Abstract
The formation of membrane contact sites between cellular organelles is required for proper organelle communication and maintenance in the compartmentalized eukaryotic cell. We recently identified a tether that links peroxisomes to the cortical ER in the yeast, Saccharomyces cerevisiae. The tether is made up of the peroxisome biogenic protein Pex3p and the peroxisome inheritance factor Inp1p, and is formed by Inp1p-mediated linkage of ER-bound Pex3p and peroxisomal Pex3p. Here we discuss how this tether is fine-tuned to ensure that peroxisomes are stably maintained over generations of yeast cells.
Keywords: peroxisome, endoplasmic reticulum, organelle tether, organelle inheritance, membrane contact site, molecular motor, high resolution microscopy
Eukaroytic cells contain a set of membrane-enclosed compartments called organelles. A hallmark of organelles is that they replicate, because it is either impossible or energetically unfavorable to make themselves anew, and partition between mother cell and daughter cell at cytokinesis. The yeast Saccharomyces cerevisiae divides asymmetrically, thereby facilitating the experimental dissection of organelle inheritance into transport and retention processes.
Transport of organelles to the yeast bud occurs along actin cables and is performed by class V myosins, which attach to individual organelles through specific adaptor molecules. Myo4p is required for the transport of cortical ER, while peroxisomes, vacuoles, mitochondria, late Golgi elements, and secretory vesicles are all carried to the bud by Myo2p.1
Organelles are retained in the mother cell by their attachment to defined structures in the cell cortex. Mitochondria and peroxisomes are anchored to the cortical ER by discrete tethering complexes made up of proteins residing in and between both compartments.2,3 The core of the ER-mitochondrion tether is made up of Mmm1p, previously believed to be a mitochondrial protein but now shown to be an integral membrane protein of the ER, and Mdm10p, a mitochondrial outer membrane β-barrel protein.2 The ER-peroxisome tether consists of the peroxisome biogenic protein Pex3p4,5 and the peroxisome inheritance factor Inp1p.6 Pex3p is an integral membrane protein that is localized to both the ER and the peroxisome. Inp1p, which contains binding sites for Pex3p at its N- and C-termini, binds Pex3p in both compartments, thus linking Pex3p molecules across the peroxisomal and ER membranes.3
How are peroxisome retention and transport balanced to ensure equitable sharing of peroxisomes between mother cell and daughter cell? In other words, how does the yeast cell “count” its peroxisomes so as not to lose control of its peroxisome population? Inp1p exhibits a marked asymmetry along the cell division axis; it is found on peroxisomes that are statically anchored in the mother cell but absent on peroxisomes that travel to the bud. Using mutants defective in peroxisome division that contain a single giant peroxisome,7,8 we showed that Inp1p is confined to that part of the peroxisome that is retained in the mother cell, while Inp2p—the adaptor that connects peroxisomes to the Myo2p motor9—enriches at the tip of the peroxisomal tubule that protrudes into the bud. Photoconversion of a peroxisomal matrix protein further demonstrated that during yeast budding, peroxisomes in the mother cell are not fully released from tethers, while peroxisomes that travel to the bud contain photoconverted reporter and are therefore the product of a peroxisome division event in the mother cell. We contend that every peroxisome replicates and splits between mother cell and daughter cell, thus ensuring that the mother cells keeps, and the daughter cell receives, its fair and equitable allotment of peroxisomes.3
Directed peroxisome transport from mother cell to bud is assured by two complementary mechanisms. First, the Myo2p motor exerts a pulling force that assists in peroxisome division and moves peroxisomes vectorially along actin cables whose (+) ends are oriented preferentially toward the bud. Second, as peroxisomes are not released from existing tethers in the mother cell and thus constantly occupy these tethers, peroxisomes arising from peroxisome division cannot reattach in the mother cell. These peroxisomes are therefore mobile, and their fate is to be segregated to the bud.
