence of HRP-2 antigens in the bloodstream for several weeks due to prior infections [3,6-8]. Another recently reported concern was a high rate of false-negative results in asymptomatic carriers of P. falciparum. False-negative results were reported only in individuals with asymptomatic infections, suggesting that parasites that fail to produce HRP-2 can cause patent bloodstream infections [9].
Asymptomatic carriers are individuals who are infected with plasmodial asexual forms, with or without gametocytes, but do not present any clinical symptoms. These individuals are a concern because they serve as reservoirs of parasites including gametocytes that are difficult to detect, and diagnosis presents challenges due to lack of distinct clinical manifestations. There is a positive correlation between number of asymptomatic carriers and high transmission, which may be due to exposure related immunity [10].
The aim of this paper is to report HRP-2 based RDT results from a recent cluster-randomized trial in Burkina Faso, which investigated the systematic, community-wide screening and treatment of asymptomatic carriers of P. falciparum with artemether-lumefantrine (AL). The main outcomes of the study have already been published [11]; however, the data on the HRP-2 test performance provide an opportunity to gain further insight on its relevance in detection of P. falciparum asymptomatic carriers in such community-based interventions.
Methods
Approach for screening of asymptomatic carriers
This analysis was carried out in a controlled, parallel, cluster-randomized study to evaluate the performance of HRP-2 based RDT in comparison with microscopy in detection of P. falciparum asymptomatic carriers in the health district of Saponé, Burkina Faso, which is an area with marked seasonal P. falciparum malaria transmission from June to November. The incidence of symptomatic malaria episodes in children (<5 years) and adults over a 12-month period was compared with asymptomatic carriers who were not treated. A total of 18 clusters, each comprising one village, were selected to participate in this trial, and were randomized in a 1:1 ratio to either the intervention or control arm.
Prior to the transmission (rainy) season, screening and treatment of asymptomatic carriers took place and all inhabitants of the intervention arm and 40% of inhabitants of the control arm participated in three community screening campaigns (campaign 1 to 3), which were approximately one month apart between January and April 2011. During each screening campaign, subjects in the intervention arm were home visited and screened for P. falciparum asexual forms using HRP-2 test. Blood smears for delayed microscopy readings were simultaneously collected. In the control arm, only blood smears for delayed microscopy readings were obtained. RDT-positive individuals from the intervention arm received treatment with AL (20 mg artemether and 120 mg lumefantrine [Coartem®, Novartis Pharma AG, Basel, Switzerland]) or AL dispersible, twice daily for three consecutive days. As inherent to the study design, results reported here are only from the intervention arm where both HRP-2 based RDT and microscopy data were concomitantly collected.
Rapid diagnostic test
First Response® Malaria Ag, (Premier Medical Corp Ltd., Nani-Daman, India) was used. This is a HRP-2 test recommended by WHO for its performance at low parasite densities, low false-positive rate, and high heat stability [12]. RDT stock was stored in an air-conditioned room with ambient temperature and humidity recorded twice daily at the Centre National de Recherche et de Formation sur le Paludisme (CNRFP). During the screening campaigns, in the absence of electricity, test kits were kept in cold boxes within the local health facility to which the study clusters reported. Storage temperature was recorded twice daily (morning and afternoon) by the study nurses.
Diagnostic microscopy
Blood films were air-dried and Giemsa-stained for examination by a light microscope fitted with 100 × oil immersion lens at a single laboratory. At least 200 thick film fields were examined before a slide was declared negative. If Plasmodium asexual forms were found, a total of 200 thick film fields were screened for Plasmodium species other than P. falciparum. If P. falciparum was present, a count of asexual forms against leukocytes was made using a tally counter. Counting was conducted based on at least 200 leukocytes in consistence with WHO standards. If less than 10 parasites were identified from the 200 leukocyte screened, the count was extended to 1,000 leukocytes. On the other hand, if P. falciparum gametocytes were seen, a gametocyte count was performed against 1,000 leukocytes. All slides were read by two independent microscopists. If the ratio of densities from the first two readings was >1.5 or < 0.67, or if less than 30 parasites were counted with an absolute difference of more than 10 in the number of parasites, the slide was evaluated by a third microscopist. The definitive result was the mean of the parasite density of the two most concordant readings. Microscopist competency was evaluated through two external quality control (EQC) programmes. The first EQC was carried out by the College of American Pathology proficiency testing and included a set of five slides provided to each microscopist for reading; three rounds of proficiency testing were conducted per year. The second EQC was performed by WHO (National Institute for Communicable Diseases) and involved the reading of a set of 20 slides every quarter by each microscopist. Only those with a score of at least 80%, graded as ‘competent’, were involved in the reading of trial participants’ slides.
