Abstract
Currently, there are limited therapeutic options against bone metastatic prostate cancer (PCA), which is primarily responsible for high mortality and morbidity in PCA patients. Enhanced osteoclastogenesis is an essential feature associated with metastatic PCA in the bone microenvironment. Silibinin, an effective chemopreventive agent, is in phase II clinical trials in PCA patients but its efficacy against PCA cells-induced osteoclastogenesis is largely unknown. Accordingly, here we examined silibinin effect on PCA cells-induced osteoclastogenesis employing human PCA (PC3MM2, PC3 and C4-2B) and murine macrophage RAW264.7 cells. We also assessed silibinin effect on receptor activator of nuclear factor B ligand (RANKL)-induced signaling associated with osteoclast differentiation in RAW264.7 cells. Further, we analyzed silibinin effect on osteomimicry biomarkers in PCA cells. Results revealed that silibinin (30–90 μM) inhibits PCA cells-induced osteoclast activity and differentiation in RAW264.7 cells via modulating expression of several cytokines (IGF-1, TGF-β, TNF-α, I-TAC, M-CSF, G-CSF, and GM-CSF etc.) that are important in osteoclastogenesis. Additionally, in RAW264.7 cells, silibinin decreased the RANKL-induced expression and nuclear localization of NFATc1, which is considered the master regulator of osteoclastogenesis. Furthermore, silibinin decreased the RANKL-induced DNA binding activity of NFATc1 and its regulators NF-κB and AP1, and the protein expression of osteoclast specific markers (TRAP, OSCAR and Cathepsin K). Importantly, silibinin also decreased the expression of osteomimicry biomarkers (RANKL, Runx2, Osteocalcin and PTHrP) in cell culture (PC3 and C4-2B cells) and/or in PC3 tumors. Together, our findings showing that silibinin inhibits PCA cells-induced osteoclastogenesis, suggest that silibinin could be useful clinically against bone metastatic PCA.
Keywords: Prostate cancer, Osteoclastogenesis, Silibinin, RANK ligand, NFATc1
INTRODUCTION
Most patients with prostate cancer (PCA) die not because of the tumor growth at the primary site, but because it has spread to the other sites. Bone is the most preferred site for PCA metastasis and virtually all patients dying of PCA experience extensive bone metastasis [1]. Patients with bone metastasis routinely suffer extreme bone pain, spinal-cord compression and fractures [2–4]. In addition, replacement of bone marrow by growing PCA cells disrupts normal haematopoiesis resulting in anemia and increased susceptibility to infections in PCA patients [3]. Therefore, bone metastasis is associated with significant morbidity and mortality in advanced PCA.
Once settled in bone, PCA cells alter the delicate balance of bone remodeling orchestrated by two types of bone cells namely osteoclast (involved in bone degradation) and osteoblast (involved in bone formation) [3–6]. PCA cells promote osteoclast maturation and activation; thereby enhance bone resorption and liberation of several growth factors including TGF-β, IGFs, BMPs, PDGF etc [2–5]. Bone degradation provides PCA cells the initial space to expand, and the released growth factors promote PCA cells survival and proliferation [3,5]. The growth factors released by bone degradation as well as additional factors secreted by PCA cells such as endothelin-1, BMPs, Wnts promote osteoblast maturation and formation of new bone [3–5,7]. Mature osteoblasts also produce growth factors which further promote PCA cells growth in bone [3–5,7]. This vicious cycle involving PCA cells, osteoclasts and osteoblasts promotes bone degradation as well as deposition of new ‘woven type bone’ (uneven/immature/embryonic), thus compromising the bone health and leads to bone complications in PCA patients. Whereas PCA bone metastasis predominantly exhibits osteoblastic features, histological findings and analyses of bone turnover markers confirm that extensive bone degradation by activated osteoclasts is also an integrated and essential phenomenon. Therefore, targeting osteoclast activity is considered one of the preventive and therapeutic options against PCA bone metastasis.
Osteoclasts are highly specialized cells originating from monocyte/macrophage lineage precursors. In addition to their critical role in skeletal development and maintenance, they are also implicated in the pathogenesis of various bone diseases such as osteoporosis, rheumatoid arthritis and cancer metastasis as detailed above [8,9]. Hence, efforts to treat and/or prevent the skeletal-related events in PCA and other cancers have focused on the inhibition of osteoclast activation [10,11]. RANKL (Receptor activator of nuclear factor B ligand) is considered as a main driver of osteoclast formation, survival, and function [9]. PCA cells also express RANKL and/or promote RANKL expression in neighboring osteoblast and stromal cells in the bone microenvironment, thereby enhance osteoclastogenesis [12]. RANKL exerts its effect by binding to its receptor RANK present on osteoclast or its precursors, and activates several major signaling pathways (NFATc1, NF-κB, Akt, MAPKs etc.) required for osteoclast differentiation and activity [13,14]. Considering the central role of RANKL in osteoclast differentiation, the identification of non-toxic agents that could target RANKL-induced signaling pathways and inhibit osteoclastogenesis would have translational relevance against bone metastatic PCA.
Silibinin, a flavonoid from Milk Thistle seed extract, is one of the most-widely consumed dietary supplements for its hepatoprotective efficacy [15,16]. Silibinin has shown remarkable efficacy both in vitro and in vivo against PCA cells, and its efficacy is currently being evaluated in PCA patients [17–22]. In the present study, we report silibinin effect on PCA cells- and RANKL-induced osteoclastogenesis in RAW264.7 cells, which is a well-established system to study osteoclastogenesis in cell culture. We also demonstrate silibinin effect on important regulators of osteoclastogenesis and osteomimicry biomarkers in PCA cells and xenografts.
MATERIALS AND METHODS
Cell Lines and Reagents
Human prostate carcinoma PC3 cells and murine macrophage RAW264.7 cells were obtained from the ATCC (Manassas, VA). C4-2B cells were from ViroMed Laboratories. PC3MM2 cells were kind gift from Dr. Isaiah J. Fidler (University of Texas M.D. Anderson Cancer Center). Anti-rabbit peroxidase conjugated secondary antibody was from Cell Signaling (Beverly, MA). α-tubulin antibody was from Neomarkers (Fremont, CA). Primary antibodies for RANKL, Runx2, Osteocalcin, TRAP, OSCAR and biotinylated anti-rabbit secondary antibody were from Santa Cruz Biotechnology (Santa Cruz, CA). NFATc1 antibody was from BD Biosciences (New Bedford, MA). PTHrP and Cathepsin K antibodies were from abcam (Cambridge, MA). Harris hematoxylin was from Sigma (St. Louis, MO) and 3,3-diaminobenzidine (DAB) was from Vector laboratories (Burlingame, CA). ECL detection kit and anti-mouse HRP conjugated secondary antibody were from GE Healthcare (Buckinghamshire, UK). Bio-Rad detergent-compatible protein assay kit was from Bio-Rad Laboratories (Hercules, CA). All other reagents were obtained in their highest purity grade available commercially.
