Abstract
3-Hydroxypropylmercapturic acid (3-HPMA) and 3-hydroxy-1-methylpropylmercapturic acid (HMPMA) are urinary metabolites of the toxicants acrolein and crotonaldehyde, respectively. Virtually all human urine samples contain these metabolites, resulting from the action of glutathione-S-transferases on acrolein and crotonaldehyde, which are lipid peroxidation products, environmental and dietary contaminants, and constituents of cigarette smoke. We have developed a high throughput liquid chromatography-tandem mass spectrometry method for quantitative analysis of 3-HPMA and HMPMA in large numbers of small urine samples, as would be required in molecular epidemiology and clinical studies relating levels of these metabolites to cancer risk. Solid-phase extraction on mixed mode reverse phase-anion exchange 96-well plates provided sufficient purification for LC-MS/MS analysis, which was performed by auto-injection using a 96-well format, and resulted in clean, readily interpretable chromatograms, with detection limits of 4.5 pmol/mL urine for 3-HPMA and 3.5 pmol/mL urine for HMPMA. Accuracy was 92% for 3-HPMA and 97% for HMPMA while inter-day precision was 9.1% (coefficient of variation) for 3-HPMA and 11.0% for HMPMA. The method was applied to more than 2600 urine samples from smokers; mean levels of 3-HPMA and HMPMA were 4800 ± 5358 (S.D.) pmol/ml and 3302 ± 3341 pmol/ml, respectively.
Keywords: High throughput, Liquid chromatography-tandem mass spectrometry, Mercapturic acids, Acrolein, Crotonaldehyde
1. Introduction
The hazards of cigarette smoking with respect to cancer risk are now so well documented that they are apparent to virtually everyone [1,2]. Smoking is by far the major cause of lung cancer, a rapidly fatal disease which kills more than 1.3 million people in the world each year [3]. Possibly less well known, smoking is also an established cause - to varying extents - of at least 18 other types of cancer [1,2]. While these dangers are widely appreciated, a significant question with respect to cancer prevention remains largely unanswered: which smoker is susceptible to cancer? Only 11–25% of lifetime smokers will get lung cancer [1]. If the vulnerable smokers could be identified at an early stage of their addiction, intensive cessation therapy and surveillance could be initiated, likely decreasing the probability of a fatal cancer. One approach to identifying susceptible smokers uses tobacco carcinogen and toxicant biomarkers [4]. These metabolites of tobacco smoke compounds could conceivably provide a metric of high risk. Rapid and reliable methods for quantitation of these biomarkers are necessary for their application in large prospective epidemiology studies which can potentially validate them as predictors of cancer susceptibility.
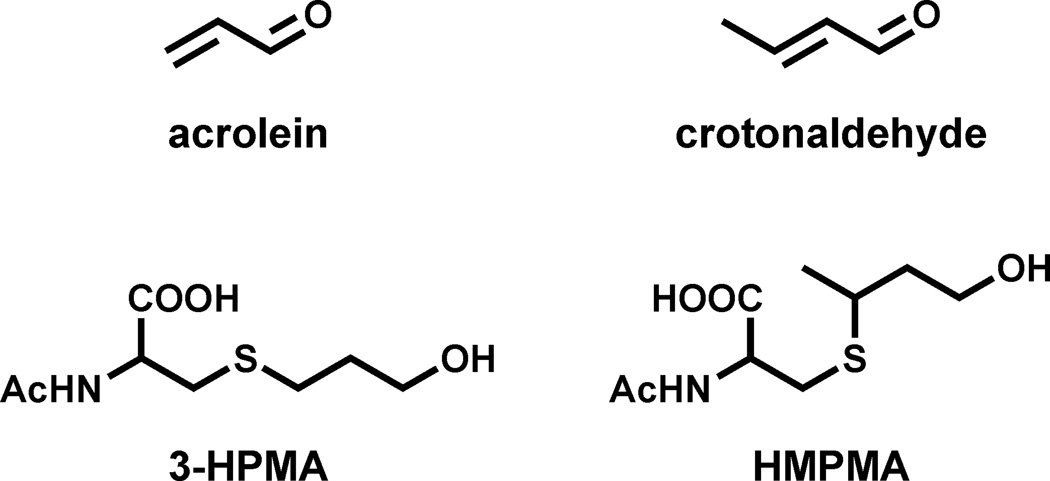
The study reported here focuses on mercapturic acids of acrolein and crotonaldehyde (Figure 1), two important constituents of cigarette smoke. Levels of acrolein in cigarette smoke are typically 50 – 220 µg per cigarette while those of crotonaldehyde have been reported as 11 – 66 µg per cigarette [1,5,6]. Acrolein is toxic to the cilia of the lung, thus contributing to lowered defenses against other noxious agents in smoke [7]. Acrolein, while not generally considered carcinogenic, does react with DNA producing a spectrum of damage in the p53 tumor suppressor gene similar to that observed in lung tumors from smokers [8–11]. Crotonaldehyde is a relatively weak hepatocarcinogen [12]. Both acrolein and crotonaldehyde-DNA adducts have been identified and quantified in DNA from human lung tissue [13,14]. Thus, these compounds could be important in tobacco carcinogenesis. Beyond that, all humans are exposed to acrolein and crotonaldehyde from endogenous lipid peroxidation as well as the diet and general environment [15–17]. Acrolein and crotonaldehyde are both scavenged by cellular glutathione, ultimately resulting in the excretion of the mercapturic acids 3-hydroxypropylmercapturic acid (3-HPMA) and 3-hydroxy-1-methylpropylmercapturic acid (HMPMA, Figure 1) [18–20]. These metabolites are present in virtually all human urine samples, but their levels are substantially higher in smokers than non-smokers, and decrease significantly upon smoking cessation [21].