Statistical methods
Data were entered into an electronic web-based case report form and were analysed using SAS® version 9.2 on UNIX according to a pre-defined analytical plan. The study sample was categorized into four age groups: zero to four years, five to nine years, 10 to 14 years, and ≥15 years. Six categories of parasite densities were considered for subjects with parasitaemia: 0–99 parasites/μl, 100–249 parasites/μl, 250–499 parasites/μl, 500–999 parasites/μl, 1,000-4,999 parasites/μl, and ≥5,000 parasites/μl.
Each HRP-2 result was categorized as true positive (TP), true negative (TN), false positive (FP), or false negative (FN). Sensitivity was calculated as TP/(TP + FN) and specificity as TN/(TN + FP). Positive predictive value (PPV) was defined as TP/(TP + FP) and negative predictive value (NPV) as TN/(TN + FN). False-positive rate was calculated as FP/(FP + TN) and false-negative rate was calculated as 1-(TN/[TN + FP]). Positive likelihood ratio was defined as the probability of a positive test result in patients with the disease divided by the probability of a positive test result in patients without the disease (i.e., sensitivity/[1 - specificity]), and negative likelihood ratio as the probability of a negative test result in patients with the disease divided by the probability of a negative test result in patients without the disease (i.e., [1 - sensitivity]/specificity). Accuracy, the proportion of all tests that yielded a correct result, was defined as (TP + TN)/(TP + TN + FP + FN). Cohen’s Kappa value, the quantitative measure of the magnitude of agreement between two tests was calculated. The test performance was evaluated across the three screening campaigns and for categories of age and parasite density. Geometric mean parasite density by age group was also reported.
Ethical aspects
The clinical trial protocol and informed consent forms were approved by the CNRFP Institutional Review Board and by the National Ethical Committee for Health Research of Burkina Faso. Prior to study initiation, a community meeting was held in each of the selected clusters to discuss the study objectives with the communities. The freedom of each individual household and household member to decide on study participation was discussed to minimize the potential influence of key opinion leaders in each cluster. Individual informed consent was obtained from each participant during a visit to the household prior to any study procedure.
Results
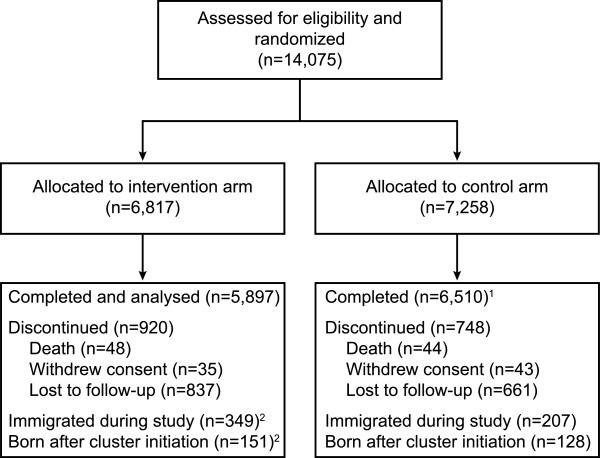
Of 14,075 subjects assessed for eligibility, a total of 6,817 subjects in the intervention arm and 7,258 subjects in the control arm were recruited and enrolled in the study (Figure 1). The intervention and control arms were similar in terms of demographic characteristics with the exception of ethnicity; the intervention arm had a higher proportion of Fulani. The primary outcomes of this study were reported by Tiono et al.[11].
Figure 1.
Consort chart. 1RDT was not done for subjects in the control arm and participants were excluded from the analysis. 2Not included in analysis as assessment was done after third campaign.
A total of 17,505 samples from 6,817 subjects in the intervention arm were examined by microscopy and HRP-2 test across the three campaigns (Table 1). About 47% of the participants were women and their mean age at baseline was 24.4 years.
Table 1.
Characteristics of study participants across three screening campaigns
| Campaign 1 | Campaign 2 | Campaign 3 | |
|---|---|---|---|
|
Number of participants |
5,633 |
5,718 |
6,154 |
|
Mean age (years) |
24.4 |
23.9 |
23.0 |
|
Number (%) of females |
2,617 (46.5) |
2,664 (46.6) |
2,863 (46.5) |
|
Prevalence of
P. falciparum
infection (by microscopy), n (%) |
|
|
|
| 0- <5 |
689 (12.2) |
205 (3.6) |
193 (3.1) |
| 5-9 |
971 (17.2) |
318 (5.6) |
271 (4.4) |
| 10-14 |
841 (14.9) |
253 (4.4) |
218 (3.5) |
| ≥15 |
1,080 (19.1) |
294 (5.1) |
230 (3.7) |
| Overall |
3,581 (63.6) |
1,070 (18.7) |
912 (14.8) |
|
Prevalence of
P. falciparum
infection (by HRP-2 test), n (%) |
|
|
|
| 0- <5 |
606 (10.8) |
238 (4.1) |
86 (1.4) |
| 5-9 |
734 (13.0) |
175 (3.0) |
52 (0.8) |
| 10-14 |
674 (12.0) |
114 (2.0) |
49 (0.8) |
| ≥15 |
1,060 (18.8) |
336 (5.9) |
190 (3.1) |
| Overall |
3,074 (54.6) |
863 (15.1) |
377 (6.1) |
|
Frequency of
P. falciparum
infection by density cut-offs (parasites/μl), n (%) |
|
|
|
| 1-99 |
798 (22.3) |
272 (25.4) |
258 (28.3) |
| 100-499 |
1,013 (28.3) |
370 (34.6) |
301 (33.0) |
| 500-999 |
599 (16.7) |
182 (17.0) |
148 (16.2) |
| 1,000-4,999 |
877 (24.5) |
199 (18.6) |
165 (18.1) |
| ≥5,000 |
294 (8.2) |
47 (4.4) |
40 (4.4) |
| Overall |
3,581 (100.0) |
1,070 (100.0) |
912 (100.0) |
|
Geometric mean
P. falciparum
density in positives by age (parasites/μl) |
|
|
|
| 0- <5 |
1,348 |
702 |
756 |
| 5-9 |
795 |
482 |
387 |
| 10-14 |
406 |
273 |
241 |
| ≥15 |
168 |
127 |
111 |
| Overall | 470 | 315 | 291 |
Age was considered at cluster initiation. Percentages in prevalence subgroups are based on the total number of subjects in the respective subgroup.
Overall, 5,563 (31.8%) samples were detected to be positive using microscopy (Table 1). The prevalence of P. falciparum infection as detected by microscopy was highest among subjects aged ≥15 years, followed by five to nine year olds and 10 to 14 year olds. Using HRP-2 test, 4,314 (24.6%) samples were detected to be positive for P. falciparum (Table 1). The prevalence of P. falciparum infection by age as detected by HRP-2 test followed the same trend as microscopy in campaign 1, but in campaigns 2 and 3, prevalence was highest among subjects aged ≥15 years, followed by <5 year olds and 10 to 14 year olds. The prevalence of P. falciparum infection by density ranged from 8.2 to 28.3% in campaign 1, 4.4 to 34.6% in campaign 2, and 4.4 to 33.0% in campaign 3 (Table 1). Across the three campaigns, the geometric mean P. falciparum density decreased with increasing age; it was highest in children <5 years of age (Table 1).
Sensitivity of HRP-2 test in asymptomatic carriers was higher in campaign 1 when compared to campaign 2 and campaign 3 (Table 2 and Figure 2). Specificity of HRP-2 test was highest in campaign 3 (Table 2 and Figure 2). Negative predictive values were high across the three campaigns, while positive predictive values ranged from 23.2 to 73.8%. An overview of the discordant results (i.e., either a false-positive or false-negative HRP-2 compared with microscopy) is given in Table 2. False-positive and false-negative rates were high in campaign 1 and campaign 3, respectively. Positive likelihood ratio was highest in campaign 3 followed by campaign 2 and campaign 1. Negative likelihood ratio was lowest in campaign 1, followed by campaign 2 and campaign 3. The proportion of all true HRP-2 test results yielded an accuracy of 0.82, 0.88, and 0.95 in campaigns 1, 2 and 3, respectively. The agreement between the two techniques, HRP-2 test and microscopy, was highest in campaign 1.
Table 2.
Overall performance of HRP-2 test in asymptomatic carriers across three screening campaigns
| Campaign 1 | Campaign 2 | Campaign 3 | |
|---|---|---|---|
|
Sensitivity, % (95% CI) |
92.4 (91.4, 93.5) |
84.0 (79.3, 88.6) |
77.8 (71.5, 84.0) |
|
Specificity, % (95% CI) |
74.6 (73.1, 76.1) |
87.9 (87.0, 88.7) |
95.9 (95.4, 96.4) |
|
Positive predictive value, % (95% CI) |
73.8 (72.2, 75.3) |
23.2 (20.3, 26.0) |
35.4 (30.5, 40.2) |
|
Negative predictive value, % (95% CI) |
92.7 (91.7, 93.7) |
99.2 (99.0, 99.5) |
99.3 (99.1, 99.5) |
|
False-positive rate |
0.25 |
0.12 |
0.04 |
|
False-negative rate |
0.08 |
0.16 |
0.22 |
|
Positive likelihood ratio |
3.64 |
6.92 |
19.01 |
|
Negative likelihood ratio |
0.10 |
0.18 |
0.23 |
|
Accuracy |
0.82 |
0.88 |
0.95 |
| Kappa, % (95% CI) | 65.2 (63.2, 67.1) | 31.9 (28.3, 35.4) | 46.6 (41.4, 51.8) |
Figure 2.
