Abstract
Connective tissues such as tendons or ligaments attach to bone across a multitissue interface with spatial gradients in composition, structure, and mechanical properties. These gradients minimize stress concentrations and mediate load transfer between the soft and hard tissues. Given the high incidence of tendon and ligament injuries and the lack of integrative solutions for their repair, interface regeneration remains a significant clinical challenge. This review begins with a description of the developmental processes and the resultant structure-function relationships that translate into the functional grading necessary for stress transfer between soft tissue and bone. It then discusses the interface healing response, with a focus on the influence of mechanical loading and the role of cell-cell interactions. The review continues with a description of current efforts in interface tissue engineering, highlighting key strategies for the regeneration of the soft tissue–to-bone interface, and concludes with a summary of challenges and future directions.
Keywords: enthesis, insertion site, interface, tendon, ligament, mechanobiology, multiphased scaffold, structure-function, tissue engineering
INTRODUCTION
The musculoskeletal system functions through the coordinated action of multiple types of tissues. These include connective tissues such as tendon, which joins muscle to bone, and ligament, which connects bone to bone. A specialized interface, called an insertion site or enthesis, integrates tendon or ligament to bone and serves to facilitate joint motion (Figure 1). These interfaces exhibit gradients in tissue organization, composition, and mechanical properties that have several functions, from effectively transferring stress between mechanically dissimilar materials (tendon/ligament and bone) to sustaining the heterotypic cellular communications required for interface function and homeostasis (1–12). A number of common orthopaedic injuries require the repair of a ruptured tendon or ligament to its bony insertion. In the shoulder, rotator cuff tendon ruptures typically necessitate surgical reattachment of the tendon to the humeral head. In the knee, torn cruciate ligaments often require reconstruction using tendon grafts. These critical junctions are not reestablished following surgical repair, with high reinjury rates associated with both procedures. Depending on the severity of the injury and the age of the patient, 20–94% of repaired rotator cuff tears fail (13). In the knee, approximately half of anterior cruciate ligament (ACL) reconstruction patients experience knee pain at one year post surgery (14), and failure to regenerate the complex soft tissue–to-bone insertion site compromises graft stability and long-term clinical outcomes (15, 16).
Figure 1.
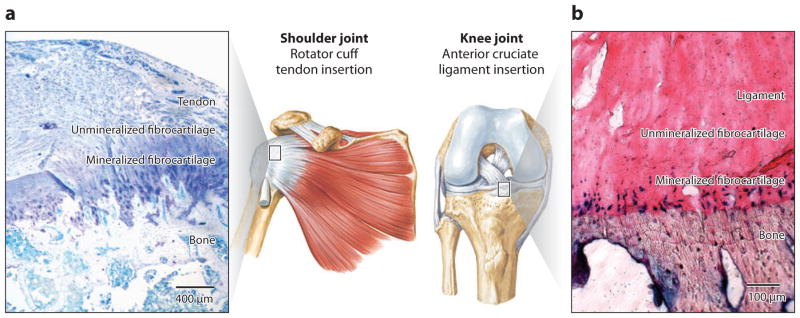
(a) Tendon and (b) ligament attach to bone across a functionally graded fibrocartilaginous transition site (a toluidine blue–stained section from an adult rat supraspinatus tendon–to-bone insertion is shown in panel a, and fast blue–stained section from a mature bovine anterior cruciate ligament insertion is shown in panel b) (3, 6).
Tendons and ligaments attach to bone via either fibrous or fibrocartilaginous insertions (17). Fibrous insertions typically occur over large areas, presumably to distribute forces and reduce stresses, and are characterized by perforating mineralized collagen fibers (18). For example, the medial collateral ligament attaches to the tibia and the deltoid tendon attaches to the humeral head across fibrous insertions. Fibrocartilaginous insertions are more common and include the bony attachments of the rotator cuff tendons, the ACL, and the Achilles tendon. Because of their clinical relevance with respect to the most common injuries to ligaments (e.g., rupture of the ACL) and shoulder (e.g., torn rotator cuff), fibrocartilaginous insertions are the focus of this review. These insertions are typically narrow (on the order of hundreds of micrometers) and have classically been categorized into four zones (17). The first zone consists of tendon/ligament, is populated by fibroblasts, and has mechanical properties similar to those of the tendon/ligament mid-substance. It is composed of linearly arrayed type I collagen fibers (6, 19). The second zone consists of fibrocartilage, is populated by fibrochondrocytes, and is composed of types I, II, and III collagen and the proteoglycan aggrecan (6, 19–22). The third zone is also populated by fibro-chondrocytes and is composed of mineralized fibrocartilage. Here, type II collagen predominates, and there are significant amounts of type X collagen as well as aggrecan (6, 19–22). The fourth zone consists of bone proper, within which osteoblasts, osteocytes, and osteoclasts reside in a matrix of mineralized type I collagen. Although the insertion site has classically been defined as containing these four zones, it is emphasized that the stratified tissue regions are compositionally distinct but structurally continuous. As described in subsequent sections, this controlled spatial distribution in matrix structure and composition is largely responsible for the functional grading necessary for minimizing stress concentrations between the connective tissue and bone.
Due to their functional significance, there exists a growing interest in the healing and regeneration of soft tissue–to-bone attachments. Furthermore, as the field of tissue engineering (23, 24) advances, the next step is to engineer complex tissues with integrated interfaces (5, 25–31). The complexity of the transition between unmineralized and mineralized tissues, however, poses significant challenges for effective interface healing and regeneration, with multiple biological and mechanical factors regulating the development as well as the healing of the soft tissue–to-bone interface. Focusing on tendon-to-bone and ligament-to-bone insertions, this review begins with a discussion of current understanding of enthesis development and maturation, with a closer look at the resultant structure-function relationships at soft tissue–to-bone attachments. Next, the mechanisms underlying the healing of the injured interface, in particular the role of mechanical loading and cellular interactions, are discussed. The final section of the review highlights current tissue engineering efforts for the regeneration of ligament- or tendon-to-bone interfaces, discussing biomimetic scaffold design as well as scaffold- and cell-based strategies for engineering a functional gradient with mechanical properties that approximate those of the native interface. This review concludes with a summary of potential challenges and future directions in an effort to understand and regenerate these critical soft tissue–to-bone attachments.
THE DEVELOPMENT OF STRUCTURE-FUNCTION RELATIONSHIPS AT THE ENTHESIS
Enthesis Development
During fetal and postnatal development, a complex transitional tissue forms between bone and connective tissues such as tendon or ligament (3, 6, 32–37). Enthesis development is initially driven by endochondral ossification: Cartilage mineralizes to form bone, and a fibrocartilaginous transition then develops at the interfaces between the bone and connective tissues. In situ hybridization and immunohistochemistry studies have revealed the spatial and temporal expression pattern for extracellular matrix components during this developmental process (Figure 2) (3, 6, 32–37). Type I collagen (characteristic of fibroblasts and osteoblasts), type II collagen (characteristic of chondrocytes), and type X collagen (characteristic of hypertrophic chondrocytes) were localized to developing tendon/ligament-to-bone insertions (3, 6). In the mouse shoulder, the fibroblasts of the supraspinatus tendon expressed type I collagen at all fetal and postnatal time points studied. The chondrocytes in the as-yet-unmineralized bone expressed type II collagen postnatally for 14 days, and those near the insertion became hypertrophic at this point and began expressing type X collagen. Mineralization proceeded via endochondral ossification of a subset of the type II collagen–expressing cells, resulting in a mineralized fibrocartilage region adjacent to the unmineralized tissue (Figure 3) (38). After this initial phase of endochondral ossification, the collagen fibers and mineral were remodeled to form a mature, well-organized, transitional tissue between the tendon and bone. Similar patterns were described for rat Achilles tendon insertions and for bovine ACL insertions postnatally and for the deltoid-humeral tuberosity attachment in utero (6, 32, 37).
Figure 2.
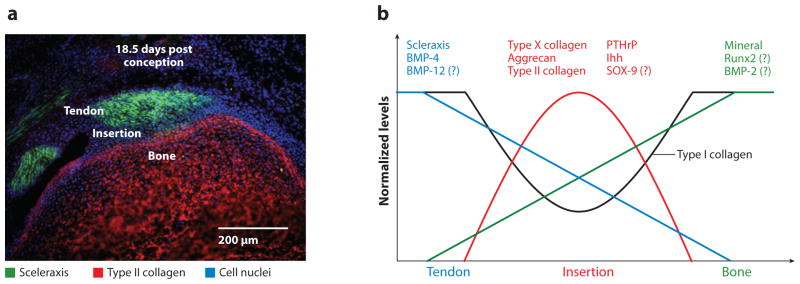
(a) At 18.5 days post conception, scleraxis expression is localized to the supraspinatus tendon and type II collagen expression is localized to the humeral head. A zone of cells at the insertion does not express either marker. (b) Spatially and temporally controlled expression of a number of transcription factors and growth factors drives enthesis development. A question mark (?) indicates that a role for the molecule is expected but has not yet been shown definitively. Abbreviations: BMP, bone morphogenetic protein; Ihh, Indian hedgehog protein; PTHrP, parathyroid hormone–related protein.
Figure 3.
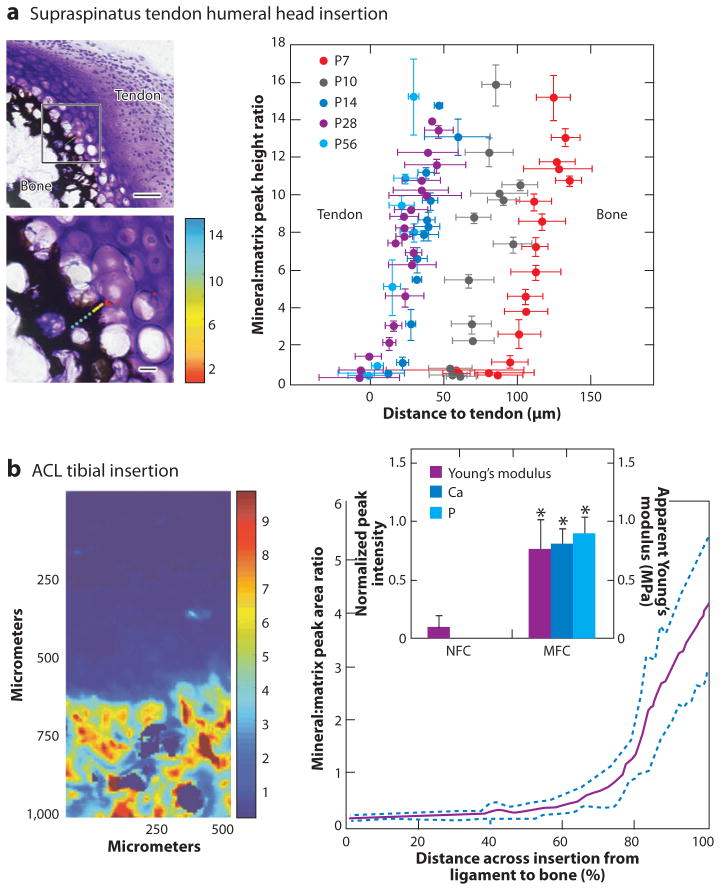
(a, left) Spatial gradients in mineral form between supraspinatus tendon and bone at the developing enthesis from the onset of endochondral ossification (age P7 in the mouse, determined via Raman spectroscopy, scale bars = 50 μm for upper left image and 10 μm for lower left image) (38). (right) The mineral gradient migrates from the center of the humeral head at P7 to the tendon attachment by P14. (b) Mineral distribution at the ACL-to-bone insertion increased exponentially across the calcified fibrocartilage region (neonatal bovine, determined via Fourier transform infrared imaging) (72). The significantly higher Ca and P content (*: p < 0.05) in the mineralized fibrocartilage (MFC) versus the nonmineralized fibrocartilage (NFC) region is accompanied by a significant increase in Young’s modulus.
A number of biologic and mechanical factors drive the development of a transitional tissue at the interface between tendon/ligament and bone (Figure 2). Many of the cellular and molecular events reported during mineralization of the enthesis follow pathways similar to those seen during chondrocyte hypertrophy in the growth plate. The hypertrophic chondrocytes of the growth plate mineralize the matrix between the cells, producing a zone of provisional calcification. The factors that modulate growth plate maturation also play a role in enthesis formation. Specifically, the Indian hedgehog (Ihh)/parathyroid hormone–related protein (PTHrP) feedback loop is critical for mineralization at the growth plate. The Ihh/PTHrP loop regulates chondrocyte differentiation and homeostasis (39–41) and plays a role in enthesis development (37, 42–46). PTHrP, for example, prevents proliferating chondrocytes in the growth plate from becoming hypertrophic chondrocytes (which eventually mineralize) (42). A population of proliferating chondrocytes can therefore be maintained by PTHrP and remain available for growth rather than for hypertrophy and mineralization. At the enthesis, graded expression of Ihh and PTHrP may regulate the formation of a graded transition between mineralized and unmineralized tissue.