Figure 1.
Structures of acrolein, crotonaldehyde, and the corresponding mercapturic acid metabolites, 3-HPMA and HMPMA, respectively.
Multiple previous studies have quantified 3-HPMA in human urine using various purification and LC-MS/MS approaches including solid-phase extraction of various types, hydrophilic interaction liquid chromatography, and ultra high performance liquid chromatography with detection by negative or positive APCI as well as negative ESI, but we are not aware of any studies in the literature that have described the details of a high throughput approach to this analysis [2,21–35]. Methods for quantitation of HMPMA in human urine have been infrequently described [21,36]. In the study reported here, we have developed a high throughput method for the analysis of 3-HPMA and HMPMA in human urine, using small samples. The method is applicable in large scale molecular epidemiology studies.
2. Materials and Methods
2.1. Urine Samples
This study was approved by the University of Minnesota Institutional Review Board. The samples originated from the Multi-Ethnic Cohort Study, an epidemiologic investigation into differences in cancer risk among U.S. racial/ethnic groups, carried out by investigators at the University of Southern California and the University of Hawaii. All of the urine samples analyzed here were from smokers. The samples were collected between 2001 and 2006, stored at −80 °C, and aliquots were shipped frozen to the University of Minnesota, where they were kept at −20 °C until analysis.
2.2. Materials
3-HPMA, [N-acetyl-D3]3-HPMA dicyclohexylamine salt, HMPMA dicyclohexylamine salt, and [N-acetyl-D3]HMPMA dicyclohexylamine salt were obtained from Toronto Research Chemicals. Initial stocks, 1 µg/µL, were made in DMSO. Oasis MAX mixed mode reverse phase anion exchange solid-phase extraction (60 mg, 60 µ, 2 mL reservoir) 96-well plates were procured from Waters Corp. Square 2 mL 96-well plates with 100 µL tapered reservoirs were purchased from Analytical Sales and Service. Sealing mats for 96-well plates, a 50 mm × 3.0 mm Synergi Max-RP C12, 2.5µ, 100Å HPLC column, and a guard column were obtained from Phenomenex. A Cerex 96 well positive pressure processor and an 8 channel Eppendorf electronic multipipetter were used to process the 96-well plates. Molnar Institute Dry Lab 2010 software for HPLC optimization assisted in the development of the chromatography parameters. All other materials and chemicals were purchased from U-Stores, University of Minnesota. All solutions and buffers were prepared freshly on the same day as the assay.
2.3. Synthesis and NMR Analysis of HMPMA Diastereomers
Crotonaldehyde (0.85 g, 0.012 mol) was added to a solution of N-acetyl(S)-cysteine (1.97 g, 0.012 mol) in methanol (25 mL) and the mixture was stirred at room temperature for 1.5 h. The pH was adjusted to 12 with aqueous NaOH, and NaBH4 (500 mg) was added. After 20 min, the solution was acidified with aqueous HCl, and HMPMA was extracted into ethyl acetate. HMPMA diastereomers were collected by HPLC using the Synergi column described above with elution by isocratic 95% 15 mM NH4OAc, 5% methanol. NMR spectra were determined with a Varian Inova 500 MHz instrument. Assignments were confirmed by 1H-1H COSY, 1H-13C HSQC, and 1H-13C HMBC.