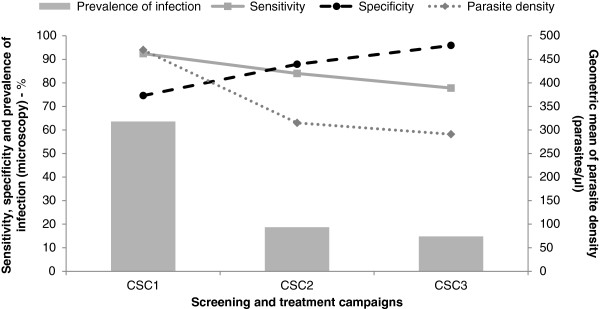
Prevalence of Plasmodium falciparum infection, parasite count and rapid diagnostic test accuracy (sensitivity and specificity) according to screening campaigns.
Generally, the sensitivity of HRP-2 test increased as parasite density increased across all the age groups (Table 3 and Figure 2). At parasite densities of 1,000-4,999/μl, HRP-2 test recorded the highest sensitivity, except for children aged 10 to 14 years.
Table 3.
Performance of HRP-2 test in asymptomatic carriers by age and parasite density
| Age group (years) | Parasite density (/μl) | N | Positive | Negative | Sensitivity % (95% CI) | Specificity % (95% CI) |
|---|---|---|---|---|---|---|
|
0- <5 |
0-99 |
2,663 |
433 |
2,230 |
74.6 (64.5, 84.8) |
|
| |
100-499 |
90 |
83 |
7 |
92.2 (86.7, 97.8) |
|
| |
500-999 |
86 |
85 |
1 |
98.8 (96.6, 100) |
|
| |
1,000-4,999 |
211 |
209 |
2 |
99.1 (97.7, 100) |
|
| |
≥5,000 |
117 |
114 |
3 |
97.4 (94.6, 100) |
|
| |
Overall |
3,167 |
924 |
2,243 |
94.6 (92.8, 96.5) |
85.3 (84.0, 86.7) |
|
5-9 |
0-99 |
2,147 |
327 |
1,820 |
84.0 (75.7, 92.3) |
|
| |
100-499 |
184 |
171 |
13 |
92.9 (89.2, 96.6) |
|
| |
500-999 |
145 |
143 |
2 |
98.6 (96.7, 100) |
|
| |
1,000-4,999 |
246 |
245 |
1 |
99.6 (98.8, 100) |
|
| |
≥5,000 |
69 |
68 |
1 |
98.6 (95.7, 100) |
|
| |
Overall |
2,791 |
954 |
1,837 |
96.0 (94.5, 97.4) |
87.3 (85.8, 88.7) |
|
10-14 |
0-99 |
2,124 |
346 |
1,778 |
85.5 (79.8, 91.2) |
|
| |
100-499 |
215 |
206 |
9 |
95.8 (93.1, 98.5) |
|
| |
500-999 |
104 |
103 |
1 |
99.0 (97.2, 100) |
|
| |
1,000-4,999 |
145 |
144 |
1 |
99.3 (98.0, 100) |
|
| |
≥5,000 |
31 |
31 |
|
100 (100, 100) |
|
| |
Overall |
2,619 |
830 |
1,789 |
95.0 (93.3, 96.7) |
88.8 (87.4, 90.2) |
|
≥15 |
0-99 |
8,287 |
1,129 |
7,158 |
73.1 (68.8, 77.5) |
|
| |
100-499 |
310 |
265 |
45 |
85.5 (81.6, 89.4) |
|
| |
500-999 |
107 |
101 |
6 |
94.4 (90.0, 98.8) |
|
| |
1,000-4,999 |
66 |
64 |
2 |
97.0 (92.8, 100) |
|
| |
≥5,000 |
17 |
10 |
7 |
58.8 (35.4, 82.2) |
|
| Overall | 8,787 | 1,569 | 7,218 | 81.4 (78.8, 83.9) | 89.4 (88.7, 90.1) |
N = number of subjects for whom RDT and microscopy results were available.
Discussion
This paper describes the performance of HRP-2 test in comparison with microscopy in the detection of asymptomatic carriers in community-wide screening and treatment campaigns from a hyperendemic region of Burkina Faso. The findings provide evidence that while HRP-2 test might be a convenient diagnostic tool in a similar community based intervention with highly demanding logistics, it has obvious limitations that could jeopardize the success of the intervention.
What lessons were learned from this analysis?