Two transcription factors necessary for chondrogenesis and tenogenesis, SOX-9 and scleraxis (Scx), respectively, also likely play important roles for tendon/ligament-to-bone development (47). SOX-9 is necessary for chondrogenesis (48), and Scx is necessary for tenogenesis (49–52). Their localized expression patterns define the transition between tendon and fibrocartilage (Figure 2).
Blitz et al. (37) recently examined the development of the deltoid tendon-humeral tuberosity attachment and described the interplay of some of these factors. The authors observed that the deltoid tuberosity formed via endochondral ossification in a two-phased process: Initiation was regulated by a signal from the tendon, whereas the subsequent growth phase was muscle dependent. Specifically, Scx regulated bone morphogenetic protein (BMP)-4 production in tendon cells at the bony insertion site. When BMP-4 expression was blocked in Scx-expressing cells, the enthesis (and associated bone ridges) did not form. It is therefore likely that BMP-4 is a key mediator of tendon-specific signaling for enthesis formation. As in other reports, the key regulators of endochondral ossification (e.g., type II collagen, Ihh, PTHrP, type X collagen) were expressed at the developing enthesis. The coordinated and spatially localized expression of these important growth and differentiation regulators drives the formation of the specialized tissue that attaches soft tissues to bone.
Role of Loading in Enthesis Development
Biophysical cues influence the development of tendons, ligaments, cartilage, and bone (53–56). All of the cell types found along the enthesis are mechanoresponsive; therefore, a role for mechanobiology during the development of this tissue is expected. Studies using genetically modified mice with muscular defects demonstrated that, although muscle loading was not required for initiation of enthesis formation, it was necessary for the subsequent growth and maturation of the enthesis (37). The role of muscle loading in the development of the tendon-to-bone insertion was also examined in a murine shoulder model by Thomopoulos et al. (35, 57) and Kim et al. (58). Rotator cuff muscles were paralyzed using either intramuscular injections of botulinum toxin A or laceration of the upper trunk of the brachial plexus. As the main events driving enthesis development of the rotator cuff occur postnatally, paralysis was induced within 24 hours of birth. Based on assessments of muscle volume, muscle mass, and force generation capacity, both methods of muscle paralysis resulted in decreases in loading across the developing enthesis (59). The reduction in muscle loading impaired mineral deposition and fibrocartilage formation and led to disorganized fiber distribution and inferior mechanical properties at the developing tendon enthesis. The amount of mineral that accumulated in the humeral head decreased when loading was removed (35, 58). Bone volume decreased and bone architecture (e.g., trabecular thickness) changed when comparing saline-injected with botulinum toxin-injected shoulders. Similar results were seen when comparing neurotomy shoulders with normal shoulders. Of particular note is that differences were not apparent at early time points, indicating that, similar to the study by Blitz et al. (37), muscle loading may not be necessary to initiate enthesis development. The lack of bone formation in unloaded insertions was at least in part due to increased levels of bone resorption, as evidenced by increased osteoclast numbers in the paralyzed group compared with the control groups. This was confirmed by the observation that suppression of osteoclast activity partially rescued the defects caused by unloading (60). These results demonstrate that mineralization at the developing enthesis is sensitive to mechanical loading. Fibrocartilage formation was impaired in paralyzed shoulders (35, 58), and a zone of hypertrophic chondrocytes was evident between the tendon and its bony insertion at early postnatal timepoints. Within 21 days, this zone matured into a graded, mineralized insertion in the loaded shoulders and remained disorganized, with little fibrocartilage, in the paralyzed shoulders. Within 56 days, insertions in the botulinum toxin-injected shoulders consisted of disorganized collagen fibers with sparse amounts of fibrocar-tilage. Consistent with the mineral accumulation results, postnatal removal of mechanical loading cues dramatically affected fibrocartilage formation at the developing enthesis. Decreased muscle loading also led to a decrease in the organization of collagen fibers at the insertion (57). The structural and compositional changes described above resulted in decreased mechanical properties in the unloaded group compared with the loaded group (57). The molecular mechanisms that control the mechanoresponsiveness of the developing enthesis remain unclear, although studies have found that expression of Ihh and PTHrP is mechanosensitive (43). In summary, the formation of a functionally graded transition between tendon and bone requires physiologic muscle loading.
Structure-Function Relationships at the Tendon/Ligament Enthesis
The tightly regulated and highly ordered enthesis developmental process leads to a robust attachment between tendon/ligament and bone. Attachment of a compliant material such as tendon or ligament (with a modulus on the order of 200 MPa) to a relatively stiff material such as bone (with a modulus on the order of 20 GPa) poses unique challenges. For example, stress concentrations would arise at the interface of these two materials if the attachment were not adjusted to alleviate the modulus mismatch. Functional grading is a well-established method to minimize stress concentrations at material interfaces (62). Similarly, the tendon- and ligament-to-bone insertion sites are natural functionally graded structures, exhibiting gradients in mechanical, compositional, and structural properties. Studies examining the supraspinatus tendon–to-bone insertions of rats have demonstrated gradations in properties along the enthesis rather than an abrupt interface between mineralized and unmineralized tissue. Along with a compositional gradient in the extracellular matrix, collagen fibers were less oriented at the insertion compared with the tendon, and mineral content increased monotonically from tendon to bone (Figure 3) (3, 38, 63). Biomechanically, both the elastic and viscous behaviors of the tissue varied from tendon to bone (3).
Direct measurement of mechanical properties at tendon/ligament-to-bone insertions has been difficult owing to the heterogeneity and small length scale of the interface (typically 100 μm to 1 mm in length) (6, 64–66). To address these challenges, the local strain distribution at the ACL-to-bone interface under uniaxial tensile load was measured using ultrasound elastography (7). The strain along the insertion site was observed to be region dependent, with the highest strain found at the ligament mid substance, followed by decreased strain progressing from the fibrocartilage interface to the bone. These regional strain differences suggest an increase in tissue stiffness from ligament to bone. Moffat et al. (8) performed microcompression experiments to determine the compressive properties of the ligament-to-bone insertion. Energy dispersive X-ray analysis was used to determine the mineral content along the insertion. Similar to the elastography findings (7), strain decreased gradually from the fibrocartilage interface to the bone (8). The elastic modulus was twice as high in the mineralized fibrocartilage when compared with the nonmineralized interface region (Figure 3). Similar significant increases in modulus were recently reported for meniscal attachments to bone (67, 68).
The region-dependent gradient in mechanical properties across the insertion was correlated to spatial changes in matrix organization and composition (Figure 3). Of particular importance is the role of mineral in increasing stiffness across the insertion. Higher matrix mineral content has been associated with greater mechanical properties in connective tissues (69–71). Evaluation of the ACL-to-bone insertion site using Fourier transform infrared imaging (FTIR-I) (72) and X-ray analysis (8) revealed an exponential increase in phosphate content progressing from the calcified fibrocartilage interface and then to the bone (Figure 3). Similar monotonic gradients in mineral were measured using Raman spectroscopy across developing supraspinatus tendon–to-bone insertions (38, 63). The increase in elastic modulus, progressing from the nonmineralized to the mineralized fibrocartilage regions, was positively correlated with the presence of calcium phosphate (8). The relationship between mineral content and stiffness was modeled recently by Genin et al. (9) using micromechanical approaches. The model predicted that the experimentally observed increase in mineral across the insertion can provide significant stiffening, but only for concentrations of mineral above a percolation threshold corresponding to formation of a mechanically continuous mineral network within each collagen fiber.
The structural organization of an insertion site is optimized to minimize stress concentrations. Although a monotonic increase in stiffness has been reported at the ACL-to-bone attachment, many anatomic locations contain interfaces with a compliant zone between unmineralized and mineralized tissues. Studies of the rotator cuff (3, 73, 74), patellar tendon (75), and meniscus (67) in adult animals demonstrated that the modulus near their bony insertions is approximately half that at the soft tissue mid substance. Using scanning acoustic microscopy, Sano et al. (74) localized this compliant region to the unmineralized fibrocartilage of the rabbit rotator cuff enthesis. Recent numerical optimizations have provided a rationale for this counterintuitive attachment system (76). Using a mathematical model of the insertion, Liu et al. (76) showed that stress concentrations can be reduced by a biomimetic grading of material properties. The authors developed an optimization scheme to determine the mechanical properties between an idealized tendon and bone that minimized stress concentrations. The solution for axisymmetric tensile loading revealed that stress concentrations were minimized when the insertion included a region more compliant than either tendon or bone, presumably found at the unmineralized region of the enthesis.
A number of additional strategies at various hierarchical levels have been identified for theoretically achieving effective stress transfer between soft tissues and bone. At the millimeter scale, decreasing the angle of attachment prevents stress singularities at the interface (4, 11, 77) and peak stresses can be reduced by optimization of the gross shape of the insertion (11). At the micrometer scale, interlocking of the tissues through interdigitation (78) increases the toughness of the interface. Grading of transitional tissue, for example, gradations in collagen fiber orientation (4, 9) and mineral content (8, 9, 63), produces a functional grading in mechanical properties across the insertion. The combination of these factors across hierarchical levels predicts the reported variation of stiffness [i.e., a compliant region followed by a stiffer region (3, 67, 68)] over the length of soft tissue–to-bone insertions. Further imaging and biomechanical experiments are necessary, however, to validate these modeling results with native tissue properties.
HEALING AND REGENERATION OF THE ENTHESIS
Current Knowledge of Interface Healing
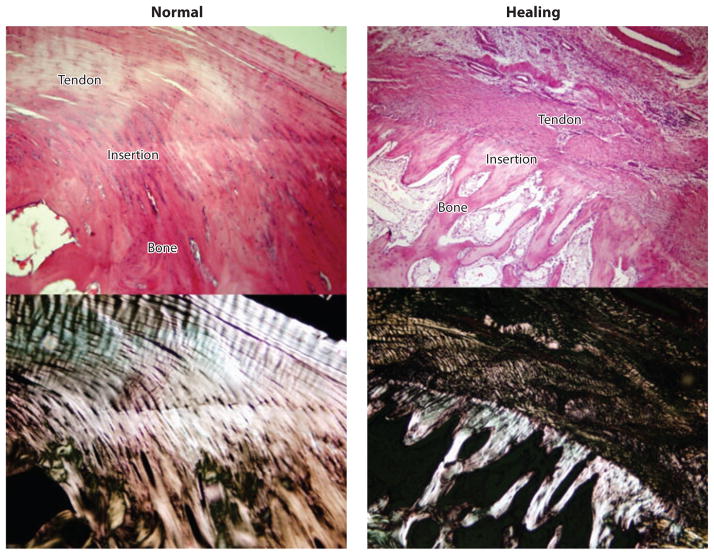
In contrast to the well-orchestrated enthesis developmental program, studies in animal models have demonstrated that soft tissue–to-bone healing occurs through formation of fibrovascular scar tissue rather than regeneration of a graded fibrocartilaginous transition (34, 79–81). In cases in which soft tissues were repaired directly to bone surfaces (e.g., for rotator cuff repair), a robust healing response was seen, but the mechanical properties of the repair did not approach physiological levels (34, 82, 83). Using a rat rotator cuff model, Thomopoulos et al. (34) showed that although the structural properties reached two-thirds of their normal levels after eight weeks of healing, the material properties remained an order of magnitude weaker than those of the control group. Histologically, a sharp boundary was evident between the soft tissue and the bone. There was no continuity of collagen fibers across the interface and no apparent gradient in mineral content. A fibrocartilaginous transition was rarely seen; rather, the tissue at the healing interface consisted of disorganized scar tissue with high levels of type III collagen. Silva et al. (84) reported similar results in a canine flexor tendon-to-bone repair model (Figure 4).
Figure 4.
The functionally graded transition between tendon and bone is not regenerated during the healing process (hematoxylin- and eosin-stained images are shown under bright field in the top row and under polarized light in the bottom row) (84).
In cases in which soft tissues were repaired into bone tunnels (e.g., for ACL reconstruction), a zone of loose fibrovascular tissue typically formed between the tendon and the wall of the bone tunnel (79, 85). Liu et al. (86) examined the morphology and matrix composition of the tendon graft–to-bone interface during healing. Two weeks after reconstruction, the tendon attached to the bone, with scar tissue filling the tendon-to-bone junction. This interface tissue was remodeled into a dense connective tissue matrix within one month, with predominantly fibroblasts present. After six weeks, contraction of the interface was prominent, and significantly less type I collagen was found in the remodeling matrix; however, type II collagen became detectable. This and other studies demonstrate that surgically juxtaposing tendon and bone does not lead to regeneration of the graded fibrocartilaginous interface (79).
Further complicating the repair of soft tissues to bone is the bone loss that typically occurs following tendon or ligament injury (87–90). Decreased bone mineral density was found at the canine distal phalanx at 10, 21, and 42 days following injury and repair, indicating that bone resorption may be a factor that contributes to the low values of repair-site failure force (90). Similar results were reported in the rat rotator cuff model (91, 92), in which bone mineral density was significantly decreased following tendon injury and repair. A delay between injury and repair resulted in inferior tendon-to-bone healing, owing in part to decreased bone quality.
The Role of Cellular Interactions During Interface Healing
Studies of ACL graft-to-bone healing suggest that cell phenotype and communication at the repair site are significant considerations for enthesis regeneration. Moreover, differentiation of multipotent cells into enthesis-relevant populations has the potential to facilitate healing at the interface. As described above, the healthy tendon/ligament-to-bone insertion consists of a progression of tissue types (tendon/ligament, fibrocartilage, mineralized fibrocartilage, and bone), each exhibiting a characteristic cellular phenotype and matrix composition. Although the cells in either soft tissue or bone have been well characterized, the phenotype of the enthesis fibrochondrocyte is not well defined. Sun et al. (93) compared the response of fibrochondrocytes isolated directly from the ligament-to-bone insertion to those of inner- and outer-ring meniscal fibrochondrocytes, as well as to ligament fibroblasts and articular chondrocytes. Aside from their mineralization potential, the ACL insertion fibrochondrocytes are more similar in growth rate and biosynthesis to articular chondrocytes than to meniscal fibrochondrocytes or ligament fibroblasts. It is likely that communication among the three resident cell populations, namely fibroblasts, fibrochondrocytes, and osteoblasts, is important for interface homeostasis and regeneration.
It has long been observed that although tendon-to-bone healing following ACL reconstruction does not lead to the reestablishment of the graded native insertion, a layer of fibrovascular tissue is formed within the bone tunnel (79, 94, 95). This observation suggests that when trauma or injury to the interface results in nonphysiologic exposure of normally segregated tissue types (e.g., bone and ligament), interactions between the resident cell populations in these tissues (e.g., osteoblasts and fibroblasts) have the potential to initiate and direct an interface repair response. Lu & Jiang (5) proposed a working hypothesis for enthesis regeneration, suggesting that osteoblast-fibroblast interactions can mediate regeneration of an enthesis through heterotypic cellular interactions leading to phenotypic changes or transdifferentiation of osteoblasts and/or fibroblasts. Furthermore, these interactions may promote the differentiation of stem cells or progenitor cells into fibrochondrocytes, leading to the regeneration of a fibrocartilage interface.
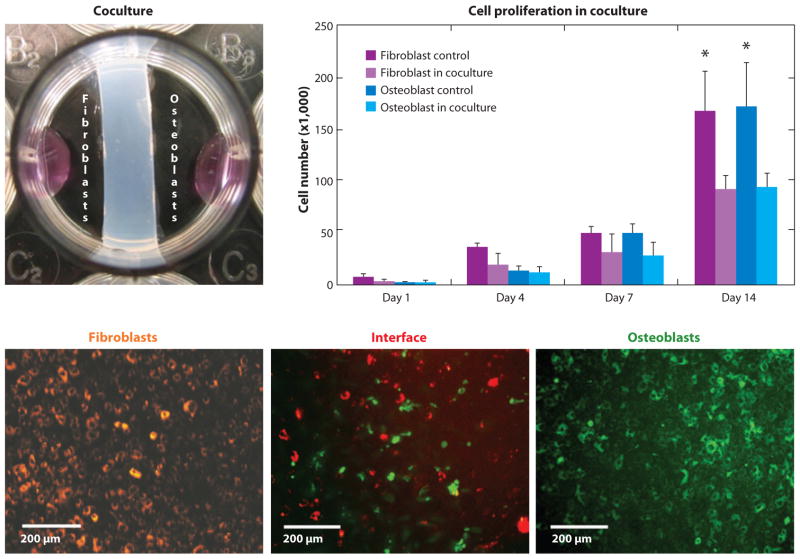
Several in vitro studies evaluating the role of heterotypic cellular interactions on interface regeneration have been reported (41, 96–98). Coculture and triculture models of interface-relevant cell populations were used to determine the effects of cellular communication on the development of fibrocartilage-specific markers in vitro. Wang et al. (98) examined the interaction between osteoblasts and ligament fibroblasts: A two-dimensional (2D) coculture model permitting both cell physical contact and soluble factor interactions was designed to emulate the in vivo condition in which the soft tissue graft is in direct contact with bone tissue following ACL reconstruction (Figure 5). Osteoblasts and fibroblasts were first separated by a hydrogel divider, and upon reaching confluence, the divider was removed, allowing the osteoblasts and fibroblasts to migrate and interact directly within the interface region (Figure 5). It was reported that these controlled interactions decreased cell proliferation (Figure 5), altered the alkaline phosphatase (ALP) activity profile, and promoted the expression of matrix proteins characteristic of the fibrocartilage interface, such as types I and II collagen, and cartilage oligomeric matrix protein (COMP). In addition to physical interaction between cells, subsequent conditioned media studies revealed that both autocrine and paracrine factors were responsible for the changes in phenotype observed during osteoblast-fibroblast coculture (99).
Figure 5.
Coculture of fibroblasts and osteoblasts (upper left) exerts spatial control of cell distribution, resulting in an interface region that contains interacting osteoblasts and fibroblasts. Fibroblast and osteoblast interactions in coculture reduced cell proliferation (*: p < 0.05 versus control) (98).
Although osteoblast-fibroblast interactions in coculture resulted in phenotypic changes and the expression of interface-relevant markers, a fibrocartilage-like interface was not formed in vitro. Interestingly, after coating tendon grafts with mesenchymal stem cells (MSCs) embedded in a fibrin gel, the formation of a zone of cartilaginous tissue between graft and bone was observed, suggesting a potential role for stem cells in fibrocartilage formation (100). In other words, fibrochondrocyte precursors or stem cells may be involved in interface regeneration, and osteoblast-fibroblast interactions may regulate fibrochondrogenic differentiation of these cells during healing. In addition, fibroblasts from tendon, ligament, and skin have been shown to exhibit fibrochondrocyte- or chondrocyte-like phenotypes under certain conditions (101–105). To test the hypothesis that stem cells may be induced toward a fibrochondrocyte lineage by factors produced from osteoblast-fibroblast interactions, Wang & Lu (106) designed a triculture system of fibroblasts, osteoblasts, and MSCs. The triculture consisted of fibroblasts and osteoblasts each seeded on cover slips on opposite sides of a well with MSCs preloaded into a hydrogel insert. Under the influence of osteoblast-fibroblast interactions, the MSCs exhibited a level of alkaline phosphatase activity similar to that of enthesis fibrochondrocytes, with both groups peaking by day 7 and decreasing thereafter. Moreover, both type II collagen production and a significantly higher level of proteoglycan synthesis were measured for the MSCs in triculture, with values approaching those of insertion fibrochondrocytes.
Findings from the in vitro coculture and triculture studies described above (a) provide preliminary validation of the hypothesis that osteoblast-fibroblast interactions can induce enthesis-specific markers in MSCs and (b) imply a role for heterotypic cellular interactions in regulating the maintenance of soft tissue–to-bone insertions. Although the mechanisms of interaction and the nature of the regulatory soluble factors that are secreted remain elusive, cell-cell communication may play an important role not only in interface regeneration in the healing setting but also in homeostasis in the uninjured setting.
The Influence of Mechanical Stimuli on Interface Healing
The role of mechanobiology during the healing process is unclear. Although all three components of the insertion site (tendon, fibrocartilage, and bone) are responsive to load when healthy, their response to load during tendon-to-bone healing has required further study. Results from animal models have demonstrated that low levels of controlled load (e.g., via cast immobilization) are optimal for healing (34, 107–109). In a rotator cuff injury-and-repair animal model, cast immobilization was demonstrated to be beneficial for tendon-to-bone healing compared with exercise (34, 107). Similarly, a study comparing early with delayed mechanical loading on tendon-to-bone healing in an ACL model demonstrated that delayed application of cyclic load improved tendon-to-bone healing (as evidenced by biomechanical and histologic assessment) more than immediate loading or prolonged immobilization did. Despite improvements in healing with controlled levels of low load, a multitissue transition was not regenerated in either model; rather, the repair site was characterized by fibrovascular scar tissue. As expected, increased loading stimulated matrix formation, leading to larger cross-sectional area in the high-load groups of the rotator cuff model compared with the cast-immobilized group. However, the orientation of the collagen fibers approached normal only in the cast-immobilized group. Furthermore, increased loading led to lower mechanical properties. Increased loading was therefore effective in stimulating matrix synthesis but ineffective in improving the mechanical properties of the repair. More material of lesser quality was produced when loading was increased. The beneficial effect of decreased loading may be due in part to a suppression of acute inflammation (109). Immobilization reduced macrophage numbers in an ACL repair model, leading to improvements in mechanical properties and tendon-bone integration. A key feature of the failed healing response in all studies was the lack of a functionally graded transition between the tendon and the bone. Instead, the transition zone was comprised of disorganized scar tissue.
The surprisingly positive outcomes in the cast-immobilized and delayed-loading groups have led to additional studies on the role of mechanical loading on tendon-to-bone healing. It was noted that the supraspinatus muscle in cast-immobilized animals was fully innervated and therefore able to generate load across the repair site in the absence of shoulder motion. The effect of complete removal of load on tendon-to-bone healing has been examined (110, 111), testing the hypothesis that complete removal of load would be detrimental to healing and guide repair. Specifically, removal of load via botulinum toxin paralysis or tenotomy was shown to impair healing (110, 111). Muscle paralysis or tenotomy combined with cast immobilization was detrimental to healing, as evidenced by decreased mechanical properties. The structural properties of the healed tendons were significantly greater in the control specimens compared with the unloaded specimens. These studies showed that when all load is removed from the healing tendon, the cross-sectional area and the structural properties decrease compared with using protective cast-immobilization alone. In conclusion, these studies demonstrate that a fine balance must be reached between excessive loads (which can lead to microdamage) and insufficient loads (which can lead to a catabolic environment) to maximize tendon-to-bone healing.
CURRENT APPROACHES TO INTERFACE REGENERATION
Tissue Engineering Strategies
Given the functional importance of the interface and its clinical significance in soft tissue repair, there is substantial interest in regenerating these critical tissue-to-tissue transitions. Utilizing a combination of cells, growth factors, and/or biomaterials, tissue engineering (23, 24) has already been applied to the formation of bone, ligament, and tendon in vitro and in vivo. Presently, a critical barrier to clinical translation in musculoskeletal tissue engineering is achieving biological fixation or functional integration of these grafts with each other and/or with the existing muscu-loskeletal system. This section reviews current tissue engineering strategies aimed at regenerating the tendon-to-bone or ligament-to-bone interfaces, focusing on biomimetic scaffolds as well as cell- and growth factor–based approaches.
The guiding principle of interface tissue engineering is a strategic recapitulation of the complex structure-function relationships inherent in native tissue interfaces. As discussed above, these tissues, each with a distinct cellular population, must operate in unison to facilitate physiologic function and maintain tissue homeostasis. It is thus not surprising that the transitions between tissue types are characterized by high levels of heterogeneous structure and composition that are crucial for musculoskeletal function. In light of the native interface’s complexity, strategic biomimicry is critical for interface regeneration and seamless graft integration, with key design parameters selected based on current understanding of interface structure-function relations as well as the mechanism of interface formation, healing, and homeostasis. Specifically, based on the multitissue matrix organization at common tendon- and ligament-to-bone insertions, interface scaffold design must support the deposition of compositionally distinct yet structurally contiguous tissues, as well as exhibit a gradient of mechanical properties similar to that of the native tissue. Typically, a stratified or multiphased scaffold can be used to capture the multitissue organization observed at the natural interface. To minimize stress concentrations, the stratified scaffold should exhibit phase-specific composition and structural organization, resulting in a gradation of mechanical properties similar to that of the native tissue-to-tissue junction.
Although a stratified scaffold is essential for recapitulating multitissue organization, a key criterion is that these strata or phases are interconnected and preintegrated with each other, thereby supporting the formation of functionally continuous multitissue regions. Moreover, phase-specific or trans-phase gradients can be engineered to more closely mimic the complexity of tissue-to-tissue transitions. In particular, given the documented progression from noncalcified to calcified interface regions at both tendon- and ligament-to-bone insertion sites, exercising spatial control over mineral distribution on the scaffold is essential. Compared with a homogeneous structure, a scaffold with predesigned, tissue-specific matrix inhomogeneity can better sustain and transmit the distribution of complex loads inherent at the multitissue interface. Furthermore, interactions between relevant cell population must be regulated and exploited, as cellular interactions play an important role in the formation, homeostasis, and repair of interfacial tissues (41, 96–98). Therefore, control over the spatial distribution of cell populations and biologic factors is another key design parameter to be considered. Finally, for biological fixation, interface regeneration must be coupled with osteointegration. Ultimately, along with engineering compositionally distinct yet structurally continuous multitissue regions, the end goal of interface tissue engineering is to establish a functional gradient of mechanical properties mimicking those of the native insertion site, an accomplishment that is essential for integrative soft tissue repair.
Ligament-to-Bone Interface Tissue Engineering
The regeneration of a multitissue transition from ligament to bone described above has long posed a significant challenge for functional ligament tissue engineering. Early attempts to improve the fixation of a ligament graft to bone focused on augmenting the surgical graft with a material that would encourage bone tissue ingrowth within the bone tunnel (112–117). For example, when calcium phosphate cement was used to fill the tendon-to-bone junction in a rabbit ACL reconstruction model, the ceramic augmented bone tissue growth and organization (113); similar findings were reported with the injection of tricalcium phosphate cement into bone tunnel (114). An alternative approach to improving tendon osteointegration included soaking ACL reconstruction grafts in a series of solutions that facilitated the formation of a calcium phosphate layer prior to implantation (118). This resulted in direct bonding between the implanted graft and the surrounding bone after three weeks. Other approaches to improve bone tunnel osteointegration include the addition of periosteum wraps to the region of the graft that interacts with bone (119–123) and of growth factors such as BMP-2 (85, 124–127), BMP-7 (128), and granulocyte colony stimulating factor (129). Additionally, the direct application of MSCs for improving graft-to-bone integration has been explored (130–132). Although these methods have enhanced graft integration within the bone tunnel, they do not yet result in the regeneration of the anatomic fibrocartilaginous interface.
Published studies in ACL tissue engineering have centered on regenerating the ligament proper (133–135), with more recent studies focusing on the integration of ACL with bone (136–139). Cooper et al. (137) reported on a multiphased design of a synthetic ACL graft fabricated from three-dimensional braiding of polylactide-coglycolide fibers with a ligament proper as well as two bony regions. In vitro (138) and in vivo (139) evaluation demonstrated biocompatibility and ligament healing in a rabbit model. Using MSCs, Ma et al. (140) formed bone-ligament-bone constructs by combining cell-engineered bone with ligament monolayers, which resulted in the self-assembly of a ligament-bone-ligament-like construct. Paxton et al. (141) incorporated both HA and RGD peptides in a polyethylene glycol hydrogel in order to engineer ligament-to-bone attachments. These novel ACL designs represent advances over single-phased ACL grafts, and the next step is to tackle the challenge of biological fixation, by incorporating the fibrocartilage interface into ACL graft design.
To this end, Spalazzi et al. (142, 143) developed a triphasic scaffold for the regeneration of the ACL-to-bone interface (Figure 6). Modeled after the native stratified transition, the scaffold consisted of three compositionally distinct yet structurally continuous phases: Phase A (PLGA 10:90 mesh) was designed for fibroblast culture and ligament formation, Phase B (sintered PLGA 85:15 microspheres) was designed as the interface region intended for fibrochondrocyte culture and fibrocartilage formation, and Phase C (sintered PLGA 85:15 and 45S5 bioactive glass composite microspheres) was designed for bone formation (144). In addition to supporting the formation of ligament, interface, and bone, predesigned structural and compositional differences between phases enabled compressive mechanical properties to increase across the stratified scaffold, with the highest elastic modulus and yield strength in Phase C (doubling those of Phase B), effectively mimicking the functional grading of the native enthesis. To form the ligament and bone regions, fibroblasts and osteoblasts were seeded onto Phase A and Phase C, respectively (Figure 6). In vitro and in vivo evaluations (142, 143) revealed extensive tissue infiltration and abundant matrix deposition on Phase A and Phase C with coculture, with tissue continuity maintained across all three scaffold phases. Interestingly, matrix production compensated for the decrease in mechanical properties accompanying scaffold degradation, and the phase-specific matrix heterogeneity was maintained in vivo (143).
Figure 6.
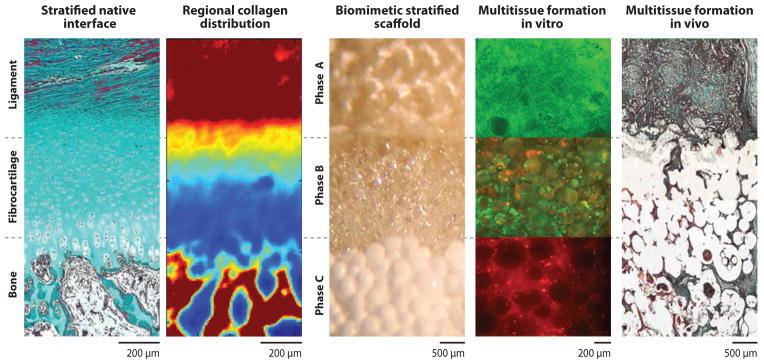
Biomimetic strategy for engineering a ligament-to-bone interface. Inspired by the native enthesis, a stratified scaffold is designed to mimic the layered tissue regions progressing from ligament to fibrocartilage to bone. Spatial control over cell distribution (fibroblasts, chondrocytes, and osteoblasts) on the triphasic scaffold resulted in the formation of compositionally distinct yet structurally continuous tissue regions mimicking those found at the native ligament-to-bone insertion site (27).
For fibrocartilage interface formation, Spalazzi et al. (143) extended their in vivo evaluation of the stratified scaffold to triculture, seeding chondrocytes along with fibroblasts and osteoblasts on their respective tissue phases. Specifically, chondrocytes were loaded via a hy-drogel carrier into Phase B, and fibroblasts and osteoblasts were preseeded onto Phase A and Phase C, respectively. At eight weeks, an extensive and contiguous collagen-rich matrix was observed across the scaffold phases (Figure 6). In addition to ligament-like matrix in Phase A and bone-like matrix in Phase C, a fibrocartilaginous tissue with chondrocyte-like cells in a matrix of types I and II collagen and glycosaminoglycans was observed in Phase B, with both cell shape and matrix morphology resembling that of the neonatal ligament-to-bone interface (6).
For clinical application, the triphasic scaffold can be used as a graft collar to guide the reestablishment of an anatomic interface directly on ACL reconstruction grafts. The feasibility of such an approach was demonstrated with a mechanoactive scaffold based on a composite of PLGA 85:15 nanofibers and sintered microspheres (145): Scaffold-induced graft compression resulted in sig-nificant matrix remodeling and the upregulation of fibrocartilage interface-related markers such as type II collagen, aggrecan, and transforming growth factor-β3 (TGF-β3). These promising results demonstrate that biomimetic stratified scaffold design coupled with spatial control over the distribution of interface-relevant cell populations can lead to interface regeneration. A key criterion to the success of these stratified scaffolds is that the strata or phases are interconnected in order to avoid delamination (146, 147), thereby enabling the scaffolds to support the formation of continuous multitissue regions.
Tendon-to-Bone Interface Tissue Engineering
Similar to the ligament-to-bone interface, the tendon-to-bone interface exhibits a zonal distribution of extracellular matrix components (Figure 1) (1, 148). Despite similarities, the biological fixation strategies for tendon-to-bone repair should be adapted to accommodate the specifics of the loading environment, mineral distribution, and surgical repair methods for the particular anatomic location.
Several groups have evaluated the feasibility of integrating tendon with bone or biomaterials through reformation of the enthesis. For example, periosteum (149) and demineralized bone matrix (DBM) (150) have been used for interface regeneration. After the periosteal flap was sutured to the end of a torn rabbit infraspinatus tendon and then attached to bone, a fibrocartilage-like matrix developed after 12 weeks, and failure load increased significantly over time. To harness the osteogenic and chondrogenic potential of DBM, Sundar et al. (150) interposed DBM between patellar tendon and osteotomized bone in an ovine model and found that DBM-augmented repair significantly improved weight bearing and fibrocartilage deposition.
The delivery of biological factors during healing has also been explored to improve tendon-to-bone integration. These have included growth factors (151–153) and matrix metalloproteinase (MMP) inhibitors (154, 155). Two recent studies demonstrated that TGF-β3 may accelerate healing (152, 153). Manning et al. (153) used TGF-β3 release to encourage tendon-to-bone healing in a rat rotator cuff repair model, which resulted in increased cellularity, vascularity, inflammation, and cell proliferation post repair. Improvements in mechanical properties were also observed compared with controls. Additionally, Rodeo et al. (151) examined the effect of a mixture of osteoinductive factors on tendon-to-bone healing in an ovine infraspinatus tendon model. Abundant formation of new bone, fibrocartilage, and soft tissue was observed, accompanied by an increase in tendon attachment strength. Recently, the influence of MMP inhibition (155) on tendon-to-bone healing was also examined in a rat model, in which the application of recombinant α-2-macroglobulin (A2M) protein (a universal MMP inhibitor) to the repaired supraspinatus tendon–to-bone interface increased fibrocartilage formation, improved collagen organization, and reduced collagen degradation.
Cell-based approaches have also been explored for enhanced tendon-to-bone healing. When Gulotta et al. (156) delivered MSCs to the cuff repair site in rats, no improvements in healing were found. Interestingly, positive results were observed after MSCs were transfected with Scx, a transcription factor critical for tendon development (157). Groups receiving Scx-transfected MSCs measured higher strength and stiffness compared with the nontransfected MSC repairs. In a similar study, MSCs transfected with membrane type 1 MMP (MT1-MMP), a factor which is upregulated during embryogenesis at tendon-to-bone insertion sites (154), showed significant improvements in healing compared with controls. Higher fibrocartilage production, as well as improvements in mechanical properties, was noted at the repair site. This approach may be further enhanced in future studies using multiple cell types delivered in a spatially graded fashion (158).
Synthetic biomaterials have also been investigated for tendon-to-bone integration. Implantation of a polyglycolide fiber mesh in a rat model led to the formation of an organized fibrovascular matrix at the infraspinatus tendon–to-bone junction (159). Recently, nanofiber scaffolds have been explored for tendon-to-bone interface tissue engineering, largely owing to their biomimetic potential and physiological relevance. These scaffolds can be tailored to match the native tendon matrix, with controlled alignment, high surface area–to-volume ratio, permeability, and porosity (160–164). Scaffold structural properties such as fiber diameter and alignment have been shown to regulate the response of tendon fibroblasts (165) and MSCs (166). In order to investigate the potential of nanofiber scaffolds for tendon tissue engineering, Moffat et al. (167) evaluated the effects of PLGA nanofiber organization (aligned versus unaligned) on human tendon fibroblast attachment and biosynthesis. Nanofiber alignment was found to be the primary factor guiding tendon fibroblast morphology, alignment, and integrin expression. Fibroblasts synthesized types I and III collagen, the dominant collagen types of the supraspinatus tendon, on nanofiber scaffolds, and collagen deposition was controlled by the underlying fiber orientation. Furthermore, scaffold tensile mechanical properties, directly related to fiber alignment, decreased as the polymer degraded but remained within range of those reported for the native supraspinatus tendon (168). Building upon these promising results, Moffat et al. (169) designed a stratified, composite nanofiber system consisting of distinct yet continuous noncalcified and calcified regions that mimic the organization of native tendon-to-bone insertion sites. The biphasic scaffold was produced by electrospinning; Phase A was comprised of aligned PLGA nanofibers to support the regeneration of the nonminer-alized fibrocartilage region, and Phase B was based on aligned PLGA nanofibers embedded with nanoparticles of hydroxyapatite (PLGA-HA) to support the regeneration of the mineralized fibro-cartilage region. The biphasic scaffold design was evaluated both in vitro (170) and in vivo (171), and chondrocyte-mediated fibrocartilage-like extracellular matrix was found in each scaffold phase. Furthermore, mineral distribution was maintained, with calcified fibrocartilage formed on Phase B, which will enable the biphasic scaffold to better integrate with the surrounding bone tissue in vivo.
Nanofiber-based scaffolds with gradients of mineral distribution have also been investigated for tendon-to-bone interface regeneration. Li et al. (172) formed a linear gradient of calcium phosphate on PLGA and poly-ε-caprolactone (PCL) nanofiber scaffolds by varying immersion time in concentrated simulated body fluid. Importantly, the mineral gradient imparted a gradation in mechanical properties along the length of the scaffold, with lower strains and higher elastic modulus corresponding to areas of higher calcium-phosphate concentration. In an alternative approach, collagen scaffolds with a compositional gradient of retroviral coating for the transcription factor RUNX2 induced fibroblasts to produce a gradient of mineralized matrix both in vitro and in vivo (173). Although the spatial resolution of these linear gradients is not yet within physiological range, these scaffold systems hold significant promise for biomimetic tendon-to-bone interface regeneration (174–176).
In summary, current strategies in tendon- and ligament-to-bone interface tissue engineering first tackle the difficult problem of soft tissue–to-bone integration ex vivo by pre-engineering the multitissue interface through multiphase scaffold design and then focus on applying the engineered constructs in the in vivo tendon-to-bone healing setting. Functional and integrative repair of tendon or ligaments may be achieved by coupling both cell-based and scaffold-based approaches and by recapitulating the complex nano- through macroscale organization of the native interface.
SUMMARY AND FUTURE DIRECTIONS
The objective of this review was to describe the current understanding of soft tissue–to-bone interfaces (tendon-to-bone and ligament-to-bone), with a focus on interface development, structure function, healing, and regeneration. The native insertion site is a functionally graded tissue, populated by multiple cell types and extracellular matrix components, which develops through a series of coordinated events that are driven by biologic and mechanical factors. Formation of a graded interface between bone and tendon or ligament is initiated by transcription factors that are specific to the various cell types at the attachment. Maturation and growth of the enthesis is then regulated by mechanical loading cues from muscle forces. Interface-relevant cell populations likely play important roles in interface repair and homeostasis. Current repairs of tendon or ligament injuries rely largely on mechanical fixation and fail to promote regeneration of the well-organized, native multitissue transition between soft tissue and bone.
Scaffold design for interface tissue engineering seeks to recapitulate the structure and function found in the native tissue; a gradation of scaffold structure, composition, and mechanical properties may promote regeneration of the interface. Therefore, stratified scaffolds have been designed with spatial control over heterotypic cell interactions to support the formation of integrated multitissue systems. These novel scaffolds can be further refined by incorporating compositional, structural, and growth factor gradients within or across phases, as well as through the use of biochemical and biomechanical stimulation. Furthermore, functional and integrative soft tissue repair may be achieved by coupling cell-, growth factor–, and scaffold-based approaches.
A number of challenges remain before these tissue engineering approaches can be applied clinically. These include the need for a greater understanding of the structure-function relationships inherent at native tissue-to-tissue interfaces as well as the mechanisms governing interface development and regeneration. There likely exists a cell type at the developing enthesis that is phenotypically distinct from fibroblasts, chondrocytes, and osteoblasts. Although many of the factors seen during endochondral ossification are also expressed during enthesis development, the manner in which an unmineralized tissue is maintained adjacent to the mineralizing cartilage remains unknown. A better understanding of the developmental process may provide targets for enhanced healing of connective tissue–to-bone interfaces or for regeneration of the native insertion. Furthermore, a better understanding is needed regarding the effects of biological, chemical, and mechanical stimulation on interface regeneration in vitro and in vivo. Tissue-engineered constructs must also be evaluated in physiologically relevant in vivo models (under healthy and degenerative conditions) to determine the clinical potential of the designed scaffolds. Finally, regulatory, medical, and ethical challenges related to engineering complex tissues must be considered if clinical translation is to be realized.
In summary, soft tissue–to-bone interfaces play a critical role in musculoskeletal function, and their regeneration via interface tissue engineering represents a promising strategy for improved clinical outcomes. Future studies expanding our understanding of the development, structure-function, and healing of attachments will allow for clinical translation of these regeneration strategies to enable functional and integrative repair of tendon and ligament injuries.
Acknowledgments
Dr. Lu thanks Philip Chuang, MS, for assistance with references and formatting and gratefully acknowledges funding support from the Presidential Early Career Award (PECASE), the National Institutes of Health (NIH-NIAMS R01-AR055580, R21-AR052402, R21-AR056459), the Wal-lace H. Coulter Foundation, and the New York State Stem Cell Science (NYSTEM C024335). Dr. Thomopoulos gratefully acknowledges support from the National Institutes of Health (R01-AR055580, R01-AR057836, R01-AR060820) and the National Science Foundation (CAREER 844607).
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Helen H. Lu, Email: hhlu@columbia.edu.
Stavros Thomopoulos, Email: ThomopoulosS@wudosis.wustl.edu.
LITERATURE CITED
- 1.Benjamin M, Evans EJ, Copp L. The histology of tendon attachments to bone in man. J Anat. 1986;149:89–100. [PMC free article] [PubMed] [Google Scholar]
- 2.Woo SL, Maynard J, Butler DL, Lyon RM, Torzilli PA, et al. Ligament, tendon, and joint capsule insertions to bone. In: Woo SL, Bulkwater JA, editors. Injury and Repair of the Musculoskeletal Soft Tissues. Rosemont, IL: Am. Acad. Orthop. Surg; 1988. pp. 133–66. [Google Scholar]
- 3.Thomopoulos S, Williams GR, Gimbel JA, Favata M, Soslowsky LJ. Variations of biomechanical, structural, and compositional properties along the tendon to bone insertion site. J Orthop Res. 2003;21:413–19. doi: 10.1016/S0736-0266(03)00057-3. [DOI] [PubMed] [Google Scholar]
- 4.Thomopoulos S, Marquez JP, Weinberger B, Birman V, Genin GM. Collagen fiber orientation at the tendon to bone insertion and its influence on stress concentrations. J Biomech. 2006;39:1842–51. doi: 10.1016/j.jbiomech.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 5.Lu HH, Jiang J. Interface tissue engineering and the formulation of multiple-tissue systems. Adv Biochem Eng Biotechnol. 2006;102:91–111. [PubMed] [Google Scholar]
- 6.Wang IE, Mitroo S, Chen FH, Lu HH, Doty SB. Age-dependent changes in matrix composition and organization at the ligament-to-bone insertion. J Orthop Res. 2006;24:1745–55. doi: 10.1002/jor.20149. [DOI] [PubMed] [Google Scholar]
- 7.Spalazzi JP, Gallina J, Fung-Kee-Fung SD, Konofagou EE, Lu HH. Elastographic imaging of strain distribution in the anterior cruciate ligament and at the ligament-bone insertions. J Orthop Res. 2006;24:2001–10. doi: 10.1002/jor.20260. [DOI] [PubMed] [Google Scholar]
- 8.Moffat KL, Sun WH, Pena PE, Chahine NO, Doty SB, et al. Characterization of the structure-function relationship at the ligament-to-bone interface. Proc Natl Acad Sci USA. 2008;105:7947–52. doi: 10.1073/pnas.0712150105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Genin GM, Kent A, Birman V, Wopenka B, Pasteris JD, et al. Functional grading of mineral and collagen in the attachment of tendon to bone. Biophys J. 2009;97:976–85. doi: 10.1016/j.bpj.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu HH, Subramony SD, Boushell MK, Zhang X. Tissue engineering strategies for the regeneration of orthopedic interfaces. Ann Biomed Eng. 2010;38:2142–54. doi: 10.1007/s10439-010-0046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, Birman V, Chen C, Thomopoulos S, Genin GM. Mechanisms of bimaterial attachment at the interface of tendon to bone. J Eng Mater Technol. 2011;133:011006. doi: 10.1115/1.4002641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomopoulos S, Birman V, Genin GM. Structural Interfaces and Attachments in Biology. New York: Springer; 2013. [Google Scholar]
- 13.Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86-A:219–24. doi: 10.2106/00004623-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Corry IS, Webb JM, Clingeleffer AJ, Pinczewski LA. Arthroscopic reconstruction of the anterior cruciate ligament. A comparison of patellar tendon autograft and four-strand hamstring tendon autograft. Am J Sports Med. 1999;27:444–54. doi: 10.1177/03635465990270040701. [DOI] [PubMed] [Google Scholar]
- 15.Friedman MJ, Sherman OH, Fox JM, Del Pizzo W, Snyder SJ, Ferkel RJ. Autogeneic anterior cruciate ligament (ACL) anterior reconstruction of the knee. A review. Clin Orthop Rel Res. 1985;196:9–14. [PubMed] [Google Scholar]
- 16.Robertson DB, Daniel DM, Biden E. Soft tissue fixation to bone. Am J Sports Med. 1986;14:398–403. doi: 10.1177/036354658601400512. [DOI] [PubMed] [Google Scholar]
- 17.Benjamin M, Kumai T, Milz S, Boszczyk BM, Boszczyk AA, Ralphs JR. The skeletal attachment of tendons—tendon “entheses. Comp Biochem Physiol A Mol Integr Physiol. 2002;133:931–45. doi: 10.1016/s1095-6433(02)00138-1. [DOI] [PubMed] [Google Scholar]
- 18.Quain J. In: Elements of Anatomy. 6 Sharpey W, Ellis GV, editors. 1–3. London: Walton & Maberly; 1856. [Google Scholar]
- 19.Waggett AD, Ralphs JR, Kwan AP, Woodnutt D, Benjamin M. Characterization of collagens and proteoglycans at the insertion of the human Achilles tendon. Matrix Biol. 1998;16:457–70. doi: 10.1016/s0945-053x(98)90017-8. [DOI] [PubMed] [Google Scholar]
- 20.Kumagai J, Sarkar K, Uhthoff HK, Okawara Y, Ooshima A. Immunohistochemical distribution of type I, II and III collagens in the rabbit supraspinatus tendon insertion. J Anat. 1994;185:279–84. [PMC free article] [PubMed] [Google Scholar]
- 21.Sagarriga VC, Kavalkovich K, Wu J, Niyibizi C. Biochemical analysis of collagens at the ligament-bone interface reveals presence of cartilage-specific collagens. Arch Biochem Biophys. 1996;328:135–42. doi: 10.1006/abbi.1996.0153. [DOI] [PubMed] [Google Scholar]
- 22.Fukuta S, Oyama M, Kavalkovich K, Fu FH, Niyibizi C. Identification of types II, IX and X collagens at the insertion site of the bovine Achilles tendon. Matrix Biol. 1998;17:65–73. doi: 10.1016/s0945-053x(98)90125-1. [DOI] [PubMed] [Google Scholar]
- 23.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–26. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 24.Skalak R. Tissue Engineering: Proceedings of a Workshop; Granlibakken, Lake Tahoe, California. February 26–29, 1988; New York: Liss; 1988. [Google Scholar]
- 25.Butler DL, Goldstein SA, Guilak F. Functional tissue engineering: the role of biomechanics. J Biomech Eng. 2000;122:570–75. doi: 10.1115/1.1318906. [DOI] [PubMed] [Google Scholar]
- 26.Mikos AG, Herring SW, Ochareon P, Elisseeff J, Lu HH, et al. Engineering complex tissues. Tissue Eng. 2006;12:3307–39. doi: 10.1089/ten.2006.12.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moffat KL, Wang IN, Rodeo SA, Lu HH. Orthopedic interface tissue engineering for the biological fixation of soft tissue grafts. Clin Sports Med. 2009;28:157–76. doi: 10.1016/j.csm.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang PJ, Temenoff JS. Engineering orthopedic tissue interfaces. Tissue Eng Part B Rev. 2009;15:127–41. doi: 10.1089/ten.teb.2008.0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Place ES, Evans ND, Stevens MM. Complexity in biomaterials for tissue engineering. Nat Mater. 2009;8:457–70. doi: 10.1038/nmat2441. [DOI] [PubMed] [Google Scholar]
- 30.Zhang X, Bogdanowicz D, Erisken C, Lee NM, Lu HH. Biomimetic scaffold design for functional and integrative tendon repair. J Shoulder Elbow Surg. 2012;21:266–77. doi: 10.1016/j.jse.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith L, Xia Y, Galatz LM, Genin GM, Thomopoulos S. Tissue-engineering strategies for the tendon/ligament-to-bone insertion. Connect Tissue Res. 2012;53:95–105. doi: 10.3109/03008207.2011.650804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fujioka H, Wang GJ, Mizuno K, Balian G, Hurwitz SR. Changes in the expression of type-X collagen in the fibrocartilage of rat Achilles tendon attachment during development. J Orthop Res. 1997;15:675–81. doi: 10.1002/jor.1100150508. [DOI] [PubMed] [Google Scholar]
- 33.Nawata K, Minamizaki T, Yamashita Y, Teshima R. Development of the attachment zones in the rat anterior cruciate ligament: changes in the distributions of proliferating cells and fibrillar collagens during postnatal growth. J Orthop Res. 2002;20:1339–44. doi: 10.1016/S0736-0266(02)00048-7. [DOI] [PubMed] [Google Scholar]
- 34.Thomopoulos S, Williams GR, Soslowsky LJ. Tendon to bone healing: differences in biomechanical, structural, and compositional properties due to a range of activity levels. J Biomech Eng. 2003;125:106–13. doi: 10.1115/1.1536660. [DOI] [PubMed] [Google Scholar]
- 35.Thomopoulos S, Kim HM, Rothermich SY, Biederstadt C, Das R, Galatz LM. Decreased muscle loading delays maturation of the tendon enthesis during postnatal development. J Orthop Res. 2007;25:1154–63. doi: 10.1002/jor.20418. [DOI] [PubMed] [Google Scholar]
- 36.Galatz L, Rothermich S, VanderPloeg K, Petersen B, Sandell L, Thomopoulos S. Development of the supraspinatus tendon-to-bone insertion: localized expression of extracellular matrix and growth factor genes. J Orthop Res. 2007;25:1621–28. doi: 10.1002/jor.20441. [DOI] [PubMed] [Google Scholar]
- 37.Blitz E, Viukov S, Sharir A, Shwartz Y, Galloway JL, et al. Bone ridge patterning during muscu-loskeletal assembly is mediated through SCX regulation of Bmp4 at the tendon-skeleton junction. Dev Cell. 2009;17:861–73. doi: 10.1016/j.devcel.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwartz AG, Pasteris JD, Genin GM, Daulton TL, Thomopoulos S. Mineral distributions at the developing tendon enthesis. PLoS One. 2012;7(11):e48630. doi: 10.1371/journal.pone.0048630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vortkamp A, Lee K, Lanske B, Segre GV, Kronenberg HM, Tabin CJ. Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science. 1996;273:613–22. doi: 10.1126/science.273.5275.613. [DOI] [PubMed] [Google Scholar]
- 40.Kronenberg HM. PTHrP and skeletal development. Ann NY Acad Sci. 2006;1068:1–13. doi: 10.1196/annals.1346.002. [DOI] [PubMed] [Google Scholar]
- 41.Jiang J, Leong NL, Mung JC, Hidaka C, Lu HH. Interaction between zonal populations of articular chondrocytes suppresses chondrocyte mineralization and this process is mediated by PTHrP. Osteoarthr Cartil. 2008;16:70–82. doi: 10.1016/j.joca.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 42.Chen X, Macica CM, Dreyer BE, Hammond VE, Hens JR, et al. Initial characterization of PTH-related protein gene-driven lacZ expression in the mouse. J Bone Miner Res. 2006;21:113–23. doi: 10.1359/JBMR.051005. [DOI] [PubMed] [Google Scholar]
- 43.Chen X, Macica C, Nasiri A, Judex S, Broadus AE. Mechanical regulation of PTHrP expression in entheses. Bone. 2007;41:752–59. doi: 10.1016/j.bone.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Broadus AE, Macica C, Chen X. The PTHrP functional domain is at the gates of endochondral bones. Ann NY Acad Sci. 2007;1116:65–81. doi: 10.1196/annals.1402.061. [DOI] [PubMed] [Google Scholar]
- 45.Koyama E, Ochiai T, Rountree RB, Kingsley DM, Enomoto-Iwamoto M, et al. Synovial joint formation during mouse limb skeletogenesis: roles of Indian hedgehog signaling. Ann NY Acad Sci. 2007;1116:100–12. doi: 10.1196/annals.1402.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gilbert SF, Singer SR. Developmental Biology. 8 Sunderland, MA: Sinauer; 2006. [Google Scholar]
- 47.Asou Y, Nifuji A, Tsuji K, Shinomiya K, Olson EN, et al. Coordinated expression of scleraxis and Sox9 genes during embryonic development of tendons and cartilage. J Orthop Res. 2002;20:827–33. doi: 10.1016/S0736-0266(01)00169-3. [DOI] [PubMed] [Google Scholar]
- 48.Huang W, Chung UI, Kronenberg HM, de Crombrugghe B. The chondrogenic transcription factor Sox9 is a target of signaling by the parathyroid hormone-related peptide in the growth plate of endochondral bones. Proc Natl Acad Sci USA. 2001;98:160–65. doi: 10.1073/pnas.011393998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cserjesi P, Brown D, Ligon KL, Lyons GE, Copeland NG, et al. Scleraxis: a basic helix-loop-helix protein that prefigures skeletal formation during mouse embryogenesis. Development. 1995;121:1099–110. doi: 10.1242/dev.121.4.1099. [DOI] [PubMed] [Google Scholar]
- 50.Schweitzer R, Chyung JH, Murtaugh LC, Brent AE, Rosen V, et al. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development. 2001;128:3855–66. doi: 10.1242/dev.128.19.3855. [DOI] [PubMed] [Google Scholar]
- 51.Brent AE, Schweitzer R, Tabin CJ. A somitic compartment of tendon progenitors. Cell. 2003;113:235–48. doi: 10.1016/s0092-8674(03)00268-x. [DOI] [PubMed] [Google Scholar]
- 52.Murchison ND, Price BA, Conner DA, Keene DR, Olson EN, et al. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development. 2007;134:2697–708. doi: 10.1242/dev.001933. [DOI] [PubMed] [Google Scholar]
- 53.Slack C, Flint MH, Thompson BM. The effect of tensional load on isolated embryonic chick tendons in organ culture. Connect Tissue Res. 1984;12:229–47. doi: 10.3109/03008208409013685. [DOI] [PubMed] [Google Scholar]
- 54.Buckwalter JA. Skeletal Growth and Development: Clinical Issues and Basic Science Advances. Rosemont, IL: Am. Acad. Orthop. Surg; 1998. [Google Scholar]
- 55.Mikic B, Johnson TL, Chhabra AB, Schalet BJ, Wong M, Hunziker EB. Differential effects of embryonic immobilization on the development of fibrocartilaginous skeletal elements. J Rehabil Res Dev. 2000;37:127–33. [PubMed] [Google Scholar]
- 56.Mikic B, Isenstein AL, Chhabra A. Mechanical modulation of cartilage structure and function during embryogenesis in the chick. Ann Biomed Eng. 2004;32:18–25. doi: 10.1023/b:abme.0000007787.39262.a7. [DOI] [PubMed] [Google Scholar]
- 57.Thomopoulos S, Genin GM, Galatz LM. The development and morphogenesis of the tendon-to-bone insertion—what development can teach us about healing. J Musculoskelet Neuronal Interact. 2010;10:35–45. [PMC free article] [PubMed] [Google Scholar]
- 58.Kim HM, Galatz LM, Das R, Patel N, Thomopoulos S. Musculoskeletal deformities secondary to neurotomy of the superior trunk of the brachial plexus in neonatal mice. J Orthop Res. 2010;28:1391–98. doi: 10.1002/jor.21128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Das R, Rich J, Kim HM, McAlinden A, Thomopoulos S. Effects of botulinum toxin-induced paralysis on postnatal development of the supraspinatus muscle. J Orthop Res. 2011;29:281–88. doi: 10.1002/jor.21234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tatara A, Lipner J, Das R, Kim HM, Patel N, et al. The effects of muscle load and osteoclast activity on bone formation at the developing tendon enthesis. Abstr 134, 57th Annu. Meet. Orthop. Res. Soc; Long Beach, CA. 2011. [Google Scholar]
- 61.Chen JM, Willers C, Xu J, Wang A, Zheng MH. Autologous tenocyte therapy using porcine-derived bioscaffolds for massive rotator cuff defect in rabbits. Tissue Eng. 2007;13:1479–91. doi: 10.1089/ten.2006.0266. [DOI] [PubMed] [Google Scholar]
- 62.Suresh S, Mortensen A. Fundamentals of Functionally Graded Materials: Processing and Thermome-chanical Behaviour of Graded Metals and Metal-Ceramic Composites. London: IOM Commun; 1998. [Google Scholar]
- 63.Wopenka B, Kent A, Pasteris JD, Yoon Y, Thomopoulos S. The tendon-to-bone transition of the rotator cuff: a preliminary Raman spectroscopic study documenting the gradual mineralization across the insertion in rat tissue samples. Appl Spectrosc. 2008;62:1285–94. doi: 10.1366/000370208786822179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cooper RR, Misol S. Tendon and ligament insertion. A light and electron microscopic study. J Bone Joint Surg Am. 1970;52:1–20. [PubMed] [Google Scholar]
- 65.Woo SL, Buckwalter JA. AAOS/NIH/ORS workshop. Injury and repair of the musculoskeletal soft tissues Savannah, Georgia, June 18–20, 1987. J Orthop Res. 1988;6:907–31. doi: 10.1002/jor.1100060615. [DOI] [PubMed] [Google Scholar]
- 66.Gao J, Messner K. Quantitative comparison of soft tissue-bone interface at chondral ligament insertions in the rabbit knee joint. J Anat. 1996;188:367–73. [PMC free article] [PubMed] [Google Scholar]
- 67.Villegas DF, Maes JA, Magee SD, Donahue TL. Failure properties and strain distribution analysis of meniscal attachments. J Biomech. 2007;40:2655–62. doi: 10.1016/j.jbiomech.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 68.Hauch KN, Oyen ML, Odegard GM, Haut Donahue TL. Nanoindentation of the insertional zones of human meniscal attachments into underlying bone. J Mech Behav Biomed Mater. 2009;2:339–47. doi: 10.1016/j.jmbbm.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Currey JD. The effect of porosity and mineral content on the Young’s modulus of elasticity of compact bone. J Biomech. 1988;21:131–39. doi: 10.1016/0021-9290(88)90006-1. [DOI] [PubMed] [Google Scholar]
- 70.Ferguson VL, Bushby AJ, Boyde A. Nanomechanical properties and mineral concentration in articular calcified cartilage and subchondral bone. J Anat. 2003;203:191–202. doi: 10.1046/j.1469-7580.2003.00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Radhakrishnan P, Lewis NT, Mao JJ. Zone-specific micromechanical properties of the extracellular matrices of growth plate cartilage. Ann Biomed Eng. 2004;32:284–91. doi: 10.1023/b:abme.0000012748.41851.b4. [DOI] [PubMed] [Google Scholar]
- 72.Spalazzi JP, Boskey AL, Lu HH. Region-dependent variations in matrix collagen and mineral distribution across the femoral and tibial anterior cruciate ligament-to-bone insertion sites. Abstr 891, 53rd Annu. Meet. Orthop. Res. Soc; San Diego, CA. 2007. [Google Scholar]
- 73.Huang CY, Wang VM, Pawluk RJ, Bucchieri JS, Levine WN, et al. Inhomogeneous mechanical behavior of the human supraspinatus tendon under uniaxial loading. J Orthop Res. 2005;23:924–30. doi: 10.1016/j.orthres.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 74.Sano H, Saijo Y, Kokubun S. Non-mineralized fibrocartilage shows the lowest elastic modulus in the rabbit supraspinatus tendon insertion: measurement with scanning acoustic microscopy. J Shoulder Elbow Surg. 2006;15:743–49. doi: 10.1016/j.jse.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 75.Stouffer DC, Butler DL, Hosny D. The relationship between crimp pattern and mechanical response of human patellar tendon-bone units. J Biomech Eng. 1985;107:158–65. doi: 10.1115/1.3138536. [DOI] [PubMed] [Google Scholar]
- 76.Liu YX, Thomopoulos S, Birman V, Lee JS, Genin GM. Bi-material attachment through a soft tissue interfacial system. Mech Mater. 2012;44:83–92. doi: 10.1016/j.mechmat.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Williams ML. Stress singularities resulting from various boundary conditions in angular corners of plates in extension. J Appl Mech. 1952;19:526–28. [Google Scholar]
- 78.Milz S, Rufai A, Buettner A, Putz R, Ralphs JR, Benjamin M. Three-dimensional reconstructions of the Achilles tendon insertion in man. J Anat. 2002;200:145–52. doi: 10.1046/j.0021-8782.2001.00016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75:1795–803. doi: 10.2106/00004623-199312000-00009. [DOI] [PubMed] [Google Scholar]
- 80.Fujioka H, Thakur R, Wang GJ, Mizuno K, Balian G, Hurwitz SR. Comparison of surgically attached and non-attached repair of the rat Achilles tendon-bone interface. Cellular organization and type X collagen expression. Connect Tissue Res. 1998;37:205–18. doi: 10.3109/03008209809002440. [DOI] [PubMed] [Google Scholar]
- 81.Aoki M, Oguma H, Fukushima S, Ishii S, Ohtani S, Murakami G. Fibrous connection to bone after immediate repair of the canine infraspinatus: the most effective bony surface for tendon attachment. J Shoulder Elbow Surg. 2001;10:123–28. doi: 10.1067/mse.2001.111963. [DOI] [PubMed] [Google Scholar]
- 82.Waggy CA, Blaha JD, Lobosky DA, Beresford WA, Clovis E. Healing of tendon to bone insertion site in rabbits: a model of the effect of partial disruption. Abstr., 40th Annu. Meet. Orthop. Res. Soc; New Orleans, LA. 1994. [Google Scholar]
- 83.St Pierre P, Olson EJ, Elliott JJ, O’Hair KC, McKinney LA, Ryan J. Tendon-healing to cortical bone compared with healing to a cancellous trough. A biomechanical and histological evaluation in goats. J Bone Joint Surg Am. 1995;77:1858–66. doi: 10.2106/00004623-199512000-00010. [DOI] [PubMed] [Google Scholar]
- 84.Silva MJ, Thomopoulos S, Kusano N, Zaegel MA, Harwood FL, et al. Early healing of flexor tendon insertion site injuries: Tunnel repair is mechanically and histologically inferior to surface repair in a canine model. J Orthop Res. 2006;24:990–1000. doi: 10.1002/jor.20084. [DOI] [PubMed] [Google Scholar]
- 85.Rodeo SA, Suzuki K, Deng XH, Wozney J, Warren RF. Use of recombinant human bone mor-phogenetic protein-2 to enhance tendon healing in a bone tunnel. Am J Sports Med. 1999;27:476–88. doi: 10.1177/03635465990270041201. [DOI] [PubMed] [Google Scholar]
- 86.Liu SH, Panossian V, al Shaikh R, Tomin E, Shepherd E, et al. Morphology and matrix composition during early tendon to bone healing. Clin Orthop Relat Res. 1997;339:253–60. doi: 10.1097/00003086-199706000-00034. [DOI] [PubMed] [Google Scholar]
- 87.Kannus P, Leppala J, Lehto M, Sievanen H, Heinonen A, Jarvinen M. A rotator cuff rupture produces permanent osteoporosis in the affected extremity, but not in those with whom shoulder function has returned to normal. J Bone Miner Res. 1995;10:1263–71. doi: 10.1002/jbmr.5650100817. [DOI] [PubMed] [Google Scholar]
- 88.Alfredson H, Nordstrom P, Lorentzon R. Prolonged progressive calcaneal bone loss despite early weightbearing rehabilitation in patients surgically treated for Achilles tendinosis. Calcif Tissue Int. 1998;62:166–71. doi: 10.1007/s002239900411. [DOI] [PubMed] [Google Scholar]
- 89.Wohl GR, Shymkiw RC, Matyas JR, Kloiber R, Zernicke RF. Periarticular cancellous bone changes following anterior cruciate ligament injury. J Appl Physiol. 2001;91:336–42. doi: 10.1152/jappl.2001.91.1.336. [DOI] [PubMed] [Google Scholar]
- 90.Ditsios K, Boyer MI, Kusano N, Gelberman RH, Silva MJ. Bone loss following tendon laceration, repair and passive mobilization. J Orthop Res. 2003;21:990–96. doi: 10.1016/S0736-0266(03)00112-8. [DOI] [PubMed] [Google Scholar]
- 91.Galatz LM, Rothermich SY, Zaegel M, Silva MJ, Havlioglu N, Thomopoulos S. Delayed repair of tendon to bone injuries leads to decreased biomechanical properties and bone loss. J Orthop Res. 2005;23:1441–47. doi: 10.1016/j.orthres.2005.05.005.1100230629. [DOI] [PubMed] [Google Scholar]
- 92.Cadet ER, Vorys GC, Rahman R, Park SH, Gardner TR, et al. Improving bone density at the rotator cuff footprint increases supraspinatus tendon failure stress in a rat model. J Orthop Res. 2010;28:308–14. doi: 10.1002/jor.20972. [DOI] [PubMed] [Google Scholar]
- 93.Sun WS, Moffat KL, Lu HH. Characterization of fibrochondrocytes derived from the ligament-bone insertion. Abstr 200, 53rd Annu. Meet. Orthop. Res. Soc; San Diego, CA. 2007. [Google Scholar]
- 94.Grana WA, Egle DM, Mahnken R, Goodhart CW. An analysis of autograft fixation after anterior cruciate ligament reconstruction in a rabbit model. Am J Sports Med. 1994;22:344–51. doi: 10.1177/036354659402200309. [DOI] [PubMed] [Google Scholar]
- 95.Blickenstaff KR, Grana WA, Egle D. Analysis of a semitendinosus autograft in a rabbit model. Am J Sports Med. 1997;25:554–59. doi: 10.1177/036354659702500420. [DOI] [PubMed] [Google Scholar]
- 96.Spalazzi JP, Dionisio KL, Jiang J, Lu HH. Osteoblast and chondrocyte interactions during cocul-ture on scaffolds. IEEE Eng Med Biol Mag. 2003;22:27–34. doi: 10.1109/memb.2003.1256269. [DOI] [PubMed] [Google Scholar]
- 97.Jiang J, Nicoll SB, Lu HH. Co-culture of osteoblasts and chondrocytes modulates cellular differentiation in vitro. Biochem Biophys Res Commun. 2005;338:762–70. doi: 10.1016/j.bbrc.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 98.Wang IE, Shan J, Choi R, Oh S, Kepler CK, et al. Role of osteoblast-fibroblast interactions in the formation of the ligament-to-bone interface. J Orthop Res. 2007;25:1609–20. doi: 10.1002/jor.20475. [DOI] [PubMed] [Google Scholar]
- 99.Shan JM, Wang IE, Lu HH. Osteoblast-fibroblast interactions modulate cell phenotypes through paracrine and autocrine regulations. Abstr 30, 53rd Annu. Meet. Orthop. Res. Soc; San Diego, CA. 2007. [Google Scholar]
- 100.Lim JK, Hui J, Li L, Thambyah A, Goh J, Lee EH. Enhancement of tendon graft osteointegration using mesenchymal stem cells in a rabbit model of anterior cruciate ligament reconstruction. Arthroscopy. 2004;20:899–910. doi: 10.1016/j.arthro.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 101.Vogel KG, Ordog A, Pogany G, Olah J. Proteoglycans in the compressed region of human tibialis posterior tendon and in ligaments. J Orthop Res. 1993;11:68–77. doi: 10.1002/jor.1100110109. [DOI] [PubMed] [Google Scholar]
- 102.Vogel KG. The effect of compressive loading on proteoglycan turnover in cultured fetal tendon. Connect Tissue Res. 1996;34:227–37. doi: 10.3109/03008209609000701. [DOI] [PubMed] [Google Scholar]
- 103.Nicoll SB, Wedrychowska A, Smith NR, Bhatnagar RS. Modulation of proteoglycan and collagen profiles in human dermal fibroblasts by high density micromass culture and treatment with lactic acid suggests change to a chondrogenic phenotype. Connect Tissue Res. 2001;42:59–69. doi: 10.3109/03008200109014249. [DOI] [PubMed] [Google Scholar]
- 104.French MM, Rose S, Canseco J, Athanasiou KA. Chondrogenic differentiation of adult dermal fibroblasts. Ann Biomed Eng. 2004;32:50–56. doi: 10.1023/b:abme.0000007790.65773.e0. [DOI] [PubMed] [Google Scholar]
- 105.Takimoto A, Oro M, Hiraki Y, Shukunami C. Direct conversion of tenocytes into chondrocytes by Sox9. Exp Cell Res. 2012;318:1492–507. doi: 10.1016/j.yexcr.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 106.Wang IE, Lu HH. Tri-culture of bovine anterior cruciate ligament fibroblasts, osteoblasts and chon-drocytes with application in interface tissue engineering. Abstr 1522, 51st Annu. Meet. Orthop. Res. Soc; Washington, DC. 2005. [Google Scholar]
- 107.Gimbel JA, Van Kleunen JP, Williams GR, Thomopoulos S, Soslowsky LJ. Long durations of immobilization in the rat result in enhanced mechanical properties of the healing supraspinatus tendon insertion site. J Biomech Eng. 2007;129:400–4. doi: 10.1115/1.2721075. [DOI] [PubMed] [Google Scholar]
- 108.Bedi A, Kovacevic D, Fox AJ, Imhauser CW, Stasiak M, et al. Effect of early and delayed mechanical loading on tendon-to-bone healing after anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2010;92:2387–401. doi: 10.2106/JBJS.I.01270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dagher E, Hays PL, Kawamura S, Godin J, Deng XH, Rodeo SA. Immobilization modulates macrophage accumulation in tendon-bone healing. Clin Orthop Relat Res. 2009;467:281–87. doi: 10.1007/s11999-008-0512-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Thomopoulos S, Zampiakis E, Das R, Silva MJ, Gelberman RH. The effect of muscle loading on flexor tendon-to-bone healing in a canine model. J Orthop Res. 2008;26:1611–17. doi: 10.1002/jor.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Galatz LM, Charlton N, Das R, Kim HM, Havlioglu N, Thomopoulos S. Complete removal of load is detrimental to rotator cuff healing. J Shoulder Elbow Surg. 2009;18:669–75. doi: 10.1016/j.jse.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 112.Ishikawa H, Koshino T, Takeuchi R, Saito T. Effects of collagen gel mixed with hydroxyapatite powder on interface between newly formed bone and grafted Achilles tendon in rabbit femoral bone tunnel. Biomaterials. 2001;22:1689–94. doi: 10.1016/s0142-9612(00)00331-8. [DOI] [PubMed] [Google Scholar]
- 113.Tien YC, Chih TT, Lin JH, Ju CP, Lin SD. Augmentation of tendon-bone healing by the use of calcium-phosphate cement. J Bone Joint Surg Br. 2004;86:1072–76. doi: 10.1302/0301-620x.86b7.14578. [DOI] [PubMed] [Google Scholar]
- 114.Huangfu X, Zhao J. Tendon-bone healing enhancement using injectable tricalcium phosphate in a dog anterior cruciate ligament reconstruction model. Arthroscopy. 2007;23:455–62. doi: 10.1016/j.arthro.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 115.Robertson WJ, Hatch JD, Rodeo SA. Evaluation of tendon graft fixation using α-BSM calcium phosphate cement. Arthroscopy. 2007;23:1087–92. doi: 10.1016/j.arthro.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 116.Gulotta LV, Kovacevic D, Ying L, Ehteshami JR, Montgomery S, Rodeo SA. Augmentation of tendon-to-bone healing with a magnesium-based bone adhesive. Am J Sports Med. 2008;36:1290–97. doi: 10.1177/0363546508314396. [DOI] [PubMed] [Google Scholar]
- 117.Shen H, Qiao G, Cao H, Jiang Y. An histological study of the influence of osteoinductive calcium phosphate ceramics on tendon healing pattern in a bone tunnel with suspensory fixation. Int Orthop. 2010;34:917–24. doi: 10.1007/s00264-009-0824-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mutsuzaki H, Sakane M, Nakajima H, Ito A, Hattori S, et al. Calcium-phosphate-hybridized tendon directly promotes regeneration of tendon-bone insertion. J Biomed Mater Res A. 2004;70:319–27. doi: 10.1002/jbm.a.30084. [DOI] [PubMed] [Google Scholar]
- 119.Ohtera K, Yamada Y, Aoki M, Sasaki T, Yamakoshi K. Effects of periosteum wrapped around tendon in a bone tunnel: a biomechanical and histological study in rabbits. Crit Rev Biomed Eng. 2000;28:115–18. doi: 10.1615/critrevbiomedeng.v28.i12.190. [DOI] [PubMed] [Google Scholar]
- 120.Kyung HS, Kim SY, Oh CW, Kim SJ. Tendon-to-bone tunnel healing in a rabbit model: the effect of periosteum augmentation at the tendon-to-bone interface. Knee Surg Sports Traumatol Arthrosc. 2003;11:9–15. doi: 10.1007/s00167-002-0317-8. [DOI] [PubMed] [Google Scholar]
- 121.Chen CH, Chen WJ, Shih CH, Yang CY, Liu SJ, Lin PY. Enveloping the tendon graft with periosteum to enhance tendon-bone healing in a bone tunnel: a biomechanical and histologic study in rabbits. Arthroscopy. 2003;19:290–96. doi: 10.1053/jars.2003.50014. [DOI] [PubMed] [Google Scholar]
- 122.Youn I, Jones DG, Andrews PJ, Cook MP, Suh JK. Periosteal augmentation of a tendon graft improves tendon healing in the bone tunnel. Clin Orthop Relat Res. 2004;419:223–31. doi: 10.1097/00003086-200402000-00037. [DOI] [PubMed] [Google Scholar]
- 123.Karaoglu S, Celik C, Korkusuz P. The effects of bone marrow or periosteum on tendon-to-bone tunnel healing in a rabbit model. Knee Surg Sports Traumatol Arthrosc. 2009;17:170–78. doi: 10.1007/s00167-008-0646-3. [DOI] [PubMed] [Google Scholar]
- 124.Martinek V, Latterman C, Usas A, Abramowitch S, Woo SL, et al. Enhancement of tendon-bone integration of anterior cruciate ligament grafts with bone morphogenetic protein-2 gene transfer: a histological and biomechanical study. J Bone Joint Surg Am. 2002;84-A:1123–31. doi: 10.2106/00004623-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 125.Ma CB, Kawamura S, Deng XH, Ying L, Schneidkraut J, et al. Bone morphogenetic proteins-signaling plays a role in tendon-to-bone healing: a study of rhBMP-2 and noggin. Am J Sports Med. 2007;35:597–604. doi: 10.1177/0363546506296312. [DOI] [PubMed] [Google Scholar]
- 126.Hashimoto Y, Yoshida G, Toyoda H, Takaoka K. Generation of tendon-to-bone interface “en-thesis” with use of recombinant BMP-2 in a rabbit model. J Orthop Res. 2007;25:1415–24. doi: 10.1002/jor.20447. [DOI] [PubMed] [Google Scholar]
- 127.Chen CH, Liu HW, Tsai CL, Yu CM, Lin IH, Hsiue GH. Photoencapsulation of bone morpho-genetic protein-2 and periosteal progenitor cells improve tendon graft healing in a bone tunnel. Am J Sports Med. 2008;36:461–73. doi: 10.1177/0363546507311098. [DOI] [PubMed] [Google Scholar]
- 128.Mihelic R, Pecina M, Jelic M, Zoricic S, Kusec V, et al. Bone morphogenetic protein-7 (osteogenic protein-1) promotes tendon graft integration in anterior cruciate ligament reconstruction in sheep. Am J Sports Med. 2004;32:1619–25. doi: 10.1177/0363546504263703. [DOI] [PubMed] [Google Scholar]
- 129.Sasaki K, Kuroda R, Ishida K, Kubo S, Matsumoto T, et al. Enhancement of tendon-bone osteo-integration of anterior cruciate ligament graft using granulocyte colony-stimulating factor. Am J Sports Med. 2008;36:1519–27. doi: 10.1177/0363546508316282. [DOI] [PubMed] [Google Scholar]
- 130.Ouyang HW, Goh JC, Lee EH. Use of bone marrow stromal cells for tendon graft-to-bone healing: histological and immunohistochemical studies in a rabbit model. Am J Sports Med. 2004;32:321–27. doi: 10.1177/0095399703258682. [DOI] [PubMed] [Google Scholar]
- 131.Soon MY, Hassan A, Hui JH, Goh JC, Lee EH. An analysis of soft tissue allograft anterior cruciate ligament reconstruction in a rabbit model: a short-term study of the use of mesenchymal stem cells to enhance tendon osteointegration. Am J Sports Med. 2007;35:962–71. doi: 10.1177/0363546507300057. [DOI] [PubMed] [Google Scholar]
- 132.Ju YJ, Muneta T, Yoshimura H, Koga H, Sekiya I. Synovial mesenchymal stem cells accelerate early remodeling of tendon-bone healing. Cell Tissue Res. 2008;332:469–78. doi: 10.1007/s00441-008-0610-z. [DOI] [PubMed] [Google Scholar]
- 133.Dunn MG, Tria AJ, Kato YP, Bechler JR, Ochner RS, et al. Anterior cruciate ligament reconstruction using a composite collagenous prosthesis. A biomechanical and histologic study in rabbits. Am J Sports Med. 1992;20:507–15. doi: 10.1177/036354659202000504. [DOI] [PubMed] [Google Scholar]
- 134.Dunn MG, Liesch JB, Tiku ML, Zawadsky JP. Development of fibroblast-seeded ligament analogs for ACL reconstruction. J Biomed Mater Res. 1995;29:1363–71. doi: 10.1002/jbm.820291107. [DOI] [PubMed] [Google Scholar]
- 135.Altman GH, Horan RL, Lu HH, Moreau J, Martin I, et al. Silk matrix for tissue engineered anterior cruciate ligaments. Biomaterials. 2002;23:4131–41. doi: 10.1016/s0142-9612(02)00156-4. [DOI] [PubMed] [Google Scholar]
- 136.Inoue N, Ikeda K, Aro HT, Frassica FJ, Sim FH, Chao EY. Biologic tendon fixation to metallic implant augmented with autogenous cancellous bone graft and bone marrow in a canine model. J Orthop Res. 2002;20:957–66. doi: 10.1016/S0736-0266(02)00037-2. [DOI] [PubMed] [Google Scholar]
- 137.Cooper JA, Lu HH, Ko FK, Freeman JW, Laurencin CT. Fiber-based tissue-engineered scaffold for ligament replacement: design considerations and in vitro evaluation. Biomaterials. 2005;26:1523–32. doi: 10.1016/j.biomaterials.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 138.Lu HH, Cooper JA, Jr, Manuel S, Freeman JW, Attawia MA, et al. Anterior cruciate ligament regeneration using braided biodegradable scaffolds: in vitro optimization studies. Biomaterials. 2005;26:4805–16. doi: 10.1016/j.biomaterials.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 139.Cooper JA, Jr, Sahota JS, Gorum WJ, Carter J, Doty SB, Laurencin CT. Biomimetic tissue-engineered anterior cruciate ligament replacement. Proc Natl Acad Sci USA. 2007;104:3049–54. doi: 10.1073/pnas.0608837104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ma J, Goble K, Smietana M, Kostrominova T, Larkin L, Arruda EM. Morphological and functional characteristics of three-dimensional engineered bone-ligament-bone constructs following implantation. J Biomech Eng. 2009;131:101017. doi: 10.1115/1.4000151. [DOI] [PubMed] [Google Scholar]
- 141.Paxton JZ, Donnelly K, Keatch RP, Baar K. Engineering the bone-ligament interface using polyethylene glycol diacrylate incorporated with hydroxyapatite. Tissue Eng Part A. 2009;15:1201–9. doi: 10.1089/ten.tea.2008.0105. [DOI] [PubMed] [Google Scholar]
- 142.Spalazzi JP, Doty SB, Moffat KL, Levine WN, Lu HH. Development of controlled matrix heterogeneity on a triphasic scaffold for orthopedic interface tissue engineering. Tissue Eng. 2006;12:3497–508. doi: 10.1089/ten.2006.12.3497. [DOI] [PubMed] [Google Scholar]
- 143.Spalazzi JP, Dagher E, Doty SB, Guo XE, Rodeo SA, Lu HH. In vivo evaluation of a multiphased scaffold designed for orthopaedic interface tissue engineering and soft tissue-to-bone integration. J Biomed Mater Res A. 2008;86A:1–12. doi: 10.1002/jbm.a.32073. [DOI] [PubMed] [Google Scholar]
- 144.Lu HH, El Amin SF, Scott KD, Laurencin CT. Three-dimensional, bioactive, biodegradable, polymer-bioactive glass composite scaffolds with improved mechanical properties support collagen synthesis and mineralization of human osteoblast-like cells in vitro. J Biomed Mater Res. 2003;64A:465–74. doi: 10.1002/jbm.a.10399. [DOI] [PubMed] [Google Scholar]
- 145.Spalazzi JP, Vyner MC, Jacobs MT, Moffat KL, Lu HH. Mechanoactive scaffold induces tendon remodeling and expression of fibrocartilage markers. Clin Orthop Relat Res. 2008;466:1938–48. doi: 10.1007/s11999-008-0310-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Suo Z, Hutchinson JW. Interface crack between two elastic layers. Int J Fract. 1990;43:1–18. [Google Scholar]
- 147.Beuth JL, Narayan SH. Residual stress-driven delamination in deposited multi-layers. Int J Solids Struct. 1996;33:65–78. [Google Scholar]
- 148.Blevins FT, Djurasovic M, Flatow EL, Vogel KG. Biology of the rotator cuff tendon. Orthop Clin North Am. 1997;28:1–16. doi: 10.1016/s0030-5898(05)70260-1. [DOI] [PubMed] [Google Scholar]
- 149.Chang CH, Chen CH, Su CY, Liu HT, Yu CM. Rotator cuff repair with periosteum for enhancing tendon-bone healing: a biomechanical and histological study in rabbits. Knee Surg Sports Traumatol Arthrosc. 2009;17:1447–53. doi: 10.1007/s00167-009-0809-x. [DOI] [PubMed] [Google Scholar]
- 150.Sundar S, Pendegrass CJ, Blunn GW. Tendon bone healing can be enhanced by demineralized bone matrix: a functional and histological study. J Biomed Mater Res B Appl Biomater. 2009;88:115–22. doi: 10.1002/jbm.b.31157. [DOI] [PubMed] [Google Scholar]
- 151.Rodeo SA, Potter HG, Kawamura S, Turner AS, Kim HJ, Atkinson BL. Biologic augmentation of rotator cuff tendon-healing with use of a mixture of osteoinductive growth factors. J Bone Joint Surg Am. 2007;89:2485–97. doi: 10.2106/JBJS.C.01627. [DOI] [PubMed] [Google Scholar]
- 152.Kovacevic D, Fox AJ, Bedi A, Ying L, Deng XH, et al. Calcium-phosphate matrix with or without TGF-β3 improves tendon-bone healing after rotator cuff repair. Am J Sports Med. 2011;39:811–19. doi: 10.1177/0363546511399378. [DOI] [PubMed] [Google Scholar]
- 153.Manning CN, Kim HM, Sakiyama-Elbert S, Galatz LM, Havlioglu N, Thomopoulos S. Sustained delivery of transforming growth factor beta three enhances tendon-to-bone healing in a rat model. J Orthop Res. 2011;29:1099–105. doi: 10.1002/jor.21301. [DOI] [PubMed] [Google Scholar]
- 154.Gulotta LV, Kovacevic D, Montgomery S, Ehteshami JR, Packer JD, Rodeo SA. Stem cells genetically modified with the developmental gene MT1-MMP improve regeneration of the supraspinatus tendon-to-bone insertion site. Am J Sports Med. 2010;38:1429–37. doi: 10.1177/0363546510361235. [DOI] [PubMed] [Google Scholar]
- 155.Bedi A, Kovacevic D, Hettrich C, Gulotta LV, Ehteshami JR, et al. The effect of matrix metallo-proteinase inhibition on tendon-to-bone healing in a rotator cuff repair model. J Shoulder Elbow Surg. 2010;19:384–91. doi: 10.1016/j.jse.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 156.Gulotta LV, Kovacevic D, Ehteshami JR, Dagher E, Packer JD, Rodeo SA. Application of bone marrow-derived mesenchymal stem cells in a rotator cuff repair model. Am J Sports Med. 2009;37:2126–33. doi: 10.1177/0363546509339582. [DOI] [PubMed] [Google Scholar]
- 157.Gulotta LV, Kovacevic D, Packer JD, Deng XH, Rodeo SA. Bone marrow-derived mesenchymal stem cells transduced with scleraxis improve rotator cuff healing in a rat model. Am J Sports Med. 2011;39:1282–89. doi: 10.1177/0363546510395485. [DOI] [PubMed] [Google Scholar]
- 158.Liu W, Zhang Y, Thomopoulos S, Xia Y. Generation of controllable gradients in cell density. Angew Chem Int Ed. 2013;52:429–32. doi: 10.1002/anie.201206060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yokoya S, Mochizuki Y, Nagata Y, Deie M, Ochi M. Tendon-bone insertion repair and regeneration using polyglycolic acid sheet in the rabbit rotator cuff injury model. Am J Sports Med. 2008;36:1298–309. doi: 10.1177/0363546508314416. [DOI] [PubMed] [Google Scholar]
- 160.Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J Biomed Mater Res. 2002;60:613–21. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 161.Ma Z, Kotaki M, Inai R, Ramakrishna S. Potential of nanofiber matrix as tissue-engineering scaffolds. Tissue Eng. 2005;11:101–9. doi: 10.1089/ten.2005.11.101. [DOI] [PubMed] [Google Scholar]
- 162.Pham QP, Sharma U, Mikos AG. Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue Eng. 2006;12:1197–211. doi: 10.1089/ten.2006.12.1197. [DOI] [PubMed] [Google Scholar]
- 163.Li WJ, Mauck RL, Cooper JA, Yuan X, Tuan RS. Engineering controllable anisotropy in electro-spun biodegradable nanofibrous scaffolds for musculoskeletal tissue engineering. J Biomech. 2007;40:1686–93. doi: 10.1016/j.jbiomech.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Liu W, Thomopoulos S, Xia Y. Electrospun nanofibers for regenerative medicine. Adv Healthc Mater. 2012;1:10–25. doi: 10.1002/adhm.201100021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Erisken C, Zhang X, Moffat KL, Levine WN, Lu H. Scaffold fiber diameter regulates human tendon fibroblast growth and differentiation. Tissue Eng Part A. 2013;19:519–28. doi: 10.1089/ten.tea.2012.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Subramony SD, Dargis BR, Castillo M, Azeloglu EU, Tracey MS, et al. Biomaterial alignment and mechanical stimulation guide stem cell differentiation. Biomaterials. 2013;34(8):1942–53. doi: 10.1016/j.biomaterials.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Moffat KL, Kwei AS, Spalazzi JP, Doty SB, Levine WN, Lu HH. Novel nanofiber-based scaffold for rotator cuff repair and augmentation. Tissue Eng Part A. 2009;15:115–26. doi: 10.1089/ten.tea.2008.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Itoi E, Berglund LJ, Grabowski JJ, Schultz FM, Growney ES, et al. Tensile properties of the supraspinatus tendon. J Orthop Res. 1995;13:578–84. doi: 10.1002/jor.1100130413. [DOI] [PubMed] [Google Scholar]
- 169.Moffat KL, Levine WN, Lu HH. In vitro evaluation of rotator cuff tendon fibroblasts on aligned composite scaffold of polymer nanofibers and hydroxyapatite nanoparticles. Abstr 1477, 54th Annu. Meeting Orthop. Res. Soc; San Francisco, CA. 2008. [Google Scholar]
- 170.Moffat KL. PhD thesis. Columbia University; New York: 2010. Biomimetic nanofiber scaffold design for tendon-to-bone interface tissue engineering. [Google Scholar]
- 171.Moffat KL, Cassilly RT, Subramony SD, Dargis BR, Zhang X, et al. In vivo evalution of a bi-phasic nanofiber-based scaffold for integrative rotator cuff repair. Abstr 184, 56th Annu. Meet. Orthop. Res. Soc; New Orleans, LA. 2010. [Google Scholar]
- 172.Li XR, Xie JW, Lipner J, Yuan XY, Thomopoulos S, Xia YN. Nanofiber scaffolds with gradations in mineral content for mimicking the tendon-to-bone insertion site. Nano Lett. 2009;9:2763–68. doi: 10.1021/nl901582f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Phillips JE, Burns KL, Le Doux JM, Guldberg RE, Garcia AJ. Engineering graded tissue interfaces. Proc Natl Acad Sci USA. 2008;105:12170–75. doi: 10.1073/pnas.0801988105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Dormer NH, Berkland CJ, Detamore MS. Emerging techniques in stratified designs and continuous gradients for tissue engineering of interfaces. Ann Biomed Eng. 2010;38:2121–41. doi: 10.1007/s10439-010-0033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Seidi A, Ramalingam M, Elloumi-Hannachi I, Ostrovidov S, Khademhosseini A. Gradient bio-materials for soft-to-hard interface tissue engineering. Acta Biomater. 2011;7:1441–51. doi: 10.1016/j.actbio.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 176.Ramalingam M, Young MF, Thomas V, Sun L, Chow LC, et al. Nanofiber scaffold gradients for interfacial tissue engineering. J Biomater Appl. 2012;27:695–705. doi: 10.1177/0885328211423783. [DOI] [PMC free article] [PubMed] [Google Scholar]