HMPMA isomer 1: 3-hydroxy-1-(S or R)-methylpropylmercapturic acid (retention time, 7.11 min): 1H NMR (500 MHz, DMSO-d6): δ 8.24-8.21 (m, 1H, -NH-), 4.40-4.33 (m, 1H, -CHCOOH), 4.18 (t, J = 7.5 Hz, 1H, -OH), 3.56-3.43 (m, 2H, -CH2OH), 2.94-2.87 (m, 2H, -CHSCH2-), 2.76-2.71 (m, 1H, -SCH2), 1.86 (s, 3H, -COCH3), 1.82-1.77 (m, 1H, -CH2CH2CH-), 1.65-1.53 (m, 1H, -CH2CH2CH-), 1.27-1.21 (m, 3H, -CHCH3). LC-MS (APCI): calc’d for [C9H16NO4S]− [M − H]− = 234.08, found m/z = 234.08.
HMPMA isomer 2: 3-hydroxy-1-(S or R)-methylpropylmercapturic acid (retention time, 7.85 min): 1H NMR (500 MHz, DMSO-d6): δ 8.26-8.21 (m, 1H, -NH-), 4.39-4.33 (m, 1H, -CHCOOH), 4.19 (t, J = 7.5 Hz, 1H, -OH), 3.57-3.45 (m, 2H, -CH2OH), 2.94-2.85 (m, 2H, -CHSCH2-), 2.77-2.71 (m, 1H, -SCH2-), 1.86 (s, 3H, -COCH3), 1.84-1.76 (m, 1H, -CH2CH2CH-), 1.69-1.51 (m, 1H, -CH2CH2CH-), 1.27-1.21 (m, 3H, -CHCH3). LC-MS (APCI): calc’d for [C9H16NO4S]− [M − H]− = 234.08, found m/z = 234.08.
2.3.1. Analysis of 3-HPMA and HMPMA in Smokers’ Urine
Urine samples (0.4 mL) were distributed into the square 2 mL 96-well plates, and a mixture of internal standards consisting of 100 ng [N-acetyl-D3]3-HPMA and 500 ng [N-acetyl-D3]HMPMA in 0.1 mL H2O was added carefully to each well. The plate was capped and vortexed gently after the addition of the internal standard mixture was complete.
The solid-phase extraction 96-well plate was pre-conditioned with 0.7 mL MeOH and 0.7 mL 2% NH4OH at a flow rate of about one drop per second. The plate containing the samples was warmed to 50 °C in an oven to dissolve any precipitate and applied to the solid-phase extraction plate with a multi-channel pipettor. The plate was washed with 0.7 mL 2% NH4OH and 0.7 mL MeOH, then dried with a stream of N2 for 5 min. Then, the plate was washed with 0.7 mL of 2% aqueous formic acid, which was discarded. Finally, the plate was washed with 0.7 mL of 30% MeOH in 2% aqueous formic acid and the eluants were collected in the square 2 mL 96-well plates.
The solvents were removed on a Speedvac without using heat, a process that can take up to 2 days. The dry 96-well collection plate was covered with a sealing mat and stored at −20 °C until LC-MS/MS analysis. Prior to analysis the samples were re-dissolved with sonication in 10 µL of methanol and then 40 µL of 15 mM ammonium acetate was added. The sample plate was centrifuged briefly on the Speedvac concentrator to deposit particulates and 2 – 5 µL of 40 µL were injected.
LC-MS/MS analyses were carried out on an Agilent 1100 HPLC system (Agilent Technologies) coupled to a TSQ Quantum Discovery Max instrument (Thermo Scientific) in the negative ion APCI mode. The HPLC solvent system was 98% 15 mM NH4OAc, pH 6.8, and 2% MeOH held for 2 min then ramped to 12% MeOH in an additional 6.5 min, then to 70% MeOH in 2.5 min, and then 2% MeOH in 0.5 min and held for 6 min. The flow rate was 0.4 mL/min and the column temperature was 25 °C. The collision energy was 13V; the peak width parameters were Q1, 0.7; Q3, 0.7; the scan width was 0.40 m/z; and the scan time was 0.1 sec. The transitions monitored were m/z 220 → m/z 91 for 3-HPMA, m/z 223 → m/z 91 for [N-acetyl-D3]3-HPMA, m/z 234 → m/z 105 for HMPMA, and m/z 237 → m/z 105 for [N-acetyl-D3]HMPMA.