Two main lessons could be drawn from the results of this trial. The first lesson is that HRP-2 test might not be suitable to use across all age groups in screening campaigns for asymptomatic carriers. Indeed, the test sensitivity decreased with age. This finding is consistent with previous reports showing that the proportion of infected individuals with low parasite densities increased with age, most likely due to age acquired immunity [13-15]. Thus, the age-dependent immune status might have lowered the HRP-2 test sensitivity [16]. This reduced sensitivity might have a serious impact on the effectiveness of mass screening and treatment (MSAT) strategies aiming to interrupt transmission. Indeed, as shown in a previous study, although gametocytes are most commonly detected in children, the proportion of asexual parasites that will develop into gametocytes may increase with age indicating the potential enormous contribution of adults for human infectious reservoir of malaria [17]. Another study that evaluated the contribution of different age groups to the human infectious reservoir found that adults may be responsible for 28 to 38% of mosquito infections [18]. This proportion could be even larger if differences in body size and exposure index to mosquitoes between adults and children were considered [19]. In total, failing to detect asexual parasites in adults in MSAT studies would thus highlight the missed opportunity in clearing the probably most important reservoir of parasites.
Overall, the HRP-2 test specificity was low and increased with age. Difference was only statistically significant when comparison was done between zero to <5 years and both 10 to 14 and ≥15 years age groups. Previous studies showed that recently treated infections may lead to false-positive results [3,6-8]. The delayed clearance and persistence of HRP-2 antigen in the blood, even after complete eradication of parasites, can limit the utility of the test [6,14,20]. The difference between age groups could be explained by the difference in incidence of clinical malaria episodes. Younger children are more prone to developing clinical symptoms and getting treated. Hence, the likelihood of detecting HRP-2 antigenaemia post treatment is high in this subpopulation. In theory, there is no potential impact of this low specificity on the effectiveness of the MSAT intervention. However, frequent false-positive findings can lead to unnecessary treatment [20].
The second important lesson from this trial is that HRP-2 might not be sufficient as a single diagnostic method in MSAT involving multiple screening campaigns. While the sensitivity and positive predictive values were high at the first campaign, a significant decrease below recommended standard by WHO [21] was observed during the next two campaigns. This trend was similar to the one observed for the prevalence of infection, and the geometric mean parasite density (Figure 2). A possible explanation is that several subjects may have harboured parasites at low densities that were below detection limit of the HRP-2 test. The proportion of subjects with parasite count <500 parasites/μl rose from 50.6% at first campaign to ~60% at second and third campaigns. This is supported by similar findings in Solomon Islands (low transmission setting) where a study conducted showed that a large proportion of asymptomatic P. falciparum and P. vivax infections had low and submicroscopic parasite densities that were not detected by RDT. The results suggested that the detection limit of the ICT combo kit was ~100 parasites/μl for P. falciparum and >300 parasites/μl for P. vivax[13]. Another study evaluated the performance of Optimal® rapid malaria test compared to expert microscopy as a tool for detecting asymptomatic malaria in an area of Thailand that is endemic for both P. falciparum and P. vivax. The low sensitivity of Optimal® rapid malaria test along with poor assay specificity and high number of false-positive cases in individuals with parasite densities <500/μl, suggested that the majority of malaria cases may not have been accurately detected during a similar surveillance programme [22].
Contrary to the sensitivity trend, the specificity and negative predictive values increased as the screening campaigns progressed. This provides evidence that the use of HRP-2 test has made it possible to comply with the overall goal of the MSAT intervention, which was to treat only those carrying malaria parasites, therefore minimizing overuse of drugs in non-parasitaemic subjects, and in turn reducing drug pressure.
Are there alternatives to RDTs in such trials?
Microscopy is widely accepted as a gold standard laboratory method. However, examining blood smears with high quality and accuracy requires extensive experience and training [23]. Furthermore, the use of microscopy may not be feasible in remote areas where malaria is prevalent [24] and results may not be readily available for treatment decision making. The experience in this trial suggests that a point-of-care malaria diagnostic tool is the best option to minimize logistical hurdles and ensure good coverage of the population. To overcome the limitations of detecting infections of lower density by microscopy and RDT, molecular diagnostic methods, such as polymerase chain reaction (PCR) have been developed. Studies using PCR in low transmission settings have revealed a high proportion of low-density parasitaemias and asymptomatic carriers that were not detected by RDT or microscopy [5,13,25,26].
Loop mediated isothermal amplification (LAMP) is an innovative gene amplification technique emerging as a simple rapid diagnostic tool for early detection and identification of microbial diseases. There is evidence that LAMP may be a fast, simple, and cost-effective diagnostic approach in detecting malaria parasites. LAMP uses DNA polymerase and amplifies a target DNA sequence with high sensitivity and specificity under isothermal conditions. Several studies have evaluated the sensitivity and specificity of LAMP for the detection and differentiation of Plasmodium species for human malaria. An advantage of LAMP is that results can be analysed with the naked eye based on turbidity or the use of fluorescent dyes [27]. Studies have also shown that LAMP is superior to expert microscopy, has diagnostic accuracy similar to PCR, and can produce results in a shorter time [28-30]. Current results and further development of LAMP may provide the possibility of its future use in the diagnosis of malaria.
What are possible scenarios for similar future interventions?
RDTs are still appealing since they are easy to use and can help overcome the challenges of microscopy in field-based trials. However, low sensitivity of RDTs in screening asymptomatic carriers limits their utility as single diagnostic tool in interventions targeting the parasite reservoirs (i.e., MSAT). While LAMP may be an option, given its ease of use in the field and diagnostic accuracy, its role in MSAT trials needs to be explored especially in settings where prevalence of malaria is high. It might not be cost effective to use this tool as stand-alone in such settings. Rational combinations with RDTs need to be explored. One possible scenario would be to use both RDT and LAMP in the first screening and treatment campaign, where RDT is used in children and LAMP in individuals ≥15 years. This approach adds cost and complexity to the trial implementation, but could help to prevent spread of the infection (by those who were not detected by RDT) in the interval before the next campaign. In subsequent campaigns, using LAMP in newcomers to the area or in the whole population, based on malaria transmission dynamics, may be targeted.
In areas with reports of high RDT sensitivity that is consistent across ages, exclusive use of RDT at the first campaign could be a better option to minimize cost. LAMP should be preferred for subsequent campaigns. Computer simulation analyses might be necessary to explore these scenarios and other possible ones to identify cost-effective solutions for better decision making.
Conclusion
The performance of HRP-2 test in P. falciparum asymptomatic carriers varied by age and parasite density. This low sensitivity in screening asymptomatic carriers may limit its utility in pre-elimination interventional settings. LAMP is a practical and more sensitive test; but cost may be a barrier to its use as single diagnostic tool in large-scale MSAT trials. Cost effective combination of HRP-2 tests and LAMP may be worth exploring.
Competing interests
ABT and AO have received honoraria from Novartis Pharma AG, Basel, Switzerland to attend Advisory Board meetings to discuss this study and manuscript. AM is an employee of Novartis Healthcare Private Limited. KH is an employee of Novartis Pharmaceuticals Corporation. AD, SC, IS, ATK, AB, and SBS declared no competing interests.
Authors’ contributions
ABT, AO, AD, SC, AM, SBS, and KH were involved in the design of the study, data interpretation, and defining the content for the manuscript. ABT, AO, AD, SC, IS, ATK, AB, and SBS were involved in data collection, while ABT, AO, AM, and KH conducted the data analysis. ABT, AM and KH were involved in writing the manuscript. All of the authors had full access to data in the study, discussed the results, critically reviewed the draft manuscript, and agreed on the final version. ABT, the corresponding author, had final responsibility for the decision to submit the manuscript for publication. Editorial assistance was provided by Ubhayabharathi Gurunath and Krishna Swetha Gummuluri, professional medical writers (Novartis Healthcare Private Limited).
Contributor Information
Alfred B Tiono, Email: t.alfred@fasonet.bf.
Alphonse Ouédraogo, Email: aouedraogo.cnrfp@fasonet.bf.
Amidou Diarra, Email: a.diarra.cnrfp@fasonet.bf.
Sam Coulibaly, Email: scad_bf@yahoo.fr.
Issiaka Soulama, Email: a.soulama.cnrfp@fasonet.bf.
Amadou T Konaté, Email: a.konate.cnlp@fasonet.bf.
Aïssata Barry, Email: assbart@hotmail.fr.
Amitava Mukhopadhyay, Email: amitava.mukhopadhyay@novartis.com.
Sodiomon B Sirima, Email: s.sirima.cnlp@fasonet.bf.
Kamal Hamed, Email: kamal.hamed@novartis.com.
Acknowledgements
We thank Jeremy Lim for providing a summary of the literature on RDTs and LAMP and for reviewing an earlier draft of the manuscript, Sarfaraz Sayyed for his assistance with statistical programming, and the many people in Saponé who took part or contributed to this study including the study participants and their communities, the CNRFP personnel, and local laboratory and health facility staff.
Novartis Pharma AG, Basel, Switzerland, provided funding for this study. The sponsors of the study were involved in study design, data analysis, data interpretation, and writing of the report.