Collection of Conditioned Media
PCA cells (PC3, PC3MM2 and C4-2B) were treated at 30–40% confluency with DMSO or silibinin (30, 60 and 90 μM) for 72 h in complete media (RPMI1640 with 10% fetal bovine serum and 1% penicillin-streptomycin). Thereafter, media was removed and cells were washed twice with 0.5% serum media and incubated for another 12 h with 0.5% serum media. Subsequently, the conditioned media was collected, centrifuged and labeled as CCM (control conditioned media), or 30SBCM, 60SBCM and 90SBCM for 30, 60 and 90 μM silibinin-treated conditioned media, respectively, and stored at −80ºC until further use. Cell number in each group was counted using a hemocytometer. In the co-culture experiments, the volume of the conditioned media was normalized with respective cell number, and a ratio of 50% conditioned media (CCM/SBCM) and 50% RAW264.7 media with 0.5% FBS was used.
Osteoclastogenesis Assay
RAW264.7 cells were incubated with CCM or SBCM collected from PC3MM2, PC3 and C4-2B cells in the presence of 5 ng/ml RANKL. Media was replaced every 48 h and after 5 days, cells were stained for tartrate resistant acid phosphatase (TRAP) using TRAP staining kit (Takara, Shiga, Japan). The number of TRAP positive cells (a measure of osteoclast activity) was determined using a light microscope. Cells were processed further as per the protocol and differentiated osteoclasts (with 4 or more nuclei) were counted. RAW264.7 cells supplemented with 5 ng/ml of RANKL only served as negative control. In another experiment, we supplemented silibinin (10, 20 and 30 μM) exogenously in the CCM and analyzed its effect on osteoclast activity and differentiation. RAW264.7 cells were also treated with RANKL (100 ng/ml) together with DMSO or silibinin (10, 20 and 30 μM) and analyzed for osteoclast activity and differentiation.
Cytokine Array
Cytokine level in CCM and SBCM was compared using Human Cytokine Antibody Array C series1000 from RayBio Technology (Norcross, GA) following vendor’s protocol. Briefly, array membranes were sequentially blocked, incubated with CCM/90SBCM, biotin-conjugated antibodies and HRP-conjugated streptavidin. Cytokines expression on the array membranes was visualized by ECL detection system followed by exposure to X-ray film. Expression of each cytokine (in duplicate) was quantified through scanning the X-ray film using Adobe Photoshop 6.0 (Adobe Systems Inc., San Jose, CA) followed by densitometry analyses using Scion Image program (National Institutes of Health, Bethesda, MD) and RayBio Analysis Tool. Cytokine location on the array 6 and 7 is illustrated in Supplementary Figure 1.
Direct Co-culture Assay
In direct co-culture assay, PC3MM2 and RAW264.7 cells were co-cultured (1:5 ratio) and treated with DMSO or silibinin (5, 10, 20 and 30 μM). The media was replaced every 48 h, and after 5 days, osteoclast activity and differentiation were determined as described above.
Western Blotting
At the end of each treatment, whole-cell lysates or nuclear and cytoplasmic extracts were prepared, and Western blotting was performed as described earlier [23–26]. In each case, blots were subjected to multiple exposures on the film to ensure that the band density was in the linear range. Few blots were multiplexed or stripped and re-probed with different antibodies including α-tubulin antibody to confirm equal protein loading. Autoradiograms/bands were scanned using Adobe Photoshop 6.0.
Confocal Microscopy
RAW264.7 cells were treated with DMSO or silibinin (30 μM) in 0.5% serum media with or without RANKL (10 ng/ml). After 24 h, cells were processed for confocal microscopy as described before [27] to detect NFATc1 level and localization.
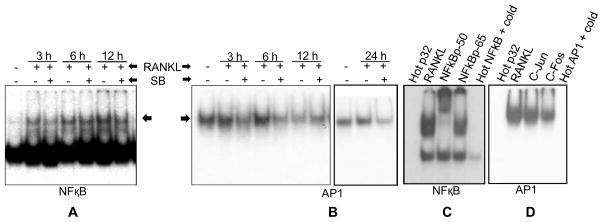
Electrophoretic Mobility Shift Assay (EMSA)
EMSA was performed as described earlier [28]. Briefly, NFATc1, NF-κB and AP1 specific oligonucleotides were end-labeled with [γ-32P]ATP using T4 polynucleotide kinase in 10X kinase buffer as per the manufacturer’s protocol (Promega, Madison, WI). Approximately 8 μg of nuclear extract was incubated with 5X gel shift binding buffer and then with γ32P end-labeled consensus oligonucleotides for 20 min at 37ºC. DNA-protein complex was resolved on 6% DNA retardation gel followed by gel drying and autoradiography. Supershift assay was performed using specific antibodies (NFATc1; p50 and p65 for NF-κB; c-Jun and c-Fos for AP1) in nuclear extract from RANKL-treatment group and competition assay was performed using excess unlabeled cold probe in the reaction mixture.
Immunohistochemistry (IHC)
We used archived paraffin-embedded PCA xenograft tissues from our previously completed PC3 orthotopic xenograft study [18] to determine in vivo effect of silibinin administration on the levels of osteomimicry biomarkers following IHC procedures detailed earlier [29]. All the microscopic IHC analyses were performed using a Zeiss Axioscope 2 microscope (Carl Zeiss) and photographs were captured (at 400X) with a Carl Zeiss AxioCam MrC5 camera with Axiovision Rel 4.5 software.
Statistical Analyses
Statistical analysis was performed using SigmaStat 2.03 software (Jandel Scientific, San Rafael, CA). Data was analyzed using one way ANOVA followed by Tukey or Bonferroni t-test and a statistically significant difference was considered at p ≤ 0.05.
RESULTS
Silibinin Inhibits PCA cells-induced Osteoclast Differentiation and Activity in RAW264.7 Cells
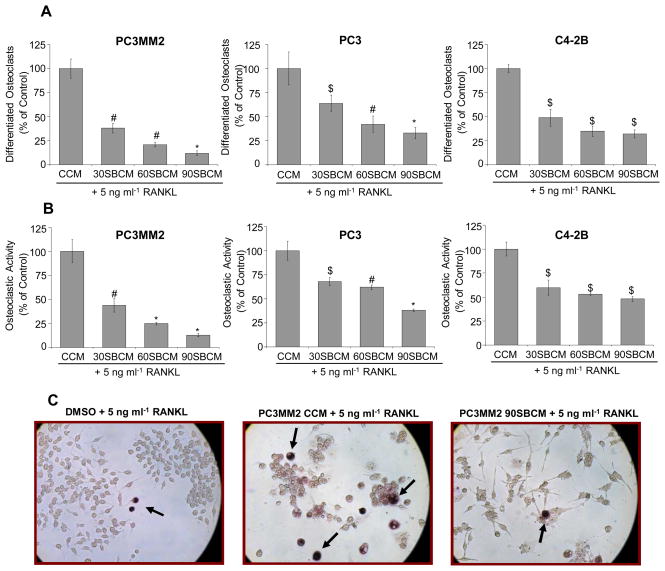
To examine the effect of silibinin on the PCA cells-induced osteoclastogenesis, we analyzed the effect of CCM and SBCM on osteoclast differentiation and activity using RAW264.7 cells. We added 5 ng/ml RANKL in CCM and SBCM to boost the osteoclastogenesis. After 5 days, we observed differentiated osteoclasts in CCM treated RAW264.7 cells while significantly lesser differentiated osteoclasts were formed in RAW264.7 cells treated with 30SBCM, 60SBCM and 90SBCM (Figure 1A). Negligible, if any, differentiated osteoclast was observed in RAW264.7 cells treated with 5 ng/ml RANKL alone. Osteoclast activity determined by TRAP staining revealed that compared to CCM, SBCM treated RAW264.7 cells have significantly lesser TRAP positive cells (Figure 1B). Silibinin effect was dose-dependent and consistent in all the three PCA cell lines, but its effect was most prominent in PC3MM2 cells (Figure 1A and 1B). The representative images of TRAP positive RAW264.7 cells (purplish red cells marked by arrows) in different treatment groups (RANKL only or CCM and 90SBCM from PC3MM2 cells) are shown in Figure 1C. Taken together, these results showed that silibinin inhibits PCA cells-induced osteoclastogenesis.