Analytes in the samples were quantified from linear calibration curves relating the ratio of varying amounts of 3-HPMA or HMPMA and a constant amount of [N-acetyl-D3]3-HPMA or [N-acetyl-D3]HMPMA to the area ratio of the appropriate analyte:internal standard MS/MS transitions. Calibration curves were established before each set of LC-MS/MS analyses. The same sample of [N-acetyl-D3]3-HPMA or [N-acetyl-D3]HMPMA used for constructing the calibration curve was also added to each sample as internal standard.
3. Results
The method reported here was designed to accommodate analysis of large numbers of relatively small (0.4 mL) urine samples. The samples and internal standards were distributed into 96-well plates, each containing positive and negative control wells to assess assay performance. Solid-phase extraction on mixed mode reverse phase-anion exchange plates provided sufficient purification for LC-MS/MS analysis, which was performed by auto-injection using a 96-well format.
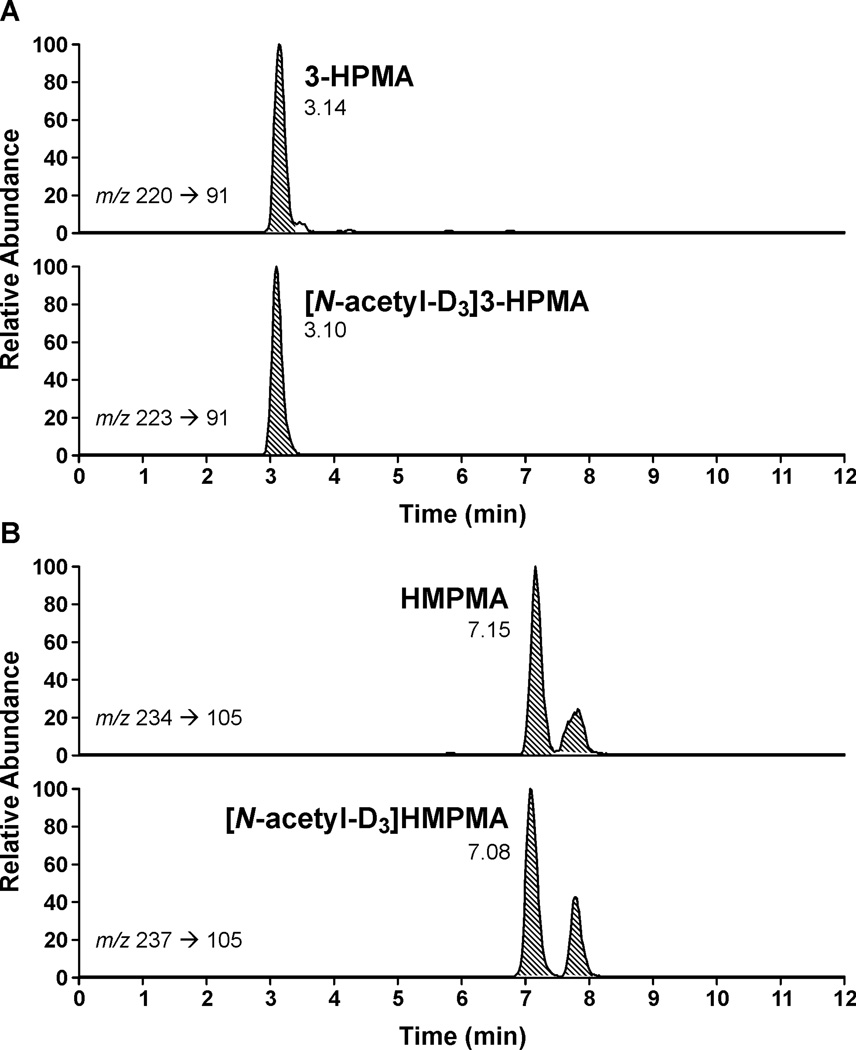
The transitions monitored, m/z 220 → m/z 91 for 3-HPMA and m/z 234 → m/z 105 for HMPMA, result from loss of -CH2CH(COOH)NHCOCH3 with negative charge retention on sulfur. Clear, readily quantifiable peaks were obtained, as illustrated in Figure 2A,B. In all cases, two peaks were observed for HMPMA. Proton NMR spectra of HMPMA, synthesized by reaction of crotonaldehyde with N-acetyl(S)-cysteine, followed by reduction with NaBH4, confirmed that these two peaks are the two diastereomers of HMPMA resulting from addition of N-acetyl(S)-cysteine to carbon 3 of crotonaldehyde, followed by reduction. In some samples, a peak eluted just after 3-HPMA. This has been identified by comparison to a standard as 2-HPMA, resulting from mercapturic acid formation from propylene oxide. A method for the quantitative analysis of 2-HPMA in urine will be described separately.
