Abstract
During tendon development collagen fibrillogenesis occurs in extracellular micro-domains defined by the tenocytes. This permits cellular regulation of the extracellular steps involved in the tissue-specific matrix assembly required for function. The hypothesis tested here is that collagen V associates with the tenocyte surface where it functions in regulation of collagen assembly and cell-directed fibril deposition. The in vitro and in vivo data demonstrate that collagen V is a quantitatively minor component of the tendon. It is preferentially localized on the tenocyte surface as distinct foci in tendons and in cell culture. In vitro data indicate that this interaction with the tenocyte is not HSPG GAG-dependent. Collagen V is present as the mature, processed form, is absent from the media, and is a significant part of the detergent-insoluble cell layer, presumably as part of a membrane-associated complex. In contrast, procollagen I is not efficiently processed and is found predominantly in the culture media. Our data suggest that the regulatory role of collagen V requires collagen V to occupy a different cellular niche from the structural collagen I. In monolayer cultures, the conversion to the tissue form of collagen V and its deposition with the cell layer suggest efficient engagement of procollagen V with pericellular receptors and processing enzymes. The secretion of collagen I into the media and inefficient processing of procollagen I suggest reduced accessibility to these pericellular molecules due to disengagement from the cell surface. This all points to differential spatial localization of collagen V as a mechanism to optimize its regulatory roles during the cell-surface directed steps in tendon collagen fibril assembly.
Keywords: Tenocyte, Fibroblast, Cell-associated, Collagen V, Collagen Fibrillogenesis, Tendon, Mouse
INTRODUCTION
Tendon fibroblasts synthesize collagen that assembles into fibrils that are further organized into a tendon-specific extracellular matrix. This assembly and organization involve a sequence of regulated steps including nucleation of protofibril assembly, linear and lateral growth of protofibrils into mature fibrils, and higher order assembly of fibrils into fibers (Banos et al., 2008; Birk et al., 1995; Birk and Zycband, 1994; Holmes et al., 1998; Izu et al., 2011; Zhang et al., 2005). The tendon fibroblast plays a critical role in regulating these distinct stages by compartmentalizing the extracellular matrix into a series of micro-domains where these processes occur (Birk DE, 1994; Birk and Trelstad, 1986; Canty et al., 2004; Zhang et al., 2005).
Collagen V has important regulatory roles in nucleating assembly of collagen into protofibrils (Banos et al., 2008; Birk and Bruckner, 2011; Blaschke et al., 2000; Wenstrup et al., 2004a; Wenstrup et al., 2004b; Wenstrup et al., 2011). The nucleation of collagen fibrillogenesis within the pericellular environment would provide a mechanism whereby collagen V serves as a focal regulatory point for fibrillogenesis (Birk and Trelstad, 1986; Canty et al., 2004; Smith et al., 2011; Sun et al., 2011). Such specific nucleation sites would ensure strict regulation of cell-orchestrated fibril formation and assembly, and the positioning of the resulting fibrils into the extracellular matrix. It has also been proposed that collagen V may define these focal nucleation points to regulate the displacement of the newly made fibrils into the matrix as new pools of intracellular collagen are brought to the cell surface (Wenstrup et al., 2011). Collagen V has distinct regulatory roles that were demonstrated to be crucial, since collagen V null mice die embryonically due to the inability to form fibrils (Wenstrup et al., 2004b; Wenstrup et al., 2006).
Collagen V co-localizes with collagen I in heterotypic fibrils within the matrix of various connective tissues (Adachi and Hayashi, 1986; Birk, 2001; Birk et al., 1988; Linsenmayer et al., 1990). Early work also suggested roles for collagen V pericellular regions (Madri et al., 1980; Martinez-Hernandez et al., 1982). However, apart from hints in that work and other early experiments describing its localization in basement membranes as part of an “exocytoskeleton” (Gay et al., 1981; Madri et al., 1980; Madri and Furthmayr, 1979), little is known about the spatial localization of collagen V relative to the tendon fibroblast surface. Our recent work demonstrated the presence of collagen V in a pericellular/cell-associated matrix extract from developing tenocytes (Smith et al., 2011). We hypothesize that collagen V is enriched at the cell surface/pericellular matrix where it is involved in cell-directed regulation of the initial stages in collagen fibril assembly. To address this, we analyzed the compartmentalization and spatial localization of this regulatory, fibril-forming collagen in a cell culture model and in early postnatal tendons. The data demonstrate that collagen V is a cell surface collagen that is preferentially retained in the insoluble pericellular matrix of cultured tendon fibroblasts while collagen I is primarily secreted into the media. The data also show that, both in vitro and in situ, the localization of collagen V at the fibroblast surface is in focal sites; cell culture experiments demonstrate that this localization is independent of heparan sulfate GAGs.
RESULTS
Minor quantitative contribution of collagen V to the collagen content in tendons
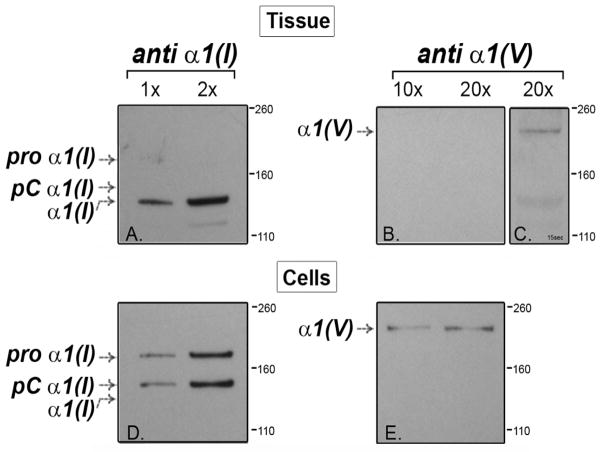
The relative contributions of collagens I and V to the tendon matrix were analyzed in extracts of postnatal day 4 (P4) tendons using immuno-blots (Fig. 1A–C). These data, obtained using different loading concentrations and exposure times, indicate an abundance of collagen I compared to collagen V, as expected (Fessler et al., 1981; Smith et al., 1986). Tenocytes grown in culture also synthesized collagen I in large excess compared to collagen V (Fig. 1D,E). Here, a large fraction of the collagen I was only partially processed and in the pC form, where the N-propeptide had been cleaved from the procollagen precursor. Both in vivo and in vitro, collagen V is a quantitatively minor component of tendon compared with collagen I. In addition, a comparable ratio of collagen I:V is synthesized in both systems.
Figure 1. Collagen V is a quantitatively minor component of tendon and comparably expressed in tenocyte cultures.
Collagens I and V were analyzed from P4 tendons (A–C) and cultured tenocytes (D, E). (A) Collagen I extracted from tendons was present as fully processed collagen. (B, C, E) Collagen V extracted from developing tendons and cultured tenocytes was present as the partially processed tissue form. Collagen V is present in significantly smaller amounts than collagen I. Based on band pixel density analysis of gels comparable to those in Panels A (anti-collagen I) and C (anti-collagen V), there is an ~80:1 ratio of extractable collagen I to collagen V in the developing tendon. (D, E) A similar minor quantitative contribution of collagen V was observed in the cell culture proteins. In tenocyte cultures collagen I was inefficiently processed with most of it present as unprocessed procollagen I or partially processed pC collagen I. Demarcations between panels indicate different exposures of a single gel to optimize signal for a specific protein. Separately bordered panels indicate different gels.
Cell-surface association of collagen V
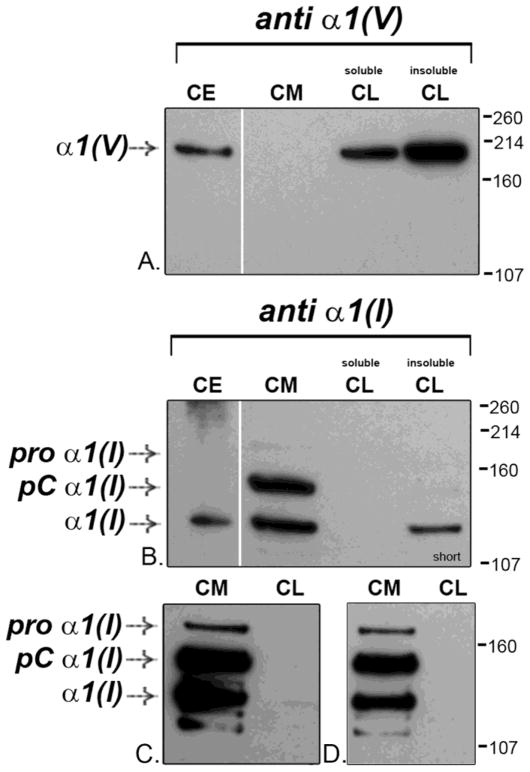
Since the relative amounts of collagen I and V were produced in both culture and tissues were comparable, the culture model was utilized to define the localization of collagens I and V relative to the tenocytes. Cultures were separated into cell media and cell layer fractions. The data demonstrate that collagen V is selectively retained in the cell layer with virtually no collagen V observed in the media fraction (Fig. 2A). In contrast, collagen I is primarily secreted into the media with little collagen I remaining in the cell layer fraction as primarily partially processed pC collagen (Fig. 2B) and with the full length precursor shown after longer exposure in panels C (same experimental sample) and D (same sample electrophoresed on a separate occasion). The data, therefore, indicate differential localization patterns for the two collagens, with collagen V localizing to the cell layer fraction and collagen I to the media fraction. There was very little overlap in the localization of these collagen types.
Figure 2. Collagen V is in the cell layer of cultured tenocytes and is enriched relative to collagen I.
Cell culture data show that collagen V is selectively retained in the cell layer (CL) while collagen I, conversely, is primarily secreted into the media (CM) and accumulates there. (A, B) Corneal extract (CE), generated in the same way as the tendon tissue extracts in this report, is used as a standard. (B) The collagen I molecules in the cell media were both the partially processed pC collagen and the mature/fully processed/tissue forms. (A) The processed, mature tissue form of collagen V is seen in the corneal extract. The collagen V from the cultured tendon cells was primarily the processed form. Processed collagen V is a major band in the insoluble CL, detergent-insoluble material that did not dissolve in our 1% Triton X-100 buffer and sedimented to the bottom of the tube when the cell lysate was spun. Panel C shows the same blot of the samples in Panel B, but at a longer exposure where the procollagen is more evident. Panel D shows the cell media (CM) from the same experiment electrophoresed on a different occasion. Demarcations between panels indicate different exposures of a single gel to optimize signal for a specific protein. Separately bordered panels indicate different gels.
Cell culture collagen I molecules are present both as the unprocessed procollagen as well as processed collagen. Conversely, the processed tissue form of collagen V is the major expressed cell culture product. This is consistent with the collagen V being extracellular and not from an intracellular, unprocessed pool. A subset of proteins in the cell layer was insoluble in Triton X-100 (insoluble CL). Collagen V is a major component of this insoluble cell layer fraction (Fig. 2A), being retained with the cell layer in a detergent-insoluble, and possibly plasma membrane-bound complex.
Collagen V is a component of the tendon pericellular matrix
Collagen V was preferentially found in the cell layer fraction of cultured tenocytes. To further address the localization, cultured tenocytes were rinsed and the cell layer biotinylated. The data indicate that collagen V is a cell-surface component of the pericellular matrix. This evidence is seen by the presence of collagen V in cell-surface biotinylated (+bio) material (Fig. 3A), where the same sample is shown blotted with anti-collagen V antibody in panel A and streptavidin-HRP in panel B. No collagen V was detected in a streptavidin bead pull-down of unbiotinylated cell layer (−bio, panel A). Since collagen V has a heparin binding domain (Greenspan et al., 1991), the possibility that collagen V-heparan sulfate interactions were responsible for the cell surface retention was examined. HS chains from the HSPGs in the culture were efficiently digested using 50 mU/ml of heparitinase; efficient digestion is demonstrated in the reactivity of select HSPGs (perlecan and syndecan-1) with the Δ-HS antibody, which recognizes a neo-epitope uncovered on the digested HS stubs indicated by the square brackets (Fig. 3D) (Govindraj et al., 2002); panel C shows the anti-perlecan (with perlecan signal denoted by the arrow) and the anti-syndecan-1 blot. Even with the HS chains removed from the culture, processed collagen V was still retained relatively exclusively in the cell layer (Fig. 3E). Therefore, HS GAG chains are not responsible for retaining collagen V at the cell surface.
Figure 3. Collagen V is tethered to the cell surface by HS GAG-independent interactions.
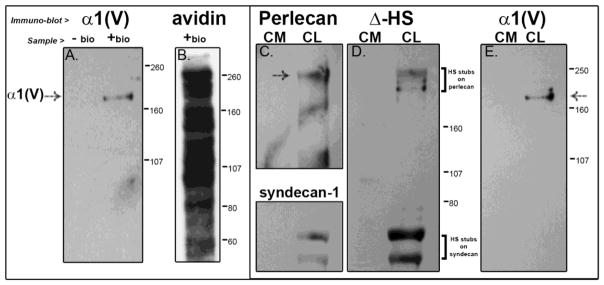
(A, E) The collagen V in the cultured cells is processed and in the mature form. (A) Further, presence of the α1 collagen V band in cell-surface biotinylated or “+ bio” (and absence in unbiotinylated or “− bio”) material from a streptavidin pull-down indicates that collagen V is a cell surface molecule of tendon fibroblasts. (B)This material is also shown blotted with streptavidin-HRP to show the full labeled complement of the numerous cell surface molecules. We further see that HS chains are not responsible for retaining collagen V at the cell surface; (C, D) even with efficient digestion of HS chains, as evidenced by reaction of the digested HSPGs perlecan (pericellular) and syndecan-1 (integral to the membrane) in panel C with the Δ-HS stub antibody in blot D, collagen V is still retained in the cell layer (panel E). Demarcations between panels indicate different exposures of a single gel to optimize signal for a specific protein. Separately bordered panels indicate different gels.
Collagen V localizes to plaques in the pericellular matrix of cultured tenocytes
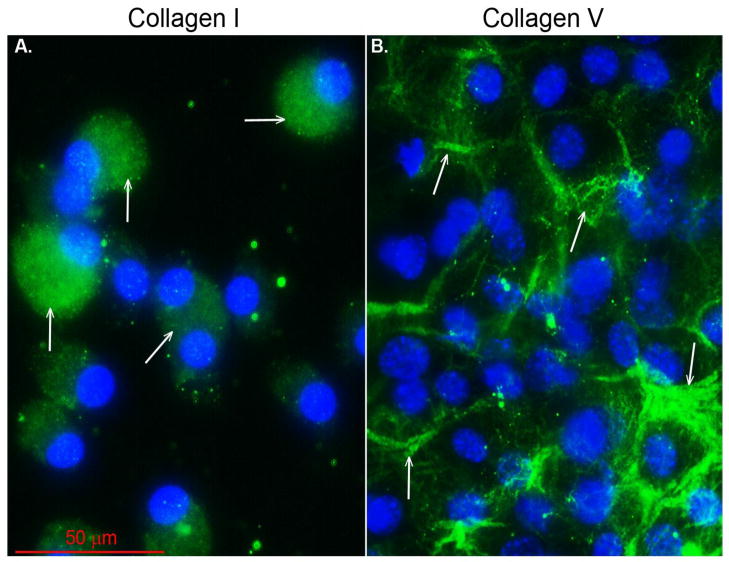
Collagen V is selectively localized to the cell surface/pericellular matrix of cultured tenocytes. Immuno-localization of collagen V demonstrated its retention in the tenocyte cell layer where it is enriched in plaque-like structures (Fig. 4B). The focal retention of collagen V in these plaques is specific and not seen for collagen I (Fig. 4A). These cultures were taken and processed for immunofluorescence microscopy at the same time as the cells processed for immuno-blots. The data support a retention and enrichment of collagen V in pericellular plaques, consistent with a regulatory role in cell-surface directed fibril and matrix assembly.
Figure 4. Collagen V is localized at extracellular focal plaques in tenocyte cultures.
(A) Immunofluorescence of cultured cells shows an intracellular localization of collagen I (green signal) only stores within the cell. No extracellular collagen I signal was observed as seen in Fig. 2 it is present in the culture medium. (B) Collagen V (green signal) selectively localizes to the cell surface, and is enriched there in a plaque-like distribution. Focal retention of collagen V in these plaques is specific to the area around the cell (e.g., white arrows). (Blue signal, DAPI for nuclei)
Collagen V is retained as focal plaques at the tenocyte surface in developing tendon
In the developing (P4) tendon, collagen I is distributed throughout the tendon, while collagen V is enriched at the fibroblast surface (Fig. 5). Immuno-localization demonstrates that collagen V reactivity is localized to focal plaques associated with the surface of the tenocyte while collagen I is homogeneously distributed in fibers throughout the tendon. Collagen V is thereby optimally positioned for a regulatory role in cell-directed assembly and deposition of collagen fibrils.
Figure 5. Collagen V preferentially localizes as focal tenocyte-associated plaques in developing tendon.
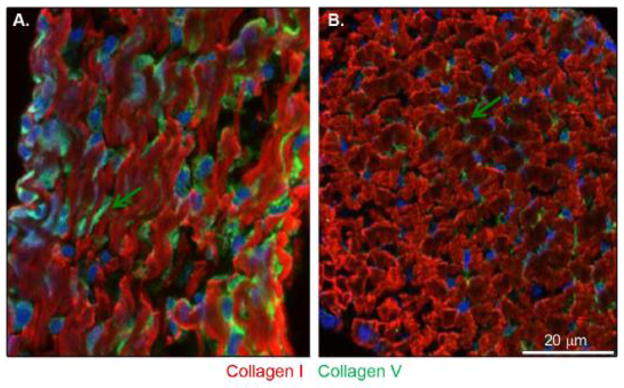
(A) Longitudinal sections: further in vivo data show that collagen I (red signal) is distributed throughout the tendon proper and shows no accumulation in a specific position relative to the cell. Conversely, collagen V (green signal) is located with the cell. This expression is enhanced at the cell surface. (B) Transverse section: collagen V expression constitutes focal plaques at the surface of the tenocytes (shown with nuclei in blue). Arrows depict the cell surface location, which is more evident in cross-section.
DISCUSSION
During tendon development, the tenocyte forms compartments where the nucleation and assembly of collagen protofibrils occur (Birk and Bruckner, 2011; Birk and Trelstad, 1984; Canty et al., 2004; Zhang et al., 2005). Collagen V is a crucial regulator of the nucleation step (Wenstrup et al., 2004a; Wenstrup et al., 2011). In this report, we demonstrate that collagen V, a regulatory fibril-forming collagen, has a different extracellular niche from collagen I, a structural fibril-forming collagen (Birk and Bruckner, 2011; Smith and Birk, 2010). We hypothesized that there is a preferential association of collagen V with the tendon fibroblast surface as matrix accumulation proceeds, allowing the cell to focally control regulation of protofibril assembly and positioning of newly assembled protofibrils into the developing matrix. Our cell culture data clearly demonstrate a differential localization of collagens I and V. Collagen V is preferentially associated with the tenocyte surface in the pericellular matrix. The cell culture and in vivo data presented in this study confirm that collagen V is a quantitatively small portion of the tendon collagen content. Despite the differences in content, the relative ratios of the two collagens remain constant across the tissue and cell culture samples. These data validate the use of the cell culture system as a model for analyses of the roles of collagen V in in vivo processes.
Our in situ data are consistent with the in vitro data and show that collagen V is enriched at the tenocyte surface in the form of focal plaques. In contrast, collagen I localized to collagen fibers, as expected. This spatial separation of collagens I and V was unexpected, since collagens I and V are known to co-assemble into heterotypic fibrils (Adachi and Hayashi, 1986; Birk, 2001; Birk et al., 1988; Birk et al., 1986; Linsenmayer et al., 1990). The focal accumulation of collagen V resulted in the generation of a distinct fluorescent signal as did the high concentration of collagen I in fibers. The co-localization of collagens I and V as heterotypic fibrils results in a signal disparity that requires an optimization of the experimental parameters to account for the extreme excess of collagen I in tendon fibrils; this was not done to address this question. In this work, the exposure was optimized to spatially localize collagens V and I which highlighted the enrichment of collagen V at the tenocyte surface rather than its co-localization with I in heterotypic fibrils. In vitro, procollagen I is secreted into the media and does not assemble into fibrils, therefore the increased staining for collagen V seen in the cultured cells. The pericellular location of collagen V is consistent with its roles in regulating collagen fibrillogenesis. At the site of procollagen processing collagen can efficiently nucleate collagen I assembly generating heterotypic collagen I/V fibrils. The ratio of collagen V to collagen I would then control fibril number and diameter. In addition, a tethering of the nucleator (collagen V) to the tenocyte surface also allows cellular control of fibril deposition and organization into the developing matrix.
Regarding the differential localization of collagens I and V in our cell culture experiments, we submit that the differential results were due to their differing roles as structural collagen I versus regulatory collagen V. The data indicate that their destinations diverge in 2D tenocyte cultures where extracellular compartmentalization is lost. We know that procollagen V processing is fundamentally different from procollagen I processing (Greenspan et al., 1991; Imamura et al., 1998; Smith and Birk, 2010; Unsold et al., 2002); differences in processing also are implicated in localization differences for collagens I and III (Gay et al., 1976). The retention of the globular region of the N-propeptide of collagen V may affect its retention at the cell surface, as the NH2-terminal non-collagenous domain interacts with cell surface molecules (Symoens et al., 2011). We also probed for collagen V in material that was surface-labeled with cell-impermeable biotin, which allowed us to further determine that collagen V was indeed in the extracellular matrix deposited by the cell and that our collagen V signal was not merely from the intracellular collagen V. We have shown in the current report that this interaction with the cell is not via HSPG GAGs, although collagen V has heparan sulfate binding sites (Greenspan et al., 1991) that could mediate direct interactions with the HSPG syndecan (Koda et al., 1985; San Antonio et al., 1994). If interaction is with the core protein, then this would be novel, as collagen V is known to interact with HSPG’s via their HS chains and its heparin binding sites.
The inefficient processing of procollagen I and accumulation of pro/collagen in the media of monolayer cultures is well known (Chan et al., 1990; Minor et al., 1986; Olsen and Berg, 1979; Rentz et al., 2007). It has been demonstrated that volume exclusion agents such as dextran or polyethylene glycol (PEG) or use of an agarose overlay will result in efficient processing (Bateman et al., 1986; Hassell et al., 2008). Use of these agents in culture enhanced conversion of procollagen to collagen, fibril formation and fibril deposition into an ECM. Presumably, these agents replace the normal cellular compartmentalization lost in monolayer culture to facilitate the interaction of procollagen I with collagen N- and C- propeptide processing enzymes at the cell surface. In our work, the processing of procollagen V to mature collagen V and its deposition into the insoluble matrix indicates that there is increased engagement between extracellular procollagen V and its processing enzymes. This suggests an intrinsic mechanism that preferentially retains collagen V at the cell surface while collagen I is released from the cell surface unprocessed and into the media as part of the secretome. In the case of collagen V processing, our report now shows that agents such as agarose, dextran or PEG appear to be unnecessary in long term culture of confluent cells, since the collagen V resides at the cell surface naturally for its protofibril nucleating role and is continuously accessible to the proteases. We speculate that this requires direct or indirect tethers (unrelated to heparan sulfate) to the plasma membrane. Recent studies of interactions between the NH2-terminal non-collagenous domain of collagen V and surfaceome proteins support this speculation (Symoens et al., 2011). This increased exposure of the procollagen as it is pericellularly tethered to the cell surface would enhance processing and deposition into the insoluble matrix (Rentz et al., 2007).
Although the precise interactions that occur in the cell-controlled deposition of an insoluble extracellular matrix are not yet elucidated, we suggest that it most likely begins with the interaction of a regulatory fibril-initiating collagen molecule, such as collagen V, with some integral membrane tether, either directly or indirectly (Wenstrup et al., 2006). Our on-going investigation into the potential tethers will tell us more about the interplay of these cell surface receptors with collagen V during matrix assembly.
EXPERIMENTAL PROCEDURES
Animals
Flexor digitorum longus (FDL) tendons from hind limbs of C57BL/6 mice at post natal (P) day 4 were used according to the IACUC guidelines of the University of South Florida.
Analysis of conditioned media and cell layer of cultured tenocytes
Cells were isolated from the tendons of P4 mice using Collagenase B (Roche). Tendon fibroblasts were cultured in DMEM supplemented with 10% FBS and 1 mM 2-phospho-L-ascorbic acid in 10 cm dishes until the cells were confluent (~6 days, with a media change on day 3). The media was then replaced with DMEM containing 1 mM ascorbate with 5% FBS for 24 hr, followed by 1% FBS for 24 hr, then serum-free DMEM, and culturing continued for an additional 72 hr. At least 3 independent cultures were analyzed. Conditioned media was pooled with 3 rinses of the cell layer and this combined volume of cell media (CM) along with an overnight 1% Triton X-100 extract of the cell layer (CL), was frozen, lyophilized and brought to 1/100th of their original volumes in 1X GLB (Invitrogen).
Heparitinase Digests
A set of cultures was treated with 50 mU/ml Heparitinase I (Seikagaku America) (Govindraj et al., 2002) from hour 69–72 of serum-free culture before the CM and CL were collected.
Cell Surface Protein Biotinylation
Finally, a set of cultures was labeled with the cell surface protein isolation kit (Pierce) according to the manufacturer’s instructions, but using a non-cleavable version of the cell-impermeable biotinylation reagent (i.e., Sulfo-NHS-LC-Biotin) to label exposed primary amines of proteins on the surface of the cells. Cell layers were then harvested, lysed and affinity-purified using NeutrAvidin Agarose Resin. The biotinylated proteins were eluted from the resin in the same volume of GLB used to reconstitute the previous samples. At least 3 independent samples were analyzed.
Generation of FDL Tendon Extracts
FDL tendons were dissected, rinsed in PBS and extracted in a 20-fold excess (volume/wet weight) of buffered 1% SDS (25 mM Tris HCl, 35 mM Tris base, 1% SDS, 0.15 mM EDTA, pH 8.5) for 24 hr at RT. At least 3 independent samples were analyzed.
Electrophoresis and Immuno-Blots
SDS-PAGE was performed using the NuPAGE Protein gel system (Invitrogen) with 4–12 % Bis-Tris gels. Samples were resolved by SDS-PAGE, transferred to nitrocellulose membranes, then processed using standard Western blot procedures with I-Block (AP Biosystems, 0.2 % in PBS) for blocking and ECL kits (Pierce) for development. The gel blots were scanned and imaged using a Bio-Rad Gel-Doc system and Quantity One software. For semi-quantitative analysis of the bands, Quantity One was used to scan the gel blots, then to outline individual bands on exposures within the linear range of the film, then to assign pixel densities (quantitatively, but in random units) to each band.
Antibodies
Anti-collagen I antibody (Chemicon) was used at 1:10,000. Anti-Δ-Heparan Sullfate [3G10 epitope] antibody (Seikagaku America) was used at 1:500. HRP- or fluor-conjugated antibodies (Invitrogen) were used at 1:5,000 and 1:200, respectively. The following primary antibodies were used at 1:1,000 in immuno-blots and 1:100 in immunofluorescence: anti-collagen V (Wenstrup et al., 2004a); anti-perlecan (generous gift from Dr. John Hassell); anti-syndecan-1 (Invitrogen).
Immuno-localization
FDL tendons were dissected and processed as previously described (Wenstrup et al., 2011). Cells were cultured in Lab-Tek™ glass chamber slides, rinsed 3 times with PBS then fixed for 15 min in 2% paraformaldehyde followed by rinsing in PBS. After fixation, tendons were rinsed in PBS, infiltrated with 10% sucrose, embedded in OCT medium, frozen and stored at −80°C. Frozen sections (3μm) were cut, mounted unto glass slides, dried, then rehydrated in PBS. Both tissues and cells were quenched with 1% glycine/PBS, and blocked for 4 hr in 2% BSA at RT°C. Primary antibody (in 1% BSA) was added overnight at 4°C, the slides were washed in PBS/0.2% (v/v) Tween-20 followed by incubation with secondary antibody for 4 hr at RT°C. The slides were then washed and mounted with medium containing DAPI before imaging using a Leica CTR 5500 fluorescence microscope (Wetzlar, Germany) and Leica DFC 340 FX digital camera. Results presented are representative of at least 3 independent experiments.
Highlights.
Collagen fibril assembly is regulated in cell-defined extracellular micro-domains
Collagen V is localized in tenocyte-associated foci plaques in vitro and in vivo
Collagen V is processed and cell-associated in the pericellular matrix in vitro
Collagen I is inefficiently processed and localized in the media in vitro
Spatial location of collagen V optimizes its regulatory roles in tendon assembly
Acknowledgments
Contract Grant Sponsor: NIH/NIAMS. Grant numbers: AR044745 and AR056937.
Supported by grants from NIH NIAMS, AR044745 and NRSA Fellowