Abstract
The experiments presented here were undertaken to determine if factor VIIa (rFVIIa, the Novo Nordisk product NovoSeven™) will directly bind to rehydrated, lyophilized (RL) platelets for the formation of a catalytic surface with an enhanced ability to generate thrombin. The interaction between rFVIIa and the RL platelet surface was examined by measuring equilibrium and non-equilibrium binding of the coagulation factor to the cells and by following the effects of the surface modification on the kinetics of thrombin generation. The association of rFVIIa with RL platelets was rapid with saturation occurring within minutes. Disassociation was slow, with over half of the coagulation factor remaining bound after two hours. Densities of over one million molecules of rFVIIa per RL platelet were obtained when high concentrations of rFVIIa were incubated with RL platelets. Thrombin generation measurements showed that RL platelet-bound rFVIIa was catalytically active. Thus we can expect that RL platelets, which have been shown to effectively bind to sites of vascular injury, will localize rFVIIa to wounds for an increase in therapeutic index. These studies indicate that rFVIIa-RL platelets are worthy of preclinical and clinical development as an infusion agent for severe bleeding.
Keywords: Platelet, factor VIIa, coagulation, thrombin, lyophilized
Introduction
The current understanding of the relationship between factor VIIa and platelets suggests that a platelet with surface bound VIIa might be a potent hemostatic agent. Recombinant factor VIIa (rFVIIa) has been shown to provide hemostasis in hemophiliacs [1, 2] as well as in bleeding disorders that are platelet-related [3–6]. The mechanisms for rFVIIa-mediated hemostasis in both of these types of disorders are thought to involve a rFVIIa interaction with the platelet surface. The ability of rFVIIa to provide hemostasis in hemophilia patients has been theorized to involve the direct activation of factor X on the surface of platelets [7, 8], thus “bypassing” the need for factors IX and VIII. In the case of thrombocytopenia (a diseased state from the lack of platelets) and/or thrombasthenia (a diseased state from the lack of proper platelet function), rFVIIa might mediate thrombin generation on the platelet surface by activating factor IX or X [7, 9]. The hypothesis that rFVIIa can play a “compensating” role for platelet function(s) by augmenting hemostatic mechanisms is supported by the clinical observation that rFVIIa can improve hemostasis in patients that are thrombocytopenic [3, 4, 10], thromboasthenic [6, 11] or those affected by both platelet defects, such as in trauma [12, 13]. However, high-doses of rFVIIa are frequently required for hemostasis, perhaps due to the low (Kd ~90 nM) affinity of the coagulation factor for the platelet surface [7].
A principle obstacle in the development of platelet-tethered therapeutics, such as rFVIIa-platelet conjugates, is the logistical difficulty of isolating fresh platelets, performing the coupling reaction, and then immediately using the product. Intermediate term storage (for days) of the product is not desirable due to storage lesion [14] and contamination issues (e.g., see Blajchman [15, 16]). Longer-term cryopreservation introduces additional logistical difficulties. This difficulty has been overcome with the development of rehydratable, lyophilized (RL) platelets with the Entegrion, Inc. (Research Triangle Park, North Carolina) trade name Stasix(R) particles. The key to the technology for preparing RL platelets is a mild aldehyde cross-linking to stabilize the cells during freezing, lyophilizing and rehydration [17, 18]. In addition to yielding a sterile product [19] that can be stored indefinitely, RL platelets have many aspects of native platelet function [17, 19–22]. Two large animal studies indicate that RL platelets will prove useful for concentrating therapeutics such as rFVIIa at sites of vascular injury. First, when red fluorescent RL platelets are infused into dogs with balloon-denuded coronary arteries, the rehydrated cells effectively bind to the site of injury [23]. Localization of RL platelets to wound sites was also demonstrated in a second study that involved pigs in severe hemorrhagic shock with washout thrombocytopenia [24]. The animal was severely coagulopathic; bleeding from 23 g venipuncture wounds to the jugular vein was indefinite. Upon infusion of RL platelets, hemostasis was restored. Postmortem examination of the venipuncture site demonstrated that the green-fluorescent RL platelets had localized to the wound site.
The molecular reason RL platelets effectively localize to wound sites has been extensively investigated. The lyophilized cells have near-normal ultra-structure as confirmed by electron microscopy [17] and have many of the molecular functions of fresh platelets. Glycoprotein IIb-IIIa and Ib-IX complexes respectively bind fibrinogen [25] and von Willebrand factor [26]. The cells also spread on foreign surfaces [17] and adhere to denuded subendothelium with Baumgartner analysis [17, 26]. RL platelets are capable of a degree of intracellular stimulus response coupling whereby intracellular protein kinases such as protein kinase C and myosin light chain kinase are stimulated by platelet agonists [21]. The result of activation-dependent intracellular signaling is that RL platelets provide positive feedback amplification for primary (cellular) and secondary (humoral) hemostasis. The lyophilized platelets degranulate, thereby leading to secretion of coagulation factors and recruitment of additional platelets, generate thromboxanes, and provide a procoagulative surface for the catalysis of prothrombin to thrombin conversion [21]. However, the thrombin receptor and ADP receptors do not strongly couple to integrin inside-out signaling processes as in native platelets [27]. The overall picture that has emerged is that RL platelet are partially “primed” and adhere to wound sites through vWf-dependent mechanisms [27].
The experiments presented here explored the hypothesis that rFVIIa will directly bind to the surface of RL platelets when incubated at super-physiological concentrations. This hypothesis was investigated by analysing the kinetics of rFVIIa association and disassociation from the surface of RL platelets. The equilibrium surface density of the coagulation factor was also measured. The effect of rFVIIa on the ability of the RL platelet to function as a catalytic surface for thrombin generation was investigated in normal plasma as well as in plasma from patients that lacked factor IX (FIX) and factor V (FV). The results of these studies demonstrate that when rFVIIa and RL platelets are incubated at super-physiological concentrations, each RL platelet can bind over a million rFVIIa molecules. The result is a potent, yet activatable, catalytic surface for thrombin generation that might be able to concentrate rFVIIa at sites of vascular injury. RL platelets are anticipated to enter phase 1 human clinical trials in 2008 as an infusion therapeutic for platelet responsive bleeding. The findings of this study indicate that a rFVIIa conjugated RL platelet could be a valuable follow-on product for controlling very severe hemorrhage, such as in trauma.
Materials and methods
rFVIIa, the Novo Nordisk NovoSeven™ product, was obtained as the infusion-grade therapeutic from the University of North Carolina Memorial Hospital Pharmacy. RL platelets were produced as described elsewhere [17] with cell separation steps being performed with tangential flow filtration. FV and FIX deficient plasmas were obtained from HRF, Inc. (Raleigh, NC, USA). The thrombin-specific fluorogenic substrate, SN17a, was obtained from Haematologic Technologies (Essex Junction, VT, USA). anti-FVII monoclonal antibodies 4F7 and 4F9 were provided by Novo Nordisk. Secondary antibodies were obtained from Sigma-Aldrich, Inc. (St. Louis, MO, USA).
RL platelet/rFVIIa interaction kinetic measurements
In preparation of the kinetic experiments rFVIIa (20 μM) was dialyzed overnight vs. citrated saline (146 mM NaCl, 5.375 mM citrate, pH = 7.4). The “on” reaction was initiated by adding calcium to a mixture of 10.0 uM rFVIIa and 9.0 × 105 RL platelets/ul in citrated saline for10 mM CaCl2. Samples were incubated with gentle rocking at 24°C. At defined times RL platelets and bound rFVIIa were separated from free rFVIIa by pelleting the cells and then performing one centrifugational wash with citrated saline. The centrifugational steps were performed at 4°C. The final RL platelet pellet was suspended in SDS-PAGE reduced sample buffer at 105 cells/ul.
In preparation for “off” reaction studies, RL platelets were loaded with rFVIIa by incubating the cells under the same conditions as described in the last paragraph for one hour. RL platelets were separated from unbound rFVIIa by centrifuging at 5 000 ×g for two minutes, then the pellets were suspended at 105/ul in citrated saline +calcium or citrated plasma (no added calcium) to initiate the “off” reaction. Samples were incubated for various times, and then the RL platelets were separated from unbound rFVIIa as described for the “on” reaction, and suspended in SDS-PAGE reduced sample buffer at 105 cells/ul.
Western analysis
Forty microlitres of the final RL platelet pellets in SDS-PAGE reduced sample buffer at 105 cells/u were applied to each lane of 10.0% polyacrylamide gels that were prepared and run as detailed elsewhere [21]. Lanes with known amounts of rFVIIa were co-electrophoresed for standards. After electrophoresis the proteins were electrotransferred to nitrocellulose and probed with anti-rFVIIa monoclonal antibodies 4F7 and 4F9 mixed 1/1 (for a final IgG concentration of 0.8 mg/ml each antibody) and diluted 1/1000 in phosphate buffered saline with 3% bovine serum albumin. Antibodies were detected with an alkaline phosphatase second antibody system [21]. rFVIIa standard and RL platelet lanes were densiometricaly scanned for quantification of the molecules rFVIIa bound/RL platelet.
Flow cytometric analysis of surface phosphatidylserine
Surface phosphatidylserine levels were analysed by measuring annexin V binding with flow cytometry as detailed elsewhere [28]. Fresh and RL platelets were measured after activation with A23187 (1/100 dilution of a 100 uM A23197 DMSO stock) or while in the resting state (1/100 dilution of DMSO).
Analysis of thrombin generation
The effect of rFVIIa and RL platelets on the kinetics of thrombin generation in plasma was investigated by following the hydrolysis of the thrombin substrate D-phe-pro-arg-ANSNH to yield a fluorescent reaction product. Experiments were performed in 96 well Costar tissue culture platelets that had been incubated for 24 hours at 37°C with 5% human serum albumin in citrated saline. In preparation for these experiments RL platelets were preincubated with rFVIIa as described for the experiments in part 1b of this section, to allow rFVIIa to associate with the platelet surface. The amount of bound rFVIIa was measured with Western analysis as detail in part 2 of this section after performing the fluorescent kinetic assays. One hundred microlitres portions of plasma/ citrated saline = 9/1 that contained rFVIIa ~ RL platelet complexes (at 1.0 × 105/ul and total bound rFVIIa of 0.02uM), rFVIIa (at 0.03 uM), unmodified RL platelets (1.0 × 105/ul) or only citrated saline were placed in triplicate in wells of a 96 well plate. The fluorometric substrate (D-phe-pro-arg-ANSNH) concentration was 50 uM. Reactions were started adding CaCl2 for a final concentration of 10 mM. The D-phe-pro-arg-ANSNH hydrolysis was followed by measuring the fluorescence at 480 nm every minute in a Polarstar Galaxy fluorescent plate reader (BMG Lab Technologies, Inc. Durham, NC, USA). Triplicate relative fluorescence values were averaged to obtain the time-course curves presented in this article.
Results
The studies presented here address the hypothesis that rFVIIa binds to the RL platelet surface to form conjugates that are stable enough to deliver active rFVIIa to sites of vascular injury. This hypothesis was investigated two ways. First, the kinetic and equilibrium properties of the association/disassociation reactions were characterized. Secondly, the functional activity (thrombin generating activity) was examined. These studies would define rFVIIa~RL platelet conjugates as “therapeutically relevant” if sufficient quantities of rFVIIa bound rapidly to the RL platelet surface, remained associated for a time period long enough for the RL platelets to localize to sites of vascular injury (≥the circulation half life), and retained catalytic functionality. If rFVIIa~RL platelet conjugates fulfill these criteria, it would be reasonable to anticipate efficacy in pre-clinical animal and clinical human studies.
The kinetic and equilibrium properties for the association/disassociation reactions
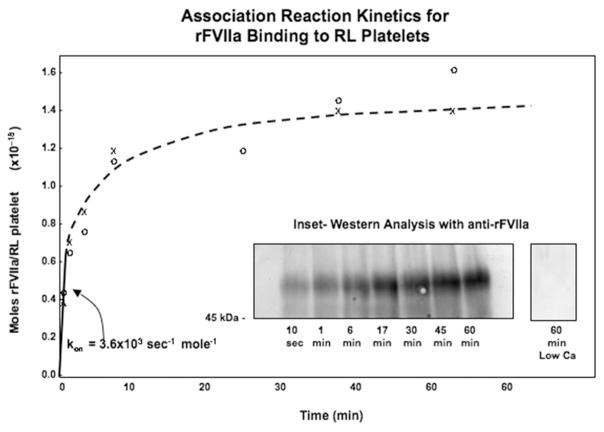
The kinetics of the association reaction were studied by mixing rFVIIa and RL platelets in calcium-containing buffer. Portions of the reaction mixture were removed at increasing time points and analysed with Western blotting to measure RL platelet-bound rFVIIa. As shown in Figure 1, a fast association phase, with an initial rate of 3.6 ×103 sec−1 moles−1, dominated the first few minutes of the study. This was followed by further binding of rFVIIa to the RL platelets and apparent equilibrium saturation by one hour. The number of molecules of rFVIIa that were bound to the platelet surface at saturation was estimated by performing Western blotting (see Inset lanes) and densitometrically comparing the lanes to co-electrophoresed standard dilutions of rFVIIa (data not shown). This analysis showed that, under the conditions of this experiment, 430 000 rFVIIa molecules bound to each RL platelet. Importantly, this association (see the right lane in the Inset in Figure 1) did not occur in the absence of added calcium to the citrated saline buffer system.
Figure 1.
Association reaction kinetics for rFVIIa binding to RL platelets. RL platelets (at 9.0 × 105/ul) were mixed with rFVIIa (at 10 uM) in citrated saline with calcium, then portions were withdrawn as a function of time and analyzed with Western Analysis as detailed in the Materials and methods section. Inset: Western analysis with and without added calcium in the reaction mixture.
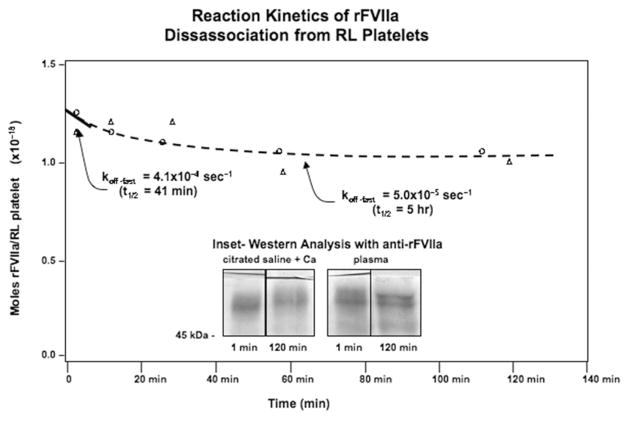
The disassociation reaction was studied by first associating rFVIIa with RL platelets with the same conditions as in Figure 1. Unbound rFVIIa was removed by spinning out the cells, then the rFVIIa~RL platelet conjugates were suspended in citrated saline with 10 mM calcium or plasma. Portions were then withdrawn as a function of time and Western blotting (see Figure 2). The disassociation reaction was best characterized as a double exponential process with an initial fast phase (koff = 4.1 × 10−4 sec−1; tau = 41 min) and a longer-term slow phase (koff = 5.0 × 10−5 sec−1; tau = 5 h). The initial on and off rates yield an equilibrium constant (Keq = koff/kon) of 110 nM. The association of rFVIIa with RL platelets is calcium-dependent, but once bound, the coagulation factor could not be disassociated from the RL platelets by centrifugational washing with EGTA (data not shown). This indicates that rFVIIa remains associated with the RL platelets in a mechanism that is at least partially calcium-independent. For example, the rFVIIa might associate surface PS in a calcium-dependent manner, and then with receptors or other cellular structures in a calcium-independent manner.
Figure 2.
Reaction kinetics of rFVIIa disassociation from RL platelets. RL platelets and rFVIIa were incubated in the presence of calcium as described in Figure 1 for an hour. As detailed in the Materials and methods section, the RL platelets were separated from unbound rFVIIa, then suspended in plasma or citrated saline to initiate the disassociation reaction. Portions of the reaction mixture were withdrawn and examined with Western analysis to quantify the amount of rFVIIa that remained associated with the RL platelets. Inset: Representative lanes from the Western analysis of rFVIIa~RL platelet conjugates one minute and two hours into the disassociation process.
Surfaced phosphatidylserine on RL platelets
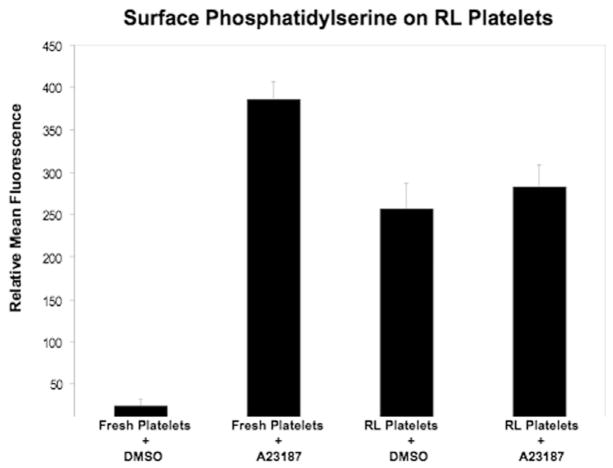
Flow cytometric analysis with annexin V (see Figure 3) revealed that the density of annexin V on the external surface of RL platelets was approximately 60% of that on fresh platelets activated with A23187. Treatment of RL platelets with the calcium ionophore did not result in a statistically significant exposure of additional phosphatidylserine. These results indicate that the association of rFVIIa with the surface membrane could be in part mediated by phosphatidylserine.
Figure 3.
Surface phosphatidylserine on RL platelets. Fresh and RL platelets were activated with A23178 or treated with DMSO carrier and then analysed with flow cytometry as detailed in the materials and methods section.
Functional activity for turning over the coagulation cascade
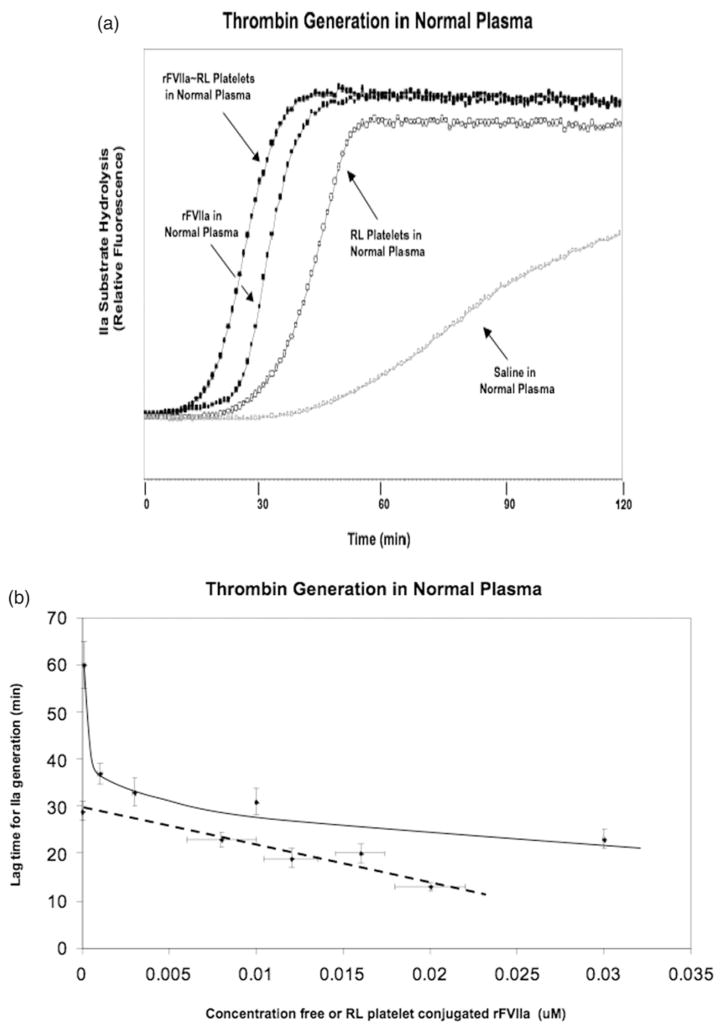
The functional activity of rFVIIa ~RL platelet conjugates for producing thrombin was examined by following the hydrolysis of a fluorometric thrombin substrate in plasma as detailed in the Materials and methods section. In preparation for these studies rFVIIa was bound to RL platelets as in Figure 1, then unbound rFVIIa was removed with a centrifugational wash. The data in Figure 4, Panel A, shows that when similar concentrations of free rFVIIa (0.03 uM) and RL platelet-bound rFVIIa (0.02 uM) were added to normal plasma, the lag time for thrombin generation was shortened; thrombin generation was faster when rFVIIa was localized to RL platelets as compared to free in solution. This effect was greatest (see Figure 4, Panel B) when lower concentrations of free and bound rFVIIa were compared. The slower thrombin generation that was measured with RL platelets alone, or with just plasma in the assay, was sensitive to corn trypsin inhibitor (data not shown), indicating that under these conditions thrombin generation was due to turnover of the intrinsic coagulation cascade.
Figure 4.
Thrombin generation in normal plasma. The effect of rFVIIa, RL platelets and rFVIIa ~RL platelet conjugates on thrombin generation in normal plasma was investigated by measuring the hydrolysis of the thrombin substrate D-phe-pro-arg-ANSNH to yield a fluorescent reaction product as detailed in the Material and methods section. rFVIIa ~RL platelet conjugates were prepared by mixing RL platelets and rFVII, then unassociated rFVIIa was removed as described in the Materials and methods section. The degree of rFVIIa modification of the RL platelets was ascertained with Western analysis. Panel A: Representative thrombin generation curves. Samples as indicated by arrows- rFVIIa ~RL platelets in normal plasma at 1.0 × 105/ul rFVIIa~RL platelets/ul for a total bound rFVIIa of 0.02 uM (filled circles); 0.03 uM rFVIIa in normal plasma (filled squares); RL platelets in normal plasma at 1.0 × 105/ul (open circles); negative control saline in normal plasma (open squares). Panel B: Dose-response relationship for effect of bound and free rFVIIa on thrombin generation in normal plasma- The concentration dependence of free rFVIIa (squares with solid line) or rFVIIa conjugated to RL platelets (circles with dashed line) on thrombin generation lag time was examined. The bound rFVIIa concentration was varied by increasing the density of rFVIIa on RL platelets; the concentration of RL platelets was held the same at 100 000 RL platelets/ul. Error bars in the vertical dimension indicate standard deviations of the triplicate measurements of the lag time for thrombin generation. Errors in the horizontal dimension represent liquid transfer errors (~1 ul) for free rFVIIa samples and deviation of duplicate rFVIIa determinations with Western blotting for conjugated rFVIIa.