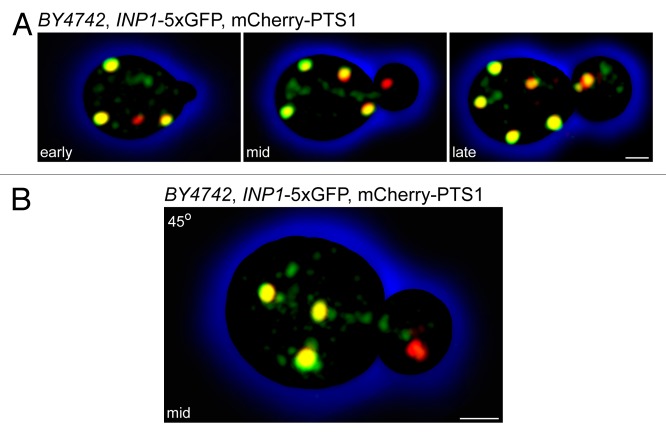
How is this elegant dance of peroxisome inheritance choreographed? We know that Inp1p has a greater affinity for ER-bound Pex3p than for peroxisomal Pex3p and that the ER-peroxisome tether is built from the ER side.3 Because Pex3p needs to be synthesized and inserted into the ER of the bud, we expect that there is a delay before Pex3p becomes available for tether formation. Could Inp1p also be made anew in the bud? This does not appear to be the case, as high-resolution microscopy of cells expressing the fluorescent peroxisomal matrix marker mCherry-PTS1 and Inp1p-5 × GFP showed strikingly that Inp1p appears to travel from mother cell to daughter cell in small vesicles that are distinct from the peroxisomal compartment itself (Fig. 1A). This is especially evident in cells containing buds of intermediate size to which Inp1p-deficient peroxisomes already have been segregated. These peroxisomes are trailed by a stream of small Inp1p-containing vesicles (Fig. 1B). While peroxisomal vesicular carriers have been characterized biochemically,10-12 this represents the first visualization of a peroxisomal precursor in living cells.
Figure 1. Inp1p is present in small vesicles that are distinct from the peroxisomal compartment. Wild-type yeast cells expressing Inp1p-5 × GFP and mCherry-PTS1 were visualized by confocal fluorescence microscopy. Images were acquired as 3D z-stacks and flattened into maximum intensity projections. (A) Time course representing early, mid, and late stages of peroxisome inheritance in a single cell. Bar, 1 μm. (B) A budding cell at the mid-stage of peroxisome inheritance. Individual z-stacks were combined and displayed at a 45° angle to enhance the 3D effect. Bar, 1 μm.
What significance is all of this? Peroxisome division likely does not occur until after peroxisomes have become immobilized, because the forces exerted by the divisional machinery to pull or constrict peroxisomes have to be counteracted by forces acting to retain peroxisomes. Peroxisome division may thus be tightly coupled to cell cycle progression and may, under standard nutrient conditions, be the only requirement for peroxisomes to multiply in a growing population of yeast cells.
We did not fail to notice that cells achieve an exacting level of control over their peroxisome populations by combining peroxisome biogenic and retention functions in a single molecule, Pex3p. Newly formed tethers in the bud are normally the recipients of peroxisomes that have been inherited from the mother cell. However, if peroxisome inheritance is blocked, Pex3p can break free from its association with Inp1p and initiate de novo peroxisome formation. While yeast cells routinely multiply their peroxisomes through growth and division,13 they do have the option of regenerating the entire peroxisome compartment from the ER.14 The ER-peroxisome tether thus contains a built-in exit strategy for Pex3p that allows it to function in de novo peroxisome biogenesis and thereby guarantees the continued maintenance of the peroxisomal compartment in a cell population.
We have come far from the view that organelle inheritance is a random event and that each dividing cell gets its fair share of organelles by chance. Our journey of discovery of how organelles, and especially peroxisomes, partition between mother and daughter cells so that both can maintain the benefits of organelle compartmentalization for generations to come continues. We expect even more exciting and surprising findings along the way.
Material and Methods
Yeast strain BY4742, INP1-5xGFP, mCherry-PTS1 (MATα, his3Δ1, leu2Δ0, lys2Δ0, ura3Δ0, inp1::INP1–5 × GFP, can1::mCherry-PTS1) was constructed by inserting a 5 × GFP cassette at the 3ʹ-end of the INP1 gene and by replacing the ORF of the CAN1 locus with sequence coding for mCherry fused at its C-terminus with the peroxisomal targeting signal 1 (Ser-Lys-Leu).3 Cells were grown to early exponential phase (OD600 = ~0.3) in YPD medium (1% yeast extract, 2% peptone, 2% glucose) and visualized using an LSM710 confocal fluorescence microscope (Carl Zeiss). Image acquisition and processing were done as previously described.3
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr Xuejun Sun and Richard Poirier for expert technical assistance. This work was supported by the Canadian Institutes of Health Research.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/26901
References
- 1.Fagarasanu A, Mast FD, Knoblach B, Rachubinski RA. Molecular mechanisms of organelle inheritance: lessons from peroxisomes in yeast. Nat Rev Mol Cell Biol. 2010;11:644–54. doi: 10.1038/nrm2960. [DOI] [PubMed] [Google Scholar]
- 2.Kornmann B, Currie E, Collins SR, Schuldiner M, Nunnari J, Weissman JS, Walter P. An ER-mitochondria tethering complex revealed by a synthetic biology screen. Science. 2009;325:477–81. doi: 10.1126/science.1175088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knoblach B, Sun X, Coquelle N, Fagarasanu A, Poirier RL, Rachubinski RA. An ER-peroxisome tether exerts peroxisome population control in yeast. EMBO J. 2013;32:2439–53. doi: 10.1038/emboj.2013.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hettema EH, Girzalsky W, van Den Berg M, Erdmann R, Distel B. Saccharomyces cerevisiae Pex3p and Pex19p are required for proper localization and stability of peroxisomal membrane proteins. EMBO J. 2000;19:223–33. doi: 10.1093/emboj/19.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang Y, Morrell JC, Jones JM, Gould SJ. PEX3 functions as a PEX19 docking factor in the import of class I peroxisomal membrane proteins. J Cell Biol. 2004;164:863–75. doi: 10.1083/jcb.200311131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fagarasanu M, Fagarasanu A, Tam YYC, Aitchison JD, Rachubinski RA. Inp1p is a peroxisomal membrane protein required for peroxisome inheritance in Saccharomyces cerevisiae. J Cell Biol. 2005;169:765–75. doi: 10.1083/jcb.200503083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoepfner D, van den Berg M, Philippsen P, Tabak HF, Hettema EH. A role for Vps1p, actin, and the Myo2p motor in peroxisome abundance and inheritance in Saccharomyces cerevisiae. J Cell Biol. 2001;155:979–90. doi: 10.1083/jcb.200107028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuravi K, Nagotu S, Krikken AM, Sjollema K, Deckers M, Erdmann R, Veenhuis M, van der Klei IJ. Dynamin-related proteins Vps1p and Dnm1p control peroxisome abundance in Saccharomyces cerevisiae. J Cell Sci. 2006;119:3994–4001. doi: 10.1242/jcs.03166. [DOI] [PubMed] [Google Scholar]
- 9.Fagarasanu A, Fagarasanu M, Eitzen GA, Aitchison JD, Rachubinski RA. The peroxisomal membrane protein Inp2p is the peroxisome-specific receptor for the myosin V motor Myo2p of Saccharomyces cerevisiae. Dev Cell. 2006;10:587–600. doi: 10.1016/j.devcel.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 10.Titorenko VI, Chan H, Rachubinski RA. Fusion of small peroxisomal vesicles in vitro reconstructs an early step in the in vivo multistep peroxisome assembly pathway of Yarrowia lipolytica. J Cell Biol. 2000;148:29–44. doi: 10.1083/jcb.148.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agrawal G, Joshi S, Subramani S. Cell-free sorting of peroxisomal membrane proteins from the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2011;108:9113–8. doi: 10.1073/pnas.1018749108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lam SK, Yoda N, Schekman R. A vesicle carrier that mediates peroxisome protein traffic from the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2011;108:E51–2. doi: 10.1073/pnas.1103526108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Motley AM, Hettema EH. Yeast peroxisomes multiply by growth and division. J Cell Biol. 2007;178:399–410. doi: 10.1083/jcb.200702167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoepfner D, Schildknegt D, Braakman I, Philippsen P, Tabak HF. Contribution of the endoplasmic reticulum to peroxisome formation. Cell. 2005;122:85–95. doi: 10.1016/j.cell.2005.04.025. [DOI] [PubMed] [Google Scholar]