Figure 1.
Effect of silibinin on PCA cells-induced osteoclast differentiation and activity in RAW264.7 cells. (A–B) PCA cells (PC3MM2, PC3 and C4-2B) were treated with silibinin (30–90 μM) and conditioned media was collected as detailed in Material and Methods’. RAW264.7 cells were treated with CCM or SBCM in presence of RANKL (5 ng/ml). In each case, media was replaced after 48 h, and after 5 days, cells stained for TRAP. Differentiated multinucleated osteoclasts (4 or more nuclei) and TRAP positive cells were counted. (C) Representative images from osteoclastogenesis assay with TRAP positive RAW264.7 cells (marked by arrows) are shown. Abbreviations: CCM: Control conditioned media; SBCM: Silibinin-treated conditioned media; *, p ≤ 0.001; #, p ≤ 0.01; $, p ≤ 0.05
Silibinin Modulates the Level of Cytokines Secreted by PCA Cells
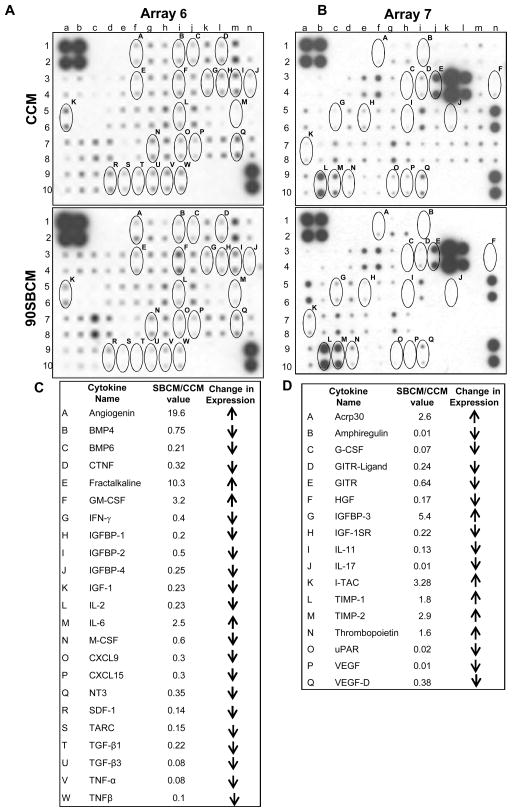
Cytokines secreted by PCA cells in the bone microenvironment play an important role in promoting osteoclastogenesis [3,4,6]. Since we observed significantly lesser osteoclastogenesis with SBCM compared to CCM, next we compared the conditioned media (CCM and 90SBCM from PC3MM2 cells) for the expression of cytokines. Cytokine arrays analyses revealed the expression of 120 cytokines in the PCA conditioned media (CCM and SBCM) suggesting the complexity of interaction involved in the CCM-induced osteoclastogenesis. Array results showed that cytokines have differential expression in SBCM compared to CCM. The exact role of individual changes in the cytokine expression on osteoclastogenesis would require more focused studies; however, we have listed few cytokines whose expression was increased (angiogenin, GM-CSF, IL-6, IGFBP-3, TIMPs etc.) or decreased (IFN-γ, IGF, TGF-β, TNFα, M-CSF, G-CSF etc.) by silibinin treatment, and these cytokines directly or indirectly affect osteoclastogenesis (Figure 2) [9,30–34]. We have circled and alphabetically labeled these selected cytokines on the arrays (Figure 2A and 2B) and presented name and changes in their expression (SBCM/CCM densitometric values) in the tables (Figure 2C and 2D).
Figure 2.
Effect of silibinin on cytokines secretion in PC3MM2 cells. Conditioned media was collected from DMSO or silibinin treated PC3MM2 cells, and processed for cytokine antibody array (volume normalized with respective cell number). (A) Expression of cytokines in CCM and SBCM on Array-6, and (B) expression of cytokines in CCM and SBCM on Array-7. Osteoclastogenesis relevant cytokines, whose expression was modulated with silibinin treatment, were labeled alphabetically on the array 6 and 7. (C–D) The change in the densitometry values of selected cytokines in 90SBCM versus CCM in Array-6 and Array-7 are presented. Abbreviations: CCM: Control conditioned media; 90SBCM: 90 μM silibinin-treated conditioned media
Silibinin Directly Inhibits PCA Conditioned media- and RANKL-induced Osteoclast Differentiation and Activity in RAW264.7 Cells
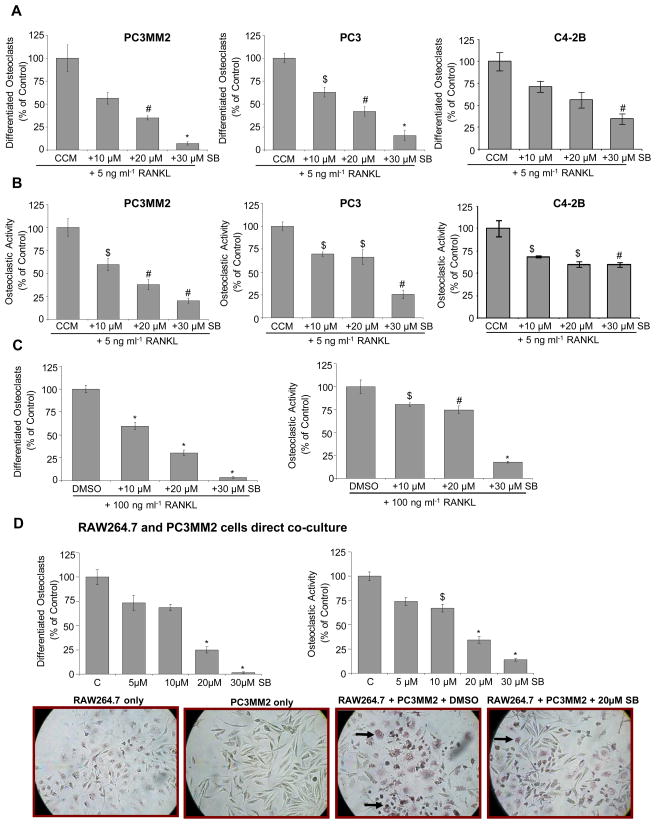
The above detailed experiment established an indirect effect of silibinin on osteoclastogenesis i.e. through targeting the secretion of various cytokines by PCA cells, which play a critical role in osteoclast differentiation and activity. Based on these results, next, we assessed whether silibinin could directly target the osteoclast differentiation and activity. First, we incubated RAW264.7 cells with CCM from PC3MM2, PC3 and C4-2B cells together with silibinin at 10, 20 and 30 μM for 5 days and analyzed the effect on osteoclast differentiation and activity. Silibinin treatment inhibited the CCM-induced osteoclast differentiation in a dose- dependent manner (Figure 3A). TRAP positive cells induced by CCM were also significantly reduced upon silibinin addition (Figure 3B). In another experiment, we stimulated RAW264.7 cells with RANKL (100 ng/ml) and treated with DMSO or silibinin (10, 20 and 30 μM). As shown in Figure 3C, silibinin treatment strongly inhibited RANKL-induced osteoclast differentiation and activity. Together, these results suggested that silibinin could also directly inhibit osteoclast differentiation and activity.