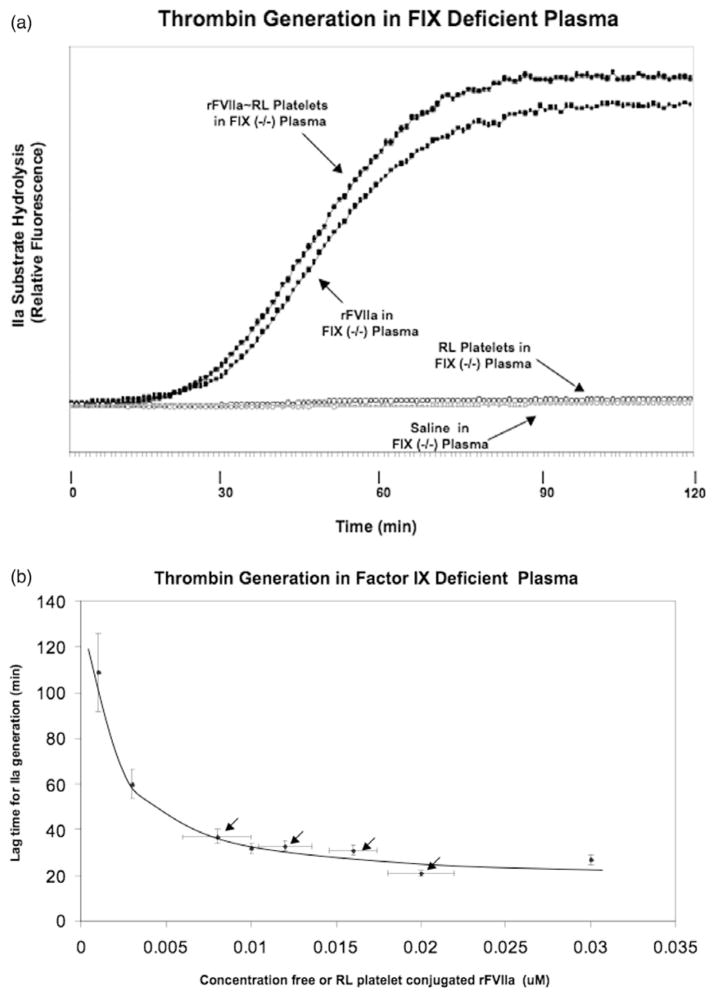
Free and RL platelet-bound rFVIIa accelerated thrombin generation in FIX-deficient plasma with similar kinetics (see Figure 5, Panel A). In contrast to normal plasma, the thrombin lag time for free (when added in the absence of RL platelets) and RL platelet conjugated rFVIIa in FIX deficient plasma were similar across a wide range of concentrations (see Figure 5, Panel B). In the absence of added (free or bound) rFVIIa thrombin generation was not measured during the two-hour experimental period (see Figure 5, Panel A).
Figure 5.
Thrombin generation in FIX deficient plasma. The effect of rFVIIa, RL platelets and rFVIIa ~RL platelet conjugates on thrombin generation in FIX deficient plasma was analysed as in Figure 6. Panel A: Representative thrombin generation curves. Samples as indicated by arrows- rFVIIa ~RL platelets in FIX deficient plasma at 1.0 ×105/ul rFVIIa ~RL platelets/ul for a total bound rFVIIa of 0.02 uM (filled circles); 0.03 uM rFVIIa in FIX deficient plasma (filled squares); RL platelets in FIX deficient plasma at 1.0 ×105/ul (open circles); negative control saline in FIX deficient plasma (open squares). Panel B: Dose-response relationship for effect of bound and free rFVIIa on thrombin generation in FIX deficient plasma. The concentration dependence of free rFVIIa (points not indicated with arrows) or rFVIIa conjugated to RL platelets (points indicated with arrows) on thrombin generation lag time was examined. Experimental details were the same as described for Figure 6, Panel B.
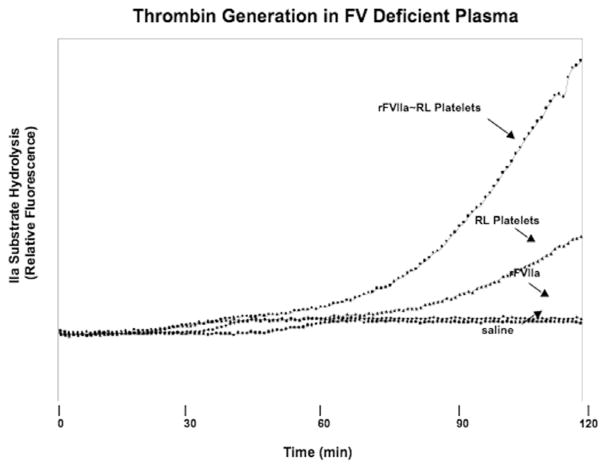
Thrombin generation experiments were performed with FV-deficient plasma to ascertain the effect of RL platelet-bound FV on coagulation cascade turnover. The data in Figure 6 show that the addition of free rFVIIa to FV-deficient plasma resulted in no measurable thrombin generation in two hours. In contrast, the addition of RL platelets generated thrombin with a lag time of approximately 75 minutes. When rFVIIa was pre-bound to the RL platelets, this lag time for thrombin generation was further reduced to approximately 50 minutes. These results confirm the importance of RL platelet-bound FV and FVa in the thrombin generation process.
Figure 6.
Thrombin generation in FV deficient plasma. The ability of rFVIIa ~RL platelet conjugates, rFVIIa, RL platelets and citrated saline (as indicated by arrows) to generate thrombin was analysed as described in Figure 5 (and detailed in the Materials and methods section) in FV deficient plasma.
Discussion
The hypothesis that was investigated in these studies was that rFVIIa can stably bind to the surfaces of RL platelets to be delivered in an active form to sites of vascular injury. The results presented here support this hypothesis in two ways. First, kinetic and equilibrium binding analysis showed that at super-physiological concentrations, rFVIIa rapidly associates with RL platelets to form complexes that are stable for long periods of time. The association and disassociation reaction kinetics reaction kinetics of rFVIIa with RL platelets were best described as a multi-step process involving at least one site with an affinity of approximately 110 nM. This affinity value is lower than the high affinity sites (and related high affinity processes) reported by others [7, 9, 29]. There could be two potential related explanations for these differences. First, we utilized super-physiological rFVIIa concentrations that were far higher than commonly used in previous studies. Secondly, the phosphatidylserine density on RL platelets is similar to that of a fully activated platelet. An affinity of 110 nM is not anticipated to be tight enough to establish the stable rFVIIa-platelet surface complexes that form with RL platelets, complexes that are tight enough to perform the centrifugational washes for disassociation reaction experiments and the thrombin generation studies. These data suggest a mixture of low affinity, calcium-dependent binding sites (possibly to phosphatidyoserine) and separate high affinity, calcium-independent binding interactions.
Current understanding of coagulation factor behavior on phosphatidylserine membranes suggests an explanation for the stability of the rFVIIa-RL platelet complex. The rFVIIa that initially binds to the platelet surface with an affinity of 110 nM might undergo one or more conformational changes for a higher affinity interaction. The observation of a very slow phase of the disassociation reaction measured in Figure 2 is consistent with this explanation; the loosely bound rFVIIa from the RL platelets disassociates within minutes, then the slower conformational transition from a more tightly bound state dominates the disassociation reaction kinetics. Several processes, that might include an insertion of hydrophobic amino acids into the membrane, additional coordination of gla-domains with the surface phosphatidylserine [30–34] and/or stable localization of rFVIIa to the surface connected open canalicular system, might be operant.
In vitro functional activity measurements provide a second finding that supports the hypothesis that rFVIIa-RL platelets will be useful for wound-site specific delivery of the coagulation factor. The finding that rFVIIa was catalytically active when associated with the RL platelet demonstrates that all (or at least for a subpopulation of the rFVIIa) catalytic site is exposed to the extracellular space. The results of functional activity measurements, based on thrombin generation kinetics, are consistent with previous studies which indicate that rFVIIa functions in coordination with platelets [7, 9, 29]. In normal plasma, rFVIIa pre-bound to RL platelets were found to catalyze thrombin generation more effectively than free rFVIIa (when added separate from the RL platelets), an effect that was most pronounced at lower rFVIIa concentrations. This result indicates that rFVIIa functions on the surface of RL platelets is at least additive with other mechanisms for RL platelet catalysis of the coagulation cascade, such as FXIIa-mediated contact activation [35, 36] and/or the functioning of rFVIIa as a “bypass factor” for factor Xa generation [7]. The observation that rFVIIa ~RL platelet conjugates restored partial thrombin generation in FV-deficient plasma, while free rFVIIa did not, indicates that the conjugated recombinant protein functionally interacts with FV and FVa that is pre-existing on the RL platelet membrane (as demonstrated with flow cytometry [37]) for formation of Xa/Va complex. In contrast to the enhanced activity of RL platelet-bound rFVIIa (as compared to free rFVIIa) that was measured in normal plasma at lower total rFVIIa concentrations (see Figure 4b), free and bound recombinant protein displayed similar catalytic functionality in FIX deficient plasma (see Figure 5b). This result indicates that the “bypass” activity of rFVIIa for factor Xa generation is rate limiting under these hemophilia conditions, and not downstream steps that occur on the platelet surface.
It is reasonable to predict that the primary advantage of a rFVIIa ~RL platelet-based therapeutic would be the ability of the RL platelets to concentrate the coagulation factor to sites of injury. Histological analysis of injured endothelium in vivo (e.g., in injured canine carotid arteries [23] or venipuncture sites in porcine jugular veins [38]) and in vitro (with Baumgartner analysis [39]) indicate that RL platelets localize to form a primary hemostatic plug for a local concentration of ≥107 RL platelets/ul (50× a normal platelet count of 200 000/ul). If each RL platelet surface-exposes 430 000 rFVIIa molecules (7.1 ×10−19 moles as obtained with the conditions in Figure 1), the local concentration of rFVIIa would be 7.1 uM. In contrast, if one therapeutic 4.8 mg (9.6 ×10−8 moles) dose of NovoSeven™ is infused into a typical human blood volume of four liters, the maximal concentration of the recombinant factor would be 2.4 ×10−10 molar, a value that is ~30 000 times lower than if the 7.1 uM rFVIIa was bound to RL platelets under conditions described in this study. The free rFVIIa would thus need to be concentrated by tissue factor at the sites of injury by a factor of ~30 000 to obtain the local concentration that RL platelets are expected to deliver. Thus, at least two clinical benefits would be gained by coupling rFVIIa to RL platelets. First, the therapeutic index would greatly increase due to the ability to concentrate rFVIIa at sites of vascular injury. A reduced systemic exposure to the recombinant factor might decrease the risk of prothrombotic complications. Secondly, the cost of such a therapeutic would be reduced, allowing for more wide spread use, particularly in combat casualty care and in clinical situations in less developed nations.
An alternative clinical paradigm for taking advantage of the affinity of rFVIIa for the RL platelets is to infuse the coagulation factor and rehydrated cells independently with the anticipation that the “on” reaction will occur at the wound site or in the systemic circulation. In this scenario, the rate of the “on” reaction is expected to be slower due to the dilution in the blood volume than if the two hemostatic agents were co-incubated at superphysiological concentrations before infusion. Pre-clinical animal studies and evaluations in the human clinic will better define the best dual use paradigms for rFVIIa and RL platelets.
In conclusion, the in vitro studies presented here show that rFVIIa binds to the RL platelet surface to form conjugates that are stable enough to be potentially useful for delivering active rFVIIa to sites of vascular injury. These studies indicate that rFVIIa-RL platelets are worthy of preclinical and clinical development as an infusion agent for severe bleeding.
Acknowledgments
The authors are grateful to Entegrion, Inc. for providing financial support for this research effort and to Novo Nordisk, Inc. for providing anti-rFVIIa antibodies, and to the Office of Naval Research staff for insightful discussions.
References
- 1.Hedner U, Glazer S, Pingel K, et al. (Successful use of recombinant factor VIIa in patient with severe haemophilia A during synovectomy. Lancet. 1988;2:1193. doi: 10.1016/s0140-6736(88)90259-0. [DOI] [PubMed] [Google Scholar]
- 2.Hedner U. Factor VIIa in the treatment of haemophilia. Blood Coagul Fibrinolysis. 1990;1:307–317. doi: 10.1097/00001721-199008000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Poon M-C, Demers C, Jobin F, Wu JWY. Recombinant factor VIIa is effective for bleeding and surgery in patients with Glanzmann Thrombasthenia. Blood. 1999;94:3951–3953. [PubMed] [Google Scholar]
- 4.Kristensen J, Killander A, Hippe E, et al. (Clinical experience with recombinant factor VIIa in patients with thrombocytopenia. Haemostasis. 1996;26 (Suppl 1):159–164. doi: 10.1159/000217260. [DOI] [PubMed] [Google Scholar]
- 5.Poon MC, d’Oiron R. Recombinant activated factor VII (NovoSeven) treatment of platelet-related bleeding disorders. International Registry on Recombinant Factor VIIa and Congenital Platelet Disorders Group. Blood Coagul Fibrinolysis. 2000;11 (Suppl 1):S55–68. [PubMed] [Google Scholar]
- 6.Tanaka KA, Waly AA, Cooper WA, Levy JH. Treatment of excessive bleeding in Jehovah’s Witness patients after cardiac surgery with recombinant factor VIIa (NovoSeven) Anesthesiology. 2003;98:1513–1515. doi: 10.1097/00000542-200306000-00034. [DOI] [PubMed] [Google Scholar]
- 7.Monroe DM, Hoffman M, Oliver JA, Roberts HR. A possible mechanism of action of activated factor VII independent of tissue factor. Blood Coagul Fibrinolysis. 1998;9 (Suppl 1):S15–20. [PubMed] [Google Scholar]
- 8.Monroe D, Hoffman M, Allen M, Roberst H. The factor VII-platelet interplay: Effectiveness of recombinant factor VIIa in the treatment of bleeding in severe thrombocytopenia. Sem Thromb Hemo. 2000;26:373–377. doi: 10.1055/s-2000-8455. [DOI] [PubMed] [Google Scholar]
- 9.Gabriel DA, Li X, Monroe DM, 3rd, Roberts HR. Recombinant human factor VIIa (rFVIIa) can activate factor FIX on activated platelets. J Thromb Haemost. 2004;2:1816–1822. doi: 10.1111/j.1538-7836.2004.01015.x. [DOI] [PubMed] [Google Scholar]
- 10.Blatt J, Gold S, Wiley J, Monahan P, Cooper H, Harvey D. Off-label use of recombinant factor VIIa in patients following bone marrow transplantation. Bone Marrow Transplantation. 2001;28:405–407. doi: 10.1038/sj.bmt.1703157. [DOI] [PubMed] [Google Scholar]
- 11.Aldouri M. The use of recombinant factor VIIa in controlling surgical bleeding in non-haemophiliac patients. Pathophysiol Haemost Thromb. 2002;32 (Suppl 1):41–46. doi: 10.1159/000057301. [DOI] [PubMed] [Google Scholar]
- 12.Dutton RP, Hess JR, Scalea TM. Recombinant factor viia for control of hemorrhage: Early experience in critically ill trauma patients. J Clin Anesth. 2003;15:184–188. doi: 10.1016/s0952-8180(03)00034-5. [DOI] [PubMed] [Google Scholar]
- 13.Martinowitz U. The use of rFVIIa as an adjunct treatment for hemorrhage control in trauma and surgery. Bloodline Rev. 2001;1:9–11. [Google Scholar]
- 14.Bode A. Platelet activation may explain the storage lesion in platelet concentrates. Blood Cells. 1990;16:109–126. [PubMed] [Google Scholar]
- 15.Blajchman M. Bacterial contamination of blood products and the value of pre-transfusion testing. Immun Invest. 1995;24:163–170. doi: 10.3109/08820139509062770. [DOI] [PubMed] [Google Scholar]
- 16.Blajchman M. Reducing the risk of bacterial contamination of cellular blood components. Dev Biol Std. 2000;102:183–193. [PubMed] [Google Scholar]
- 17.Read MS, Reddick RL, Bode AP, et al. (Preservation of hemostatic and structural properties of rehydrated lyophilized platelets: Potential for long-term storage of dried platelets for transfusion. Proc Natl Acad Sci. 1995;92:397–401. doi: 10.1073/pnas.92.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bode A, Read M, Summaria L. Lyophilized platelets: Nearing clinical trials. Platelets. 1997;8:436–437. [Google Scholar]
- 19.Bode A, Read M, Reddick R. Activation and adherence of lyophilized human platelets on canine vessel strips in the Baumgartner perfusion chamber. J Lab Clin Med. 1999;133:200–211. doi: 10.1016/s0022-2143(99)90013-6. [DOI] [PubMed] [Google Scholar]
- 20.Fischer TH, Khandelwas G, Merricks E, Raymer R, Nichols T, Bode A, Bellinger D, Russel K, Reddick R, Sanders W, et al. (Thrombus formation and lysis with rehydrated, lyophilized platelets. Hematology. 2002;7:359–369. doi: 10.1080/1024533021000047954. [DOI] [PubMed] [Google Scholar]
- 21.Fischer TH, Merricks EP, Russell KE, et al. (Intracellular function in rehydrated lyophilized platelets. Br J Haematol. 2000;111:167–174. doi: 10.1046/j.1365-2141.2000.02343.x. [DOI] [PubMed] [Google Scholar]
- 22.Bode AaRMS. Lyophilized platelets for transfusion. In: Seghatchian E, JaS, editors. Platelet therapy: Current status and future challenges. Elsevier Sci; Amsterdam: 2001. pp. 132–167. [Google Scholar]
- 23.Read MS, Reddick RL, Bode AP, et al. Preservation of hemostatic and structural properties of rehydrated lyophilized platelets: Potential for long-term storage of dried platelets for transfusion. Proc Natl Acad Sci USA. 1995;92:397–401. doi: 10.1073/pnas.92.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fischer TH, Merricks E, Raymer R, Nichols T, Hayes P, Bode A, Pearce L, Manning J. The co-infusion of rehydrated lyopholized platelets wth HBOC-201 for hemostasis in dilutional thrombocytopenia. Blood. 2001b;98:2250. [Google Scholar]
- 25.Fischer TH, Merricks EP, Bode AP, et al. (Thrombus formation with rehydrated, lyophilized platelets. Hematology. 2002;7:359–369. doi: 10.1080/1024533021000047954. [DOI] [PubMed] [Google Scholar]
- 26.Khandelwal G, Sanders W, Bode A, Nichols T, Erickson G, Read M. von Willebrand factor binding to rehydrated lophilized platelet surface GP1b and inhibition by monoclonal antibody to GP1b. FASEB J. 1997;11:1812. [Google Scholar]
- 27.Fischer TH, Bode AP, Parker BR, et al. (Primary and secondary hemostatic functionalities of rehydrated, lyophilized platelets. Transfusion. 2006;46:1943–1950. doi: 10.1111/j.1537-2995.2006.01002.x. [DOI] [PubMed] [Google Scholar]
- 28.Bode AP, Hickerson DH. Characterization and quantitation by flow cytometry of membranous microparticles formed during activation of platelet suspensions with ionophore or thrombin. Platelets. 2000;11:259–271. doi: 10.1080/09537100050129279. [DOI] [PubMed] [Google Scholar]
- 29.Monroe DM, Hoffman M, Oliver JA, Roberts HR. Platelet activity of high-dose factor VIIa is independent of tissue factor. Br J Haematol. 1997;99:542–547. doi: 10.1046/j.1365-2141.1997.4463256.x. [DOI] [PubMed] [Google Scholar]
- 30.Hagen FS, Gray CL, O’Hara P, et al. (Characterization of a cDNA coding for human factor VII. Proc Natl Acad Sci. 1986;83:2412–2416. doi: 10.1073/pnas.83.8.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soriano-Garcia M, Padmanabhan K, de Vos AM, Tulinsky A. The Ca2+ ion and membrane binding structure of the Gla domain of Ca-prothrombin fragment 1. Biochemistry. 1992;31:2554–2566. doi: 10.1021/bi00124a016. [DOI] [PubMed] [Google Scholar]
- 32.Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the calcium ion-bound gamma-carboxyglutamic acid-rich domain of factor IX. Biochemistry. 1995;34:12126–12137. doi: 10.1021/bi00038a005. [DOI] [PubMed] [Google Scholar]
- 33.Ellison EH, Castellino FJ. Adsorption of vitamin K-dependent blood coagulation proteins to spread phospholipid monolayers as determined from combined measurements of the surface pressure and surface protein concentration. Biochemistry. 1998;37:7997–8003. doi: 10.1021/bi973118+. [DOI] [PubMed] [Google Scholar]
- 34.Huang M, Rigby AC, Morelli X, et al. (Structural basis of membrane binding by Gla domains of vitamin K-dependent proteins. Nat Struct Biol. 2003;10:751–756. doi: 10.1038/nsb971. [DOI] [PubMed] [Google Scholar]
- 35.Colman RW, Schmaier AH. The contact activation system: Biochemistry and interactions of these surface-mediated defense reactions. Crit Rev Oncol Hematol. 1986;5:57–85. doi: 10.1016/s1040-8428(86)80053-1. [DOI] [PubMed] [Google Scholar]
- 36.Fischer TH, Thatte HS, Nichols TC, Bender-Neal DE, Bellinger DA, Vournakis JN. Synergistic platelet integrin signaling and factor XII activation in poly-N-acetyl glucosamine fiber mediated hemostasis. Biomaterials. 2005;26:5433–5443. doi: 10.1016/j.biomaterials.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 37.Valeri CR, Macgregor H, Barnard MR, Summaria L, Michelson AD, Ragno G. In vitro testing of fresh and lyophilized reconstituted human and baboon platelets. Transfusion. 2004;44:1505–1512. doi: 10.1111/j.1537-2995.2004.04135.x. [DOI] [PubMed] [Google Scholar]
- 38.Fischer TH, Merricks E, Raymer R, Nichols T, Hayes P, Bode A, Pearce L, Manning J. The co-infusion of rehydrated lyopholized platelets wth HBOC-201 for hemostasis in dilutional thrombocytopenia. Blood. 2001;98:2250. [Google Scholar]
- 39.Bode AP, Read MS, Reddick RL. Activation and adherence of lyophilized human platelets on canine vessel strips in the Baumgartner perfusion chamber. J Lab Clin Med. 1999;133:200–211. doi: 10.1016/s0022-2143(99)90013-6. [DOI] [PubMed] [Google Scholar]