Figure 2.
Typical LC-MS/MS chromatograms obtained upon the analysis of human urine samples for A, 3-HPMA and B, HMPMA. Upper panels, analytes; lower panels, internal standards. The transitions monitored were m/z 220 → m/z 91 for 3-HPMA, m/z 223 → m/z 91 for [N-acetyl-D3]3-HPMA, m/z 234 → m/z 105 for HMPMA, and m/z 237 → m/z 105 for [N-acetyl-D3]HBMA. The two peaks in the HMPMA chromatograms have been identified here as diastereomers of HMPMA.
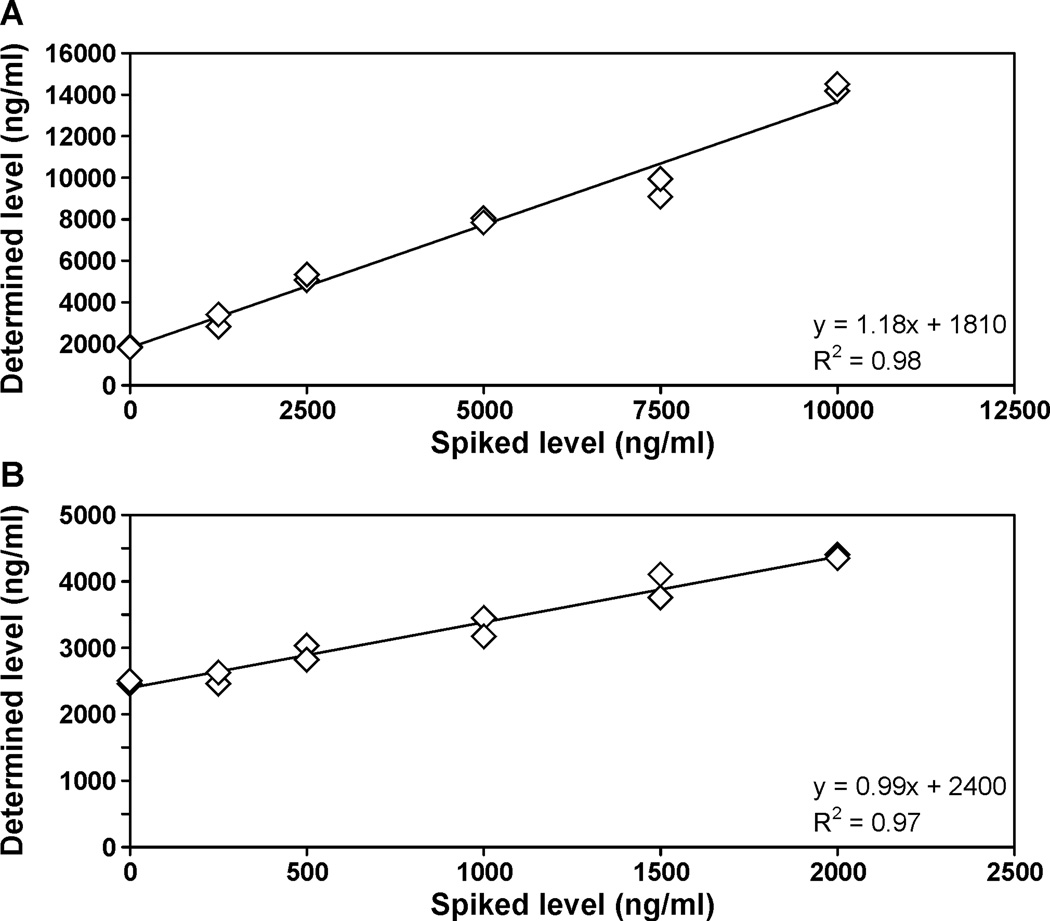
Calibration curves for 3-HPMA and HMPMA were linear in the range in which the analytes were quantified (R2 = 0.99). The on-column detection limits were approximately 0.2 pmol for 3-HPMA and 0.08 pmol for HMPMA. The detection limits in urine samples were approximately 4.5 pmol/mL (0.99 ng/mL) for 3-HPMA and 3.5 pmol/mL (0.82 ng/mL) for HMPMA, starting with 0.4 mL of urine. The limits of quantitation were estimated to be 15 pmol/mL for 3-HPMA and 12 pmol/mL for HMPMA. Accuracy was determined by adding increasing amounts of 3-HPMA or HMPMA to a pooled smokers’ urine sample and carrying out the analysis. The results are presented in Figure 3A,B. The correlation coefficients between the expected and observed values were R2 = 0.98 and R2 = 0.97 for 3-HPMA and HMPMA, respectively, and accuracy was 92% for 3-HPMA and 97% for HMPMA. Precision was evaluated from the positive control samples included with each 96-well plate. The inter-day precision data were 9.1% (coefficient of variation) for 3-HPMA and 11.0% for HMPMA. These validation data are summarized in Table 1.