Figure 3.
(A–B) Direct effect of silibinin on osteoclast differentiation and activity induced by PCA cells conditioned media and RANKL. RAW264.7 cells were treated with CCM from PC3MM2, PC3 and C4-2B cells in presence of DMSO or silibinin (10, 20 and 30 μM) together with RANKL (5 ng/ml). After 5 days of treatment, osteoclast differentiation and activity was measured. (C) Effect of silibinin on RANKL-induced osteoclast differentiation and activity. RAW264.7 cells were treated with DMSO or silibinin (10, 20 and 30 μM) in the presence of RANKL (100 ng/ml), and after 5 days of treatment, osteoclast differentiation and activity was measured. (D) Effect of silibinin on osteoclast differentiation and activity in RAW264.7 cells co-cultured with PC3MM2 cells. PC3MM2 and RAW264.7 cells were co-cultured (1:5 ratio) and treated with DMSO or silibinin (5–30 μM). Media was replaced after 48 h, and after 5 days of treatment, osteoclast differentiation and activity was measured. Representative microscopic images from direct co-culture assay with TRAP positive cells (marked by arrows) are shown. Abbreviations: SB: Silibinin; CCM: Control conditioned media; SBCM: Silibinin-treated conditioned media; *, p ≤ 0.001; #, p ≤ 0.01; $, p ≤ 0.05
Silibinin Inhibits Osteoclast Differentiation and Activity in RAW264.7 Cells Co-cultured with PC3MM2 Cells
Next, to more closely simulate bone microenvironment condition, we examined whether silibinin could inhibit osteoclast formation when PCA cells are in direct contact with RAW264.7 cells. As shown in Figure 3D (bar diagram and photomicrographs), the presence of PC3MM2 cells induced the osteoclast differentiation and activity in RAW264.7 cells, which was inhibited by silibinin treatment; while TRAP positive cells were not observed in RAW264.7 or PC3MM2 cells cultured alone.
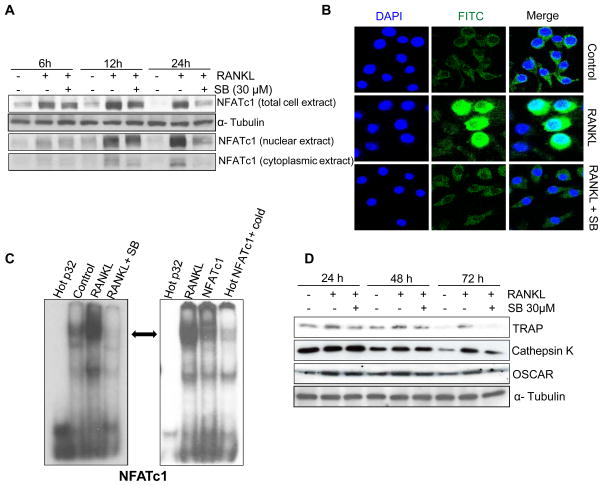
Silibinin Suppresses RANKL-induced NFATc1 Expression in RAW264.7 Cells
NFATc1, a member of the NFAT family of transcription factors, is strongly up-regulated during RANKL-induced osteoclastogenesis [35]. It has been reported that NFATc1-deficient embryonic stem cells fail to differentiate into osteoclasts, and ectopic expression of NFATc1 causes precursors to undergo efficient osteoclast differentiation even in the absence of RANKL [35]. Hence, NFATc1 has been coined as ‘master regulator of osteoclastogenesis’. To understand the mechanism/s responsible for the inhibitory effect of silibinin on osteoclastogenesis, we next analyzed its effect on RANKL-induced NFATc1 expression in RAW264.7 cells. As shown in Figure 4A, silibinin (30 μM) inhibited the RANKL-induced NFATc1 expression after 6, 12 and 24 h of treatment. Furthermore, silibinin treatment strongly decreased the RANKL-induced NFATc1 protein expression in both nuclear and cytoplasmic fractions (Figure 4A). These results were further confirmed by immunofluorescence, where RANKL stimulation (24 h) increased the NFATc1 expression (Alexa Fluor 488-green staining) in both nucleus and cytoplasm, while silibinin treatment decreased the overall RANKL-induced NFATc1 expression (Figure 4B).
Figure 4.
Effect of silibinin on RANKL-induced NFATc1 activation and osteoclast specific markers in RAW264.7 cells. (A) RAW264.7 cells were treated with or without RANKL (10 ng/ml) and silibinin (30 μM) for the indicated time in 0.5% serum media. Whole-cell lysates or nuclear and cytoplasmic extracts were prepared and analyzed for NFATc1 expression by Western blotting. (B) Effect of silibinin on the RANKL-induced expression and cellular localization of NFATc1 was analyzed by immunofluorescence. Images were captured at 1000X magnification on a Nikon inverted confocal microscope using 488/405 nm laser wavelengths to detect Alexa Fluor 488 (green) and DAPI (blue) emissions, respectively. (C) Nuclear extracts were prepared after 24 h of RANKL and silibinin treatment in RAW264.7 cells and analyzed for NFATc1 DNA binding activity by EMSA (left panel). Super shift and competition assays were performed using nuclear extract from RANKL-treatment group to confirm the specificity of NFATc1 binding (right panel). (D) Effect of silibinin on osteoclast specific markers (TRAP, Cathepsin K and OSCAR) was analyzed by Western blotting in whole-cell lysates. Abbreviation: SB: Silibinin
NFATc1 binds to specific region of the DNA and controls the transcription of genes that are critically important in the osteoclastogenesis [9,36]. Accordingly, next, we examined silibinin effect on the DNA binding of NFATc1 by EMSA. Our results clearly showed that silibinin treatment strongly inhibits the NFATc1 DNA binding (Figure 4C, left panel). EMSA results were confirmed using the NFATc1 antibody and competition with excess of cold probe (Figure 4C, right panel). Furthermore, silibinin treatment strongly inhibited RANKL-caused increase in the expression of osteoclast specific markers TRAP, Cathepsin K and OSCAR (Fig. 4D), which are controlled by NFATc1 [35,37]. Together, these data suggest that silibinin effectively inhibits RANKL-induced NFATc1 activation and its transcriptional targets that result in the inhibition of osteoclastogenesis.