References
- WHO. Guidelines for the treatment of malaria. Second. Geneva: World Health Organization; 2010. [PubMed] [Google Scholar]
- WHO. Good practices for selecting and procuring rapid diagnostic tests for malaria. Geneva: World Health Organization; 2011. [Google Scholar]
- Maltha J, Gillet P, Jacobs J. Malaria rapid diagnostic tests in travel medicine. Clin Microbiol Infect. 2013;19:408–415. doi: 10.1111/1469-0691.12152. [DOI] [PubMed] [Google Scholar]
- WHO. New perspectives: Malaria diagnosis: Report of joint WHO/USAID informal consultation. Geneva: World Health Organization; 1999. [Google Scholar]
- McMorrow ML, Aidoo M, Kachur SP. Malaria rapid diagnostic tests in elimination settings - can they find the last parasite? Clin Microbiol Infect. 2011;17:1624–1631. doi: 10.1111/j.1469-0691.2011.03639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyabayinze DJ, Tibenderana JK, Odong GW, Rwakimari JB, Counihan H. Operational accuracy and comparative persistent antigenicity of HRP2 rapid diagnostic tests for Plasmodium falciparum malaria in a hyperendemic region of Uganda. Malar J. 2008;7:221. doi: 10.1186/1475-2875-7-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent A, Schellenberg J, Shirima K, Ketende SC, Alonso PL, Mshinda H, Tanner M, Schellenberg D. Performance of HRP-2 based rapid diagnostic test for malaria and its variation with age in an area of intense malaria transmission in southern Tanzania. Malar J. 2010;9:294. doi: 10.1186/1475-2875-9-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly AB, Tall A, Perry R, Baril L, Badiane A, Faye J, Rogier C, Touré A, Sokhna C, Trape JF, Michel R. Use of HRP-2 based rapid diagnostic test for Plasmodium falciparum malaria: assessing accuracy and cost-effectiveness in the villages of Dielmo and Ndiop. Senegal. Malar J. 2010;9:153. doi: 10.1186/1475-2875-9-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koita OA, Doumbo OK, Ouattara A, Tall LK, Konaré A, Diakité M, Diallo M, Sagara I, Masinde GL, Doumbo SN, Dolo A, Tounkara A, Traoré I, Krogstad DJ. False-negative rapid diagnostic tests for malaria and deletion of the histidine-rich repeat region of the HRP2 gene. Am J Trop Med Hyg. 2012;86:194–198. doi: 10.4269/ajtmh.2012.10-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laishram DD, Sutton PL, Nanda N, Sharma VL, Sobti RC, Carlton JM, Joshi H. The complexities of malaria disease manifestations with a focus on asymptomatic malaria. Malar J. 2012;11:29. doi: 10.1186/1475-2875-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiono AB, Ouédraogo A, Ogutu B, Diarra A, Coulibaly S, Gansané A, Sirima SB, O’Neil G, Mukhopadhyay A, Hamed K. A controlled, parallel, cluster-randomized trial of community-wide screening and treatment of asymptomatic carriers of Plasmodium falciparum in Burkina Faso. Malar J. 2013;12:79. doi: 10.1186/1475-2875-12-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Malaria rapid diagnostic test performance: Results of WHO product testing of malaria RDTs (Round 4) Geneva: World Health Organization; 2012. [Google Scholar]
- Harris I, Sharrock WW, Bain LM, Gray KA, Bobogare A, Boaz L, Lilley K, Krause D, Vallely A, Johnson ML, Gatton ML, Shanks GD, Cheng Q. A large proportion of asymptomatic Plasmodium infections with low and sub-microscopic parasite densities in the low transmission setting of Temotu Province. Solomon Islands: challenges for malaria diagnostics in an elimination setting. Malar J. 2010;9:254. doi: 10.1186/1475-2875-9-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams HA, Causer L, Metta E, Malila A, O’Reilly T, Abdulla S, Kachur SP, Bloland PB. Dispensary level pilot implementation of rapid diagnostic tests: an evaluation of RDT acceptance and usage by providers and patients–Tanzania, 2005. Malar J. 2008;7:239. doi: 10.1186/1475-2875-7-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okell LC, Ghani AC, Lyons E, Drakeley CJ. Submicroscopic infection in Plasmodium falciparum-endemic populations: a systematic review and meta-analysis. J Infect Dis. 2009;200:1509–1517. doi: 10.1086/644781. [DOI] [PubMed] [Google Scholar]
- Fryauff DJ, Gomez-Saladin E, Purnomo, Sumawinata I, Sutamihardja MA, Tuti S, Subianto B, Richie TL. Comparative performance of the ParaSight F test for detection of Plasmodium falciparum in malaria-immune and nonimmune populations in Irian Jaya, Indonesia. Bull World Health Organ. 1997;75:547–552. [PMC free article] [PubMed] [Google Scholar]
- Ouédraogo AL, Bousema T, de Vlas SJ, Cuzin-Ouattara N, Verhave JP, Drakeley C, Luty AJ, Sauerwein R. The plasticity of Plasmodium falciparum gametocytaemia in relation to age in Burkina Faso. Malar J. 2010;9:281. doi: 10.1186/1475-2875-9-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drakeley CJ, Akim NI, Sauerwein RW, Greenwood BM, Targett GA. Estimates of the infectious reservoir of Plasmodium falciparum malaria in The Gambia and in Tanzania. Trans R Soc Trop Med Hyg. 2000;94:472–476. doi: 10.1016/S0035-9203(00)90056-7. [DOI] [PubMed] [Google Scholar]
- Port GR, Boreham PFL, Bryan JH. The relationship of host size to feeding by mosquitoes of the Anopheles gambiae Giles complex (Diptera: Culicidae) Bull Entomol Res. 1980;70:133–144. doi: 10.1017/S0007485300009834. [DOI] [Google Scholar]
- Nyunt MH, Kyaw MP, Win KK, Myint KM, Nyunt KM. Field evaluation of HRP-2 and pan pLDH-based immunochromatographic assay in therapeutic monitoring of uncomplicated falciparum malaria in Myanmar. Malar J. 2013;12:123. doi: 10.1186/1475-2875-12-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell D, Peeling RW. WHO-Regional Office for the Western Pacific/TDR. Evaluation of rapid diagnostic tests: malaria. Nat Rev Microbiol. 2006;4(9 Suppl):S34–S38. doi: 10.1038/nrmicro1524. [DOI] [PubMed] [Google Scholar]
- Coleman RE, Maneechai N, Ponlawat A, Kumpitak C, Rachapaew N, Miller RS, Sattabongkot J. Short report: Failure of the OptiMAL rapid malaria test as a tool for the detection of asymptomatic malaria in an area of Thailand endemic for Plasmodium falciparum and P. vivax. Am J Trop Med Hyg. 2002;67:563–565. doi: 10.4269/ajtmh.2002.67.563. [DOI] [PubMed] [Google Scholar]
- Wanji S, Kimbi HK, Eyong JE, Tendongfor N, Ndamukong JL. Performance and the usefulness of the Hexagon rapid diagnostic test in children with asymptomatic malaria living in the Mount Cameroon region. Malar J. 2008;7:89. doi: 10.1186/1475-2875-7-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltha J, Gillet P, Jacobs J. Malaria rapid diagnostic tests in endemic settings. Clin Microbiol Infect. 2013;19:399–407. doi: 10.1111/1469-0691.12151. [DOI] [PubMed] [Google Scholar]
- Nicastri E, Bevilacqua N, Sañé Schepisi M, Paglia MG, Meschi S, Ame SM, Mohamed JA, Mangi S, Fumakule R, Di Caro A, Capobianchi MR, Kitua A, Molteni F, Racalbuto V, Ippolito G. Accuracy of malaria diagnosis by microscopy, rapid diagnostic test, and PCR methods and evidence of antimalarial overprescription in non-severe febrile patients in two Tanzanian hospitals. Am J Trop Med Hyg. 2009;80:712–717. [PubMed] [Google Scholar]
- Alves FP, Gil LH, Marrelli MT, Ribolla PE, Camargo EP, Da Silva LH. Asymptomatic carriers of Plasmodium spp. as infection source for malaria vector mosquitoes in the Brazilian Amazon. J Med Entomol. 2005;42:777–779. doi: 10.1603/0022-2585(2005)042[0777:ACOPSA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Han ET. Loop-mediated isothermal amplification test for the molecular diagnosis of malaria. Expert Rev Mol Diagn. 2013;13:205–218. doi: 10.1586/erm.12.144. [DOI] [PubMed] [Google Scholar]
- Poon LL, Wong BW, Ma EH, Chan KH, Chow LM, Abeyewickreme W, Tangpukdee N, Yuen KY, Guan Y, Looareesuwan S, Peiris JS. Sensitive and inexpensive molecular test for falciparum malaria: detecting Plasmodium falciparum DNA directly from heat-treated blood by loop-mediated isothermal amplification. Clin Chem. 2006;52:303–306. doi: 10.1373/clinchem.2005.057901. [DOI] [PubMed] [Google Scholar]
- Han ET, Watanabe R, Sattabongkot J, Khuntirat B, Sirichaisinthop J, Iriko H, Jin L, Takeo S, Tsuboi T. Detection of four Plasmodium species by genus- and species-specific loop-mediated isothermal amplification for clinical diagnosis. J Clin Microbiol. 2007;45:2521–2528. doi: 10.1128/JCM.02117-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley SD, González IJ, Mohamed D, Daly R, Bowers K, Watson J, Mewse E, Armstrong M, Gray C, Perkins MD, Bell D, Kanda H, Tomita N, Kubota Y, Mori Y, Chiodini PL, Sutherland CJ. Clinical evaluation of a loop-mediated amplification kit for diagnosis of imported malaria. J Infect Dis. 2013;208:637–644. doi: 10.1093/infdis/jit183. [DOI] [PMC free article] [PubMed] [Google Scholar]