Figure 3.
Determination of the accuracy of the A, 3-HPMA and B, HMPMA analyses. A pooled smokers’ urine sample was spiked with five increasing amounts of the appropriate analyte and the analysis was carried out as described in Materials and Methods.
Table 1.
Validation data for the analysis of 3-HPMA and HMPMAa
| Limit of detection (pmol/mL urine) |
Limit of quantitation (pmol/mL urine) |
Precision (CV, %) | Accuracy (%) | ||
|---|---|---|---|---|---|
| Intra-day | Inter-day | ||||
| 3-HPMA | 4.5 | 15 | 5.3 | 9.1 | 92 |
| HMPMA | 3.5 | 12 | 6.7 | 11.0 | 97 |
Starting with 0.4 mL urine
Mean (± S.D.) and median levels of 3-HPMA in the 2613 smokers’ urine samples analyzed here were 4800 ± 5358 pmol/ml and 3392 pmol/ml, respectively, while the corresponding values for HMPMA were 3302 ± 3341 pmol/ml and 2360 pmol/ml. There were no samples below the limit of quantitation. Levels of 3-HPMA and HMPMA correlated, R2 = 0.67.
4. Discussion
This paper presents accurate, precise, and robust high throughput methodology for the determination of 3-HPMA and HMPMA in small samples of human urine. The method has been tested and validated with spiked smokers’ urine samples and positive control samples. It was applied in the ongoing Multi-Ethnic Cohort study of smokers from five different ethnic groups in which more than 2600 urine samples, each with a total volume of 0.4 mL, were analyzed for these two metabolites. The statistical analysis of 3-HPMA and HMPMA levels with respect to ethnicity, genetic polymorphisms, and lung cancer risk in the Multi-Ethnic Cohort study will be described in a separate publication.
While numerous methods have been described for the quantitation of urinary 3-HPMA, this is, to our knowledge, the first to present details of a 96-well format approach [4]. With respect to HMPMA, the literature is relatively sparse. We have confirmed the identity of the diastereomeric peaks observed in this analysis and provide methodology appropriate for large studies.
The mean amount of 3-HPMA reported here, 4800 pmol/ml (2900 –7700 nmol/24h based on urine excretion of 0.6 – 1.6 L per day) is somewhat less than the 9180 nmol/24 h reported in the “total exposure study” of 3585 smokers or than our own previous data of 10,020 nmol/24h in smokers [21,37]. The mean amount of HMPMA, 3302 pmol/mL (1800 – 5300 nmol/24h) compares to values of 9825 – 26,000 nmol/24h in smokers in the relatively few studies of HMPMA reported in the literature [4]. The relatively lower values observed in the present study may relate to the fact that most of the subjects in this study were fairly light smokers, smoking less than 15 cigarettes per day.
One previous study has examined the relationship of mercapturic acid metabolites of acrolein and crotonaldehyde, as well as benzene, 1,3-butadiene, and ethylene oxide to lung cancer in smokers [38]. Based on urine samples collected prospectively from 343 lung cancer cases and 392 controls, all smokers, in the Shanghai Cohort Study, 3-HPMA and HMPMA as well as the other mercapturic acids, were not independent risk predictors of lung cancer after adjustment for urinary cotinine, which was an independent risk factor. Further large scale studies are required to confirm this finding. As in the current study, levels of 3-HPMA and HMPMA correlated.
In addition to the Multi-Ethnic Cohort study, the present method is being applied in a large clinical study examining the effects of consumption of compounds derived from broccoli sprouts on mercapturic acid metabolism of acrolein and crotonaldehyde. In a preliminary study, we observed statistically significant increases in the levels of these urinary metabolites after consumption of two broccoli sprout-derived beverages, one rich in sulforaphane and the other in glucoraphanin [39]. This is consistent with the known ability of sulforaphane to induce phase II enzymes such as glutathione-S-transferases involved in the mercapturic acid pathway.
In summary, the method described here will be applicable to large clinical studies analyzing the urinary mercapturic acid metabolites of the toxicants acrolein and crotonaldehyde. This will be important in assessing further their possible contribution to a predictive algorithm for lung cancer susceptibility in smokers, as well as for other studies involving modulation of the mercapturic acid pathway.
Highlights.