Silibinin Inhibits RANKL-induced NF-κB and AP1 Activation in RAW264.7 Cells
Upon RANKL binding, RANK activates several signaling pathways including NF-κB and AP1, which directly or indirectly play a role in the NFATc1 activation and osteoclastogenesis [9,13,14,38]. Therefore, next we examined silibinin effect on RANKL-induced activity of NF-κB and AP1. EMSA results clearly showed that RANKL increased the NF-κB DNA binding after 3, 6 and 12 h; and RANKL-induced NF-κB DNA binding was inhibited by silibinin (30 μM) treatment (Figure 5A). RANKL also increased the AP1 DNA binding at 3 and 6 h time-points, even though increase was not as strong as NF-κB; and even a decrease in AP1 DNA binding was observed after 12 h of RANKL exposure (Figure 5B, left panel). However, an increase in AP1 DNA binding with RANKL was evident again at 24 h time-point (Figure 5B, right panel). Silibinin (30 μM) treatment strongly reduced the RANKL-induced DNA binding of AP1 at all time-points studied except 12 h (Figure 5B). These results clearly showed the inhibitory effect of silibinin on RANKL-induced NF-κB and AP1 activation in RAW264.7 cells. These results also suggested a time-dependent RANKL effect on AP1 activation and demands in-depth time-course studies to clearly establish RANKL effect on AP1 DNA binding.
Figure 5.
Effect of silibinin on RANKL-induced NF-κB and AP1 activation in RAW264.7 cells. (A–D) RAW264.7 cells were treated with silibinin (30 μM) for the indicated time in the presence of RANKL (10 ng/ml), and nuclear extracts were prepared and analyzed for (A) NF-κB and (B) AP1 DNA binding activity by EMSA. Super shift and competition assays were performed using nuclear extract from RANKL-treatment group to confirm the specificity of (C) NF-κB and (D) AP1 binding. Abbreviation: SB: Silibinin
Subsequently, EMSA results were confirmed using specific antibodies (p50, p65, c-Jun and c-Fos) in supershift assay and competition with excess of cold probe (Figure 5C and 5D). A clear supershift in NF-κB was observed with p50 antibody (Figure 5C). Though, we did not observe a supershift in AP1 in the presence of c-Jun or c-Fos antibody but only a decrease in the gel shift (Figure 5D), and these results indirectly implied a partial presence of c-Jun and c-Fos in the AP1-DNA complex. More studies are required to identify the other possible constituents of AP1 (JunB, JunD, Fra1-2, or ATF1-7) in AP1-DNA complex. Together, these results suggest that silibinin inhibits RANKL-induced signaling pathways that have an important role in regulating NFATc1 expression as well as osteoclastogenesis.
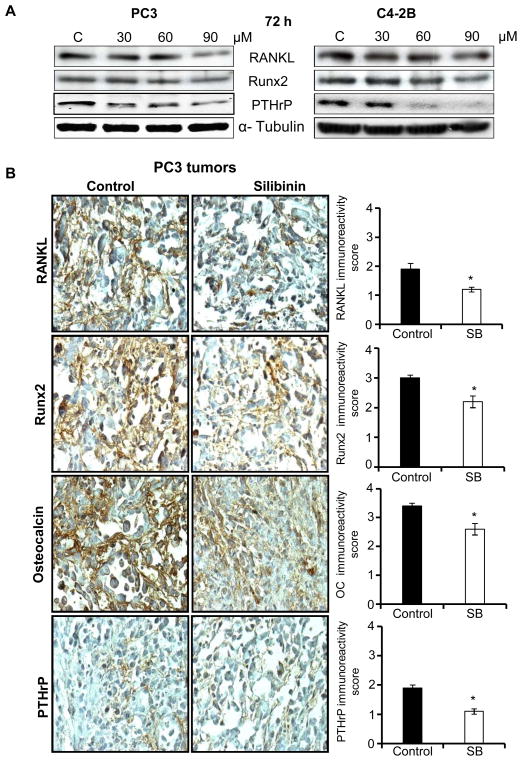
Silibinin Down-regulates Expression of Osteomimicry Markers in PCA Cells both in vitro and in vivo
PCA cells have a unique characteristic of adapting to the bone microenvironment through expressing several proteins whose expression is usually limited to bone cells, a phenomenon termed as ‘osteomimicry’ [39,40]. Accordingly, we also examined silibinin effect on osteomimicry biomarkers in PCA cells including RANKL, Runx2 and PTHrP. As shown in Figure 6A, silibinin treatment down-regulated the expression of these molecules in PC3 and C4-2B cells with a strong effect observed only at 90 μM dose. Next, we confirmed these cell culture results in vivo by analyzing PC3 orthotopic xenograft tissues. Earlier, we have reported that silibinin feeding (100 mg/kg body weight for 7 weeks) inhibits the growth of PC3 tumors in the prostate microenvironment [18]. IHC analyses of these PC3 tumor tissues revealed a significant reduction in the expression of RANKL, Runx2, Osteocalcin and PTHrP in silibinin-treated group in comparison to control (Fig. 6B). Taken together, these results clearly suggest that silibinin inhibits osteomimicry markers in PCA cells both in vitro and in vivo.
Figure 6.
Effect of silibinin on osteomimicry biomarkers in PCA cells. (A) PC3 and C4-2B cells were treated with silibinin at indicated doses for 72 h. At the end of the treatment, whole-cell lysates were prepared and analyzed for the expression of RANKL, Runx2 and PTHrP by Western blotting. (B) Paraffin-embedded PCA xenograft tissues from previously completed PC3 orthotopic xenograft study were analyzed to determine effect of silibinin administration on the expression of RANKL, Runx2, Osteocalcin (OC), and PTHrP by IHC. Immunoreactivity (represented by brown staining) of these biomarkers was scored as 0+ (no staining), 1+ (weak staining), 2+ (moderate staining), 3+ (strong staining), 4+ (very strong staining). The data shown in the bar diagram represents mean ± SEM of 4–5 samples for each group. Abbreviations: SB: Silibinin; *, p ≤ 0.001
DISCUSSION
PCA continues to be the most common non-cutaneous cancer and the second leading cause of cancer-related deaths among American men [41]. According to American Cancer Society report, about 241,740 incidences (29% of total estimated new cases) and 28,170 deaths (9% of total estimated deaths) due to PCA are estimated in American men in 2012 [41]. Bone metastasis is considered primarily responsible for high morbidity and mortality in PCA patients. To alleviate pain, PCA patients with bone metastasis are generally treated with bisphosphonates which are osteoclast inhibitors [42]. Denosumab (trade name ‘Xgeva’), a human monoclonal antibody against RANKL, has been reported to delay bone metastasis in men with PCA [11,43]. Recently, denosumab was approved for the prevention of skeletal-related events in patients with breast or prostate cancer. But both bisphosphonates and denosumab have considerable adverse effects such as osteonecrosis and hypocalcaemia, and there are still doubts about their beneficial effect on the overall survival of the patients [11]. Therefore, targeting PCA cells as well as osteoclastogenesis through non-toxic natural agents is relevant, and in the present study, we demonstrate the effect of one such natural agent, silibinin, on the PCA cells-induced osteoclastogenesis.
In recent years, silibinin has been extensively studied in pre-clinical models as well as clinically for its efficacy against variety of cancers including PCA [44]. Anti-cancer effects of silibinin have been shown by targeting proliferation, apoptosis, angiogenesis, epithelial-mesenchymal transition (EMT), and metastasis [44,45]. Silibinin also targets tumor microenvironment components such as endothelial and macrophage cells towards inhibiting angiogenesis in lung tumors [46]. There have been earlier reports that silibinin inhibits osteoclastogenesis while enhances osteoblastogenesis [30,34]. Kim et al. have shown that silibinin treatment does not affect mature osteoclast function but inhibits the resorption pits formation via targeting osteoclastogenesis [34]. Despite these earlier studies, the effect of silibinin on the PCA cells interaction with bone microenvironment component (such as osteoclast) remained unknown. Results from present study for the first time revealed that silibinin targets PCA cells-induced osteoclast differentiation and activity in RAW264.7 cells. Another highlight of the present study is that we report inhibitory effect of silibinin on several osteomimicry biomarkers (RANKL, Runx2, osteocalcin, and PTHrP) in PCA cells. The inhibition of osteomimicry biomarkers’ expression might compromise PCA cells’ ability to adapt to the bone microenvironment and result in the inhibition of bone metastatic growth.
Osteoclastogenesis is a complex phenomenon and in addition to RANKL, it is affected by several growth factors and cytokines [31,32,34,47–57]. Cytokine array results showed that silibinin treatment altered the expression of several cytokines secreted by PCA cells (Figure 2); and these changes in cytokines level could be responsible for the significant inhibition of osteoclastogenesis observed with SBCM compared to CCM (Figure 1). For example, silibinin decreased the level of several cytokines (including IGF-1, TGF-β, TNF-α, HGF, TARC, and IL-17) that are known to promote osteoclastogenesis [32,34,51,54–56]. Furthermore, silibinin treatment enhanced the level of several cytokines (such as angiogenin, Acrp30, IGFBP-3, I-TAC, and TIMPs) that could directly or indirectly inhibit osteoclastogenesis [48–50,52,53]. Similarly, silibinin treatment decreased the level of M-CSF and G-CSF, but enhanced the level of GM-CSF. Importantly, both M-CSF and G-CSF promote osteoclastogenesis, while GM-CSF inhibits osteoclastogenesis [31,47,54,57]. Together, cytokine array results suggested that silibinin could target multiple circuitries towards inhibiting osteoclastogenesis; however extensive studies are required to validate the role of individual cytokine targeted by silibinin.
As mentioned above, RANKL-RANK signaling is the main regulator of osteoclast differentiation process [9,14]. RANKL binding to its receptor RANK results in the recruitment of adaptor molecule TRAF6, activation of NF-κB, and subsequent initial induction of NFATc1 [9,13]. Thereafter, AP1 complex containing c-Fos plays a role in the auto-amplification of NFATc1, and enables a robust induction of NFATc1 [9]. RANKL also activates MAPKs which could affect NFATc1 expression via modulating AP-1 activation [9,13,58]. NFATc1 cooperates with other transcriptional factors (AP1, PU.1, MITF etc.) to activate osteoclast-specific genes such as TRAP, Cathepsin K, OSCAR [9,36,59]. Results from present study showed that silibinin targeted multiple signaling molecules (NF-κB, AP-1, and NFATc1) towards inhibiting osteoclastogenesis. For example, silibinin strongly decreased the RANKL-induced NFATc1 expression as well as its DNA binding activity resulting in a decreased expression of NFATc1 regulated genes (TRAP, Cathepsin K, and OSCAR). Similarly, silibinin treatment inhibited the RANKL-induced DNA binding of NF-κB and AP1. However, more studies are needed in future to establish the relative importance of these signaling molecules in silibinin-caused inhibition of osteoclastogenesis.
Overall, results from the present study clearly established that silibinin inhibits PCA cells-induced osteoclastogenesis. Molecular studies showed that silibinin inhibited multiple signaling molecules but NFATc1 seems to be the central target in osteoclastogenesis inhibition by silibinin. Considering the fact that silibinin consumption is extremely safe and that it has already been tested in PCA patients, our results suggest translational utility of silibinin in PCA patients with advanced metastatic disease.
Supplementary Material
(A–B) The position of individual cytokines on the cytokine array 6 and array 7.
Acknowledgments
Grant Support: This work was supported by NCI RO1 grant CA102514.
Abbreviations
- Acrp30
Adipocyte complement related protein of 30 kDa
- AP1
Activator protein 1
- BMP
Bone morphogenetic protein
- CCM
Control conditioned media
- DAPI
4′,6-diamidino-2-phenylindole
- DMSO
Dimethyl sulfoxide
- EMSA
Electrophoretic mobility shift assay
- G-CSF
Granulocyte-colony stimulating factor
- GM-CSF
Granulocyte macrophage-colony stimulating factor
- HGF
Hepatocyte growth factor
- IFN-γ
Interferon gamma
- IGF
Insulin-like growth factor
- IGFBP-3
Insulin-like growth factor binding protein 3
- IL-6
Interleukin 6
- I-TAC
Interferon-inducible T-cell alpha chemoattractant
- MAPK
Mitogen activated protein kinase
- M-CSF
Macrophage-colony stimulating factor
- NFATc1
Nuclear factor of activated T-cells
- NF-κB
Nuclear factor-kappa B
- OSCAR
Osteoclast-associated receptor
- PCA
prostate cancer
- PTHrP
Parathyroid hormone-related protein
- RANKL
Receptor activator of nuclear factor κB ligand
- Runx2
Runt-related transcription factors 2
- SBCM
Silibinin-treated conditioned media
- TARC
Thymus activation regulated cytokine
- TGF-β
Transforming growth factor beta
- TIMP
Tissue inhibitor of metalloproteinase
- TNFα
Tumor necrosis factor alpha
- TRAP
Tartrate resistant acid phosphatase
References
- 1.Roudier MP, True LD, Higano CS, et al. Phenotypic heterogeneity of end-stage prostate carcinoma metastatic to bone. Hum Pathol. 2003;34:646–653. doi: 10.1016/s0046-8177(03)00190-4. [DOI] [PubMed] [Google Scholar]
- 2.Saad F, Markus R, Goessl C. Targeting the receptor activator of nuclear factor-kappaB (RANK) ligand in prostate cancer bone metastases. BJU Int. 2008;101:1071–1075. doi: 10.1111/j.1464-410X.2007.07364.x. [DOI] [PubMed] [Google Scholar]
- 3.Msaouel P, Pissimissis N, Halapas A, Koutsilieris M. Mechanisms of bone metastasis in prostate cancer: clinical implications. Best Pract Res Clin Endocrinol Metab. 2008;22:341–355. doi: 10.1016/j.beem.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Buijs JT, van der Pluijm G. Osteotropic cancers: from primary tumor to bone. Cancer Lett. 2009;273:177–193. doi: 10.1016/j.canlet.2008.05.044. [DOI] [PubMed] [Google Scholar]
- 5.Casimiro S, Guise TA, Chirgwin J. The critical role of the bone microenvironment in cancer metastases. Mol Cell Endocrinol. 2009;310:71–81. doi: 10.1016/j.mce.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Clarke NW, Hart CA, Brown MD. Molecular mechanisms of metastasis in prostate cancer. Asian J Androl. 2009;11:57–67. doi: 10.1038/aja.2008.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ibrahim T, Flamini E, Fabbri L, et al. Multidisciplinary approach to the treatment of bone metastases: Osteo-Oncology Center, a new organizational model. Tumori. 2009;95:291–297. doi: 10.1177/030089160909500304. [DOI] [PubMed] [Google Scholar]
- 8.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 9.Asagiri M, Takayanagi H. The molecular understanding of osteoclast differentiation. Bone. 2007;40:251–264. doi: 10.1016/j.bone.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 10.Body JJ. Denosumab for the management of bone disease in patients with solid tumors. Expert Rev Anticancer Ther. 2011 doi: 10.1586/era.11.204. [DOI] [PubMed] [Google Scholar]
- 11.Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377:813–822. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 13.Lee ZH, Kim HH. Signal transduction by receptor activator of nuclear factor kappa B in osteoclasts. Biochemical and biophysical research communications. 2003;305:211–214. doi: 10.1016/s0006-291x(03)00695-8. [DOI] [PubMed] [Google Scholar]
- 14.Feng X. RANKing intracellular signaling in osteoclasts. IUBMB Life. 2005;57:389–395. doi: 10.1080/15216540500137669. [DOI] [PubMed] [Google Scholar]
- 15.Agarwal R, Agarwal C, Ichikawa H, Singh RP, Aggarwal BB. Anticancer potential of silymarin: from bench to bed side. Anticancer Res. 2006;26:4457–4498. [PubMed] [Google Scholar]
- 16.Pradhan SC, Girish C. Hepatoprotective herbal drug, silymarin from experimental pharmacology to clinical medicine. Indian J Med Res. 2006;124:491–504. [PubMed] [Google Scholar]
- 17.Deep G, Singh RP, Agarwal C, Kroll DJ, Agarwal R. Silymarin and silibinin cause G1 and G2-M cell cycle arrest via distinct circuitries in human prostate cancer PC3 cells: a comparison of flavanone silibinin with flavanolignan mixture silymarin. Oncogene. 2006;25:1053–1069. doi: 10.1038/sj.onc.1209146. [DOI] [PubMed] [Google Scholar]
- 18.Singh RP, Raina K, Deep G, Chan D, Agarwal R. Silibinin suppresses growth of human prostate carcinoma PC-3 orthotopic xenograft via activation of extracellular signal-regulated kinase 1/2 and inhibition of signal transducers and activators of transcription signaling. Clinical cancer research: an official journal of the American Association for Cancer Research. 2009;15:613–621. doi: 10.1158/1078-0432.CCR-08-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raina K, Rajamanickam S, Singh RP, Deep G, Chittezhath M, Agarwal R. Stage-specific inhibitory effects and associated mechanisms of silibinin on tumor progression and metastasis in transgenic adenocarcinoma of the mouse prostate model. Cancer research. 2008;68:6822–6830. doi: 10.1158/0008-5472.CAN-08-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh RP, Raina K, Sharma G, Agarwal R. Silibinin inhibits established prostate tumor growth, progression, invasion, and metastasis and suppresses tumor angiogenesis and epithelial-mesenchymal transition in transgenic adenocarcinoma of the mouse prostate model mice. Clinical cancer research: an official journal of the American Association for Cancer Research. 2008;14:7773–7780. doi: 10.1158/1078-0432.CCR-08-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flaig TW, Gustafson DL, Su LJ, et al. A phase I and pharmacokinetic study of silybin-phytosome in prostate cancer patients. Invest New Drugs. 2007;25:139–146. doi: 10.1007/s10637-006-9019-2. [DOI] [PubMed] [Google Scholar]
- 22.Flaig TW, Glode M, Gustafson D, et al. A study of high-dose oral silybin-phytosome followed by prostatectomy in patients with localized prostate cancer. The Prostate. 2010;70:848–855. doi: 10.1002/pros.21118. [DOI] [PubMed] [Google Scholar]
- 23.Muller MM, Schreiber E, Schaffner W, Matthias P. Rapid test for in vivo stability and DNA binding of mutated octamer binding proteins with ‘mini-extracts’ prepared from transfected cells. Nucleic Acids Res. 1989;17:6420. doi: 10.1093/nar/17.15.6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zi X, Feyes DK, Agarwal R. Anticarcinogenic effect of a flavonoid antioxidant, silymarin, in human breast cancer cells MDA-MB 468: induction of G1 arrest through an increase in Cip1/p21 concomitant with a decrease in kinase activity of cyclin-dependent kinases and associated cyclins. Clinical cancer research: an official journal of the American Association for Cancer Research. 1998;4:1055–1064. [PubMed] [Google Scholar]
- 25.Kaur M, Velmurugan B, Tyagi A, et al. Silibinin suppresses growth and induces apoptotic death of human colorectal carcinoma LoVo cells in culture and tumor xenograft. Mol Cancer Ther. 2009;8:2366–2374. doi: 10.1158/1535-7163.MCT-09-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agarwal C, Singh RP, Dhanalakshmi S, et al. Silibinin upregulates the expression of cyclin-dependent kinase inhibitors and causes cell cycle arrest and apoptosis in human colon carcinoma HT-29 cells. Oncogene. 2003;22:8271–8282. doi: 10.1038/sj.onc.1207158. [DOI] [PubMed] [Google Scholar]
- 27.Deep G, Oberlies NH, Kroll DJ, Agarwal R. Isosilybin B causes androgen receptor degradation in human prostate carcinoma cells via PI3K-Akt-Mdm2-mediated pathway. Oncogene. 2008;27:3986–3998. doi: 10.1038/onc.2008.45. [DOI] [PubMed] [Google Scholar]
- 28.Singh RP, Dhanalakshmi S, Mohan S, Agarwal C, Agarwal R. Silibinin inhibits UVB- and epidermal growth factor-induced mitogenic and cell survival signaling involving activator protein-1 and nuclear factor-kappaB in mouse epidermal JB6 cells. Mol Cancer Ther. 2006;5:1145–1153. doi: 10.1158/1535-7163.MCT-05-0478. [DOI] [PubMed] [Google Scholar]
- 29.Deep G, Raina K, Singh RP, Oberlies NH, Kroll DJ, Agarwal R. Isosilibinin inhibits advanced human prostate cancer growth in athymic nude mice: comparison with silymarin and silibinin. Int J Cancer. 2008;123:2750–2758. doi: 10.1002/ijc.23879. [DOI] [PubMed] [Google Scholar]
- 30.Kim JL, Kang SW, Kang MK, et al. Osteoblastogenesis and osteoprotection enhanced by flavonolignan silibinin in osteoblasts and osteoclasts. J Cell Biochem. 2012;113:247–259. doi: 10.1002/jcb.23351. [DOI] [PubMed] [Google Scholar]
- 31.Hiasa M, Abe M, Nakano A, et al. GM-CSF and IL-4 induce dendritic cell differentiation and disrupt osteoclastogenesis through M-CSF receptor shedding by up-regulation of TNF-alpha converting enzyme (TACE) Blood. 2009;114:4517–4526. doi: 10.1182/blood-2009-04-215020. [DOI] [PubMed] [Google Scholar]
- 32.Hemingway F, Taylor R, Knowles HJ, Athanasou NA. RANKL-independent human osteoclast formation with APRIL, BAFF, NGF, IGF I and IGF II. Bone. 2011;48:938–944. doi: 10.1016/j.bone.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 33.Kim JH, Jin HM, Kim K, et al. The mechanism of osteoclast differentiation induced by IL-1. J Immunol. 2009;183:1862–1870. doi: 10.4049/jimmunol.0803007. [DOI] [PubMed] [Google Scholar]
- 34.Kim JH, Kim K, Jin HM, et al. Silibinin inhibits osteoclast differentiation mediated by TNF family members. Molecules and Cells. 2009;28:201–207. doi: 10.1007/s10059-009-0123-y. [DOI] [PubMed] [Google Scholar]
- 35.Takayanagi H, Kim S, Koga T, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3:889–901. doi: 10.1016/s1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 36.Zhao Q, Wang X, Liu Y, He A, Jia R. NFATc1: functions in osteoclasts. Int J Biochem Cell Biol. 2010;42:576–579. doi: 10.1016/j.biocel.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 37.Kim Y, Sato K, Asagiri M, Morita I, Soma K, Takayanagi H. Contribution of nuclear factor of activated T cells c1 to the transcriptional control of immunoreceptor osteoclast-associated receptor but not triggering receptor expressed by myeloid cells-2 during osteoclastogenesis. J Biol Chem. 2005;280:32905–32913. doi: 10.1074/jbc.M505820200. [DOI] [PubMed] [Google Scholar]
- 38.Matsumoto M, Sudo T, Saito T, Osada H, Tsujimoto M. Involvement of p38 mitogen-activated protein kinase signaling pathway in osteoclastogenesis mediated by receptor activator of NF-kappa B ligand (RANKL) J Biol Chem. 2000;275:31155–31161. doi: 10.1074/jbc.M001229200. [DOI] [PubMed] [Google Scholar]
- 39.Rucci N, Teti A. Osteomimicry: how tumor cells try to deceive the bone. Front Biosci (Schol Ed) 2010;2:907–915. doi: 10.2741/s110. [DOI] [PubMed] [Google Scholar]
- 40.Josson S, Matsuoka Y, Chung LW, Zhau HE, Wang R. Tumor-stroma co-evolution in prostate cancer progression and metastasis. Semin Cell Dev Biol. 2010;21:26–32. doi: 10.1016/j.semcdb.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA: a cancer journal for clinicians. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 42.Aapro M, Saad F, Costa L. Optimizing clinical benefits of bisphosphonates in cancer patients with bone metastases. Oncologist. 2010;15:1147–1158. doi: 10.1634/theoncologist.2007-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith MR, Saad F, Coleman R, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet. 2012;379:39–46. doi: 10.1016/S0140-6736(11)61226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deep G, Agarwal R. Antimetastatic efficacy of silibinin: molecular mechanisms and therapeutic potential against cancer. Cancer Metastasis Rev. 2010;29:447–463. doi: 10.1007/s10555-010-9237-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deep G, Gangar SC, Agarwal C, Agarwal R. Role of E-cadherin in antimigratory and antiinvasive efficacy of silibinin in prostate cancer cells. Cancer Prev Res (Phila) 2011;4:1222–1232. doi: 10.1158/1940-6207.CAPR-10-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tyagi A, Singh RP, Ramasamy K, et al. Growth inhibition and regression of lung tumors by silibinin: modulation of angiogenesis by macrophage-associated cytokines and nuclear factor-kappaB and signal transducers and activators of transcription 3. Cancer Prev Res (Phila Pa) 2009;2:74–83. doi: 10.1158/1940-6207.CAPR-08-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Atanga E, Dolder S, Dauwalder T, Wetterwald A, Hofstetter W. TNFalpha inhibits the development of osteoclasts through osteoblast-derived GM-CSF. Bone. 2011;49:1090–1100. doi: 10.1016/j.bone.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 48.Qian Y, Huang HZ. The role of RANKL and MMP-9 in the bone resorption caused by ameloblastoma. Journal of oral pathology & medicine: official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology. 2010;39:592–598. doi: 10.1111/j.1600-0714.2009.00882.x. [DOI] [PubMed] [Google Scholar]
- 49.Tu Q, Zhang J, Dong LQ, et al. Adiponectin inhibits osteoclastogenesis and bone resorption via APPL1-mediated suppression of Akt1. The Journal of biological chemistry. 2011;286:12542–12553. doi: 10.1074/jbc.M110.152405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oshima K, Nampei A, Matsuda M, et al. Adiponectin increases bone mass by suppressing osteoclast and activating osteoblast. Biochemical and biophysical research communications. 2005;331:520–526. doi: 10.1016/j.bbrc.2005.03.210. [DOI] [PubMed] [Google Scholar]
- 51.Wang Y, Nishida S, Elalieh HZ, Long RK, Halloran BP, Bikle DD. Role of IGF-I signaling in regulating osteoclastogenesis. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2006;21:1350–1358. doi: 10.1359/jbmr.060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coelho LF, Magno de Freitas Almeida G, Mennechet FJ, Blangy A, Uze G. Interferon-alpha and -beta differentially regulate osteoclastogenesis: role of differential induction of chemokine CXCL11 expression. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:11917–11922. doi: 10.1073/pnas.0502188102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morita Y, Ono A, Serizawa A, et al. Purification and identification of lactoperoxidase in milk basic proteins as an inhibitor of osteoclastogenesis. Journal of dairy science. 2011;94:2270–2279. doi: 10.3168/jds.2010-4039. [DOI] [PubMed] [Google Scholar]
- 54.Braun T, Zwerina J. Positive regulators of osteoclastogenesis and bone resorption in rheumatoid arthritis. Arthritis research & therapy. 2011;13:235. doi: 10.1186/ar3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Adamopoulos IE, Xia Z, Lau YS, Athanasou NA. Hepatocyte growth factor can substitute for M-CSF to support osteoclastogenesis. Biochemical and biophysical research communications. 2006;350:478–483. doi: 10.1016/j.bbrc.2006.09.076. [DOI] [PubMed] [Google Scholar]
- 56.Cadosch D, Gautschi OP, Chan E, Simmen HP, Filgueira L. Titanium induced production of chemokines CCL17/TARC and CCL22/MDC in human osteoclasts and osteoblasts. Journal of biomedical materials research Part A. 2010;92:475–483. doi: 10.1002/jbm.a.32390. [DOI] [PubMed] [Google Scholar]
- 57.Takamatsu Y, Simmons PJ, Moore RJ, Morris HA, To LB, Levesque JP. Osteoclast-mediated bone resorption is stimulated during short-term administration of granulocyte colony-stimulating factor but is not responsible for hematopoietic progenitor cell mobilization. Blood. 1998;92:3465–3473. [PubMed] [Google Scholar]
- 58.Miyazaki T, Katagiri H, Kanegae Y, et al. Reciprocal role of ERK and NF-kappaB pathways in survival and activation of osteoclasts. J Cell Biol. 2000;148:333–342. doi: 10.1083/jcb.148.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takayanagi H. The role of NFAT in osteoclast formation. Ann N Y Acad Sci. 2007;1116:227–237. doi: 10.1196/annals.1402.071. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A–B) The position of individual cytokines on the cytokine array 6 and array 7.