A method to analyze mercapturic acids of acrolein and crotonaldehyde is described
The method was validated for accuracy and precision
The method was applied in the analysis of more than 2600 urine samples from smokers
Mean smokers’ urinary levels were 4800±5358 (3-HPMA) and 3302±3341 pmol/ml (HMPMA)
Acknowledgements
This study was supported by grant PO1 CA-138338 from the National Cancer Institute. Mass spectrometry was carried out in the Analytical Biochemistry Shared Resource of the Masonic Cancer Center, University of Minnesota, funded in part by Cancer Center Support Grant CA-77598. We thank Peter Villalta and Brock Matter for help with mass spectrometry, and Bob Carlson for editorial assistance.
References
- 1.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 2004;Vol. 83:33–1187. [PMC free article] [PubMed] [Google Scholar]
- 2.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. v. 100E. Lyon, FR: IARC; 2012. pp. 43–167. [Google Scholar]
- 3.World Health Organization. Cancer Fact Sheet No. 297. 2012 Feb; http://www.who.int/mediacentre/factsheets/fs297/en/index.html 2012. [Google Scholar]
- 4.Hecht SS, Yuan J-M, Hatsukami DK. Chem. Res. Toxicol. 2010;23:1001–1008. doi: 10.1021/tx100056m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roemer E, Stabbert R, Rustemeier K, Veltel DJ, Meisgen TJ, Reininghaus W, Carchman RA, Gaworski CL, Podraza KF. Toxicology. 2004;195:31–52. doi: 10.1016/j.tox.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Counts ME, Hsu FS, Laffoon SW, Dwyer RW, Cox RH. Regul. Toxicol. Pharmacol. 2004;39:111–134. doi: 10.1016/j.yrtph.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Kensler CJ, Battista SP, Engl N. J. Med. 1963;269:1161–1166. doi: 10.1056/NEJM196311282692202. [DOI] [PubMed] [Google Scholar]
- 8.Chung FL, Young R, Hecht SS. Cancer Res. 1984;44:990–995. [PubMed] [Google Scholar]
- 9.Minko IG, Kozekov ID, Harris TM, Rizzo CJ, Lloyd RS, Stone MP. Chem Res Toxicol. 2009;22:759–778. doi: 10.1021/tx9000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emami A, Dyba M, Cheema AK, Pan J, Nath RG, Chung FL. Anal. Biochem. 2008;374:163–172. doi: 10.1016/j.ab.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng Z, Hu W, Hu Y, Tang MS. Proc. Natl. Acad. Sci. USA. 2006;103:15404–15409. doi: 10.1073/pnas.0607031103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung FL, Tanaka T, Hecht SS. Cancer Res. 1986;46:1285–1289. [PubMed] [Google Scholar]
- 13.Zhang S, Villalta PW, Wang M, Hecht SS. Chem. Res. Toxicol. 2006;19:1386–1392. doi: 10.1021/tx060154d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang S, Villalta PW, Wang M, Hecht SS. Chem. Res. Toxicol. 2007;20:565–571. doi: 10.1021/tx700023z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stevens JF, Maier CS. Mol Nutr. Food Res. 2008;52:7–25. doi: 10.1002/mnfr.200700412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung FL, Zhang L, Ocando JE, Nath RG. In: Exocyclic DNA Adducts in Mutagenesis and Carcinogenesis, International Agency for Research on Cancer. Singer B, Bartsch H, editors. Lyon, France: 1999. pp. 45–54. [Google Scholar]
- 17.Pan J, Chung FL. Chem. Res. Toxicol. 2002;15:367–372. doi: 10.1021/tx010136q. [DOI] [PubMed] [Google Scholar]
- 18.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 63. Lyon, France: IARC; 1995. pp. 337–372. [Google Scholar]
- 19.Parent RA, Paust DE, Schrimpf MK, Talaat RE, Doane RA, Caravello HE, Lee SJ, Sharp DE. Toxicol. Sci. 1998;43:110–120. doi: 10.1006/toxs.1998.2462. [DOI] [PubMed] [Google Scholar]
- 20.Perbellini L, Veronese N, Princivalle A, Chromatogr J. B Analyt. Technol. Biomed. Life Sci. 2002;781:269–290. doi: 10.1016/s1570-0232(02)00501-9. [DOI] [PubMed] [Google Scholar]
- 21.Carmella SG, Chen M, Han S, Briggs A, Jensen J, Hatsukami DK, Hecht SS. Chem. Res. Toxicol. 2009;22:734–741. doi: 10.1021/tx800479s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stanek W, Krenmayr P, Scherer G, Schmid ER. Biol. Mass Spectrom. 1993;22:133–142. doi: 10.1002/bms.1200220206. [DOI] [PubMed] [Google Scholar]
- 23.Mascher DG, Mascher HJ, Scherer G, Schmid ER, Chromatogr J. B Biomed. Sci. Appl. 2001;750:163–169. doi: 10.1016/s0378-4347(00)00385-6. [DOI] [PubMed] [Google Scholar]
- 24.Scherer G, Engl J, Urban M, Gilch G, Janket D, Riedel K. Regul. Toxicol. Pharmacol. 2007;47:171–183. doi: 10.1016/j.yrtph.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Carmella SG, Chen M, Zhang Y, Zhang S, Hatsukami DK, Hecht SS. Chem. Res. Toxicol. 2007;20:986–990. doi: 10.1021/tx700075y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roethig HJ, Zedler BK, Kinser RD, Feng S, Nelson BL, Liang Q, Clin J. Pharmacol. 2007;47:518–530. doi: 10.1177/0091270006297686. [DOI] [PubMed] [Google Scholar]
- 27.Sarkar M, Kapur S, Frost-Pineda K, Feng S, Wang J, Liang Q, Roethig H. Nicotine Tob. Res. 2008;10:1761–1772. doi: 10.1080/14622200802443718. [DOI] [PubMed] [Google Scholar]
- 28.Roethig HJ, Feng S, Liang Q, Liu J, Rees WA, Zedler BK. J Clin Pharmacol. 2008;48:580–591. doi: 10.1177/0091270008315316. [DOI] [PubMed] [Google Scholar]
- 29.Schettgen T, Musiol A, Kraus T. Rapid Commun. Mass Spectrom. 2008;22:2629–2638. doi: 10.1002/rcm.3659. [DOI] [PubMed] [Google Scholar]
- 30.Ding YS, Blount BC, Valentin-Blasini L, Applewhite HS, Xia Y, Watson CH, Ashley DL. Chem. Res. Toxicol. 2009;22:1018–1025. doi: 10.1021/tx800468w. [DOI] [PubMed] [Google Scholar]
- 31.Eckert E, Drexler H, Goen T, Chromatogr J. B Analyt. Technol. Biomed. Life Sci. 2010;878:2506–2514. doi: 10.1016/j.jchromb.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Hou H, Xiong W, Gao N, Song D, Tang G, Hu Q. Se. Pu. 2011;29:31–35. doi: 10.3724/sp.j.1123.2011.00031. [DOI] [PubMed] [Google Scholar]
- 33.Alwis KU, Blount BC, Britt AS, Patel D, Ashley DL. Anal. Chim. Acta. 2012;750:152–160. doi: 10.1016/j.aca.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watzek N, Scherbl D, Feld J, Berger F, Doroshyenko O, Fuhr U, Tomalik-Scharte D, Baum M, Eisenbrand G, Richling E. Mol. Nutr. Food Res. 2012;56:1825–1837. doi: 10.1002/mnfr.201200323. [DOI] [PubMed] [Google Scholar]
- 35.Yoshida M, Mikami T, Higashi K, Saiki R, Mizoi M, Fukuda K, Nakamura T, Ishii I, Nishimura K, Toida T, Tomitori H, Kashiwagi K, Igarashi K. Clin. Chim. Acta. 2012;413:753–759. doi: 10.1016/j.cca.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 36.Scherer G, Urban M, Hagedorn HW, Feng S, Kinser RD, Sarkar M, Liang Q, Roethig HJ. Hum Exp Toxicol. 2007;26:37–47. doi: 10.1177/0960327107073829. [DOI] [PubMed] [Google Scholar]
- 37.Roethig HJ, Munjal S, Feng S, Liang Q, Sarkar M, Walk RA, Mendes PE. Nicotine Tob. Res. 2009;11:1216–1225. doi: 10.1093/ntr/ntp126. [DOI] [PubMed] [Google Scholar]
- 38.Yuan JM, Gao YT, Wang R, Chen M, Carmella SG, Hecht SS. Carcinogenesis. 2012;33:804–809. doi: 10.1093/carcin/bgs026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kensler TW, Ng D, Carmella SG, Chen M, Jacobson LP, Munoz A, Egner PA, Chen JG, Qian GS, Chen TY, Fahey JW, Talalay P, Groopman JD, Yuan JM, Hecht SS. Carcinogenesis. 2012;33:101–107. doi: 10.1093/carcin/bgr229. [DOI] [PMC free article] [PubMed] [Google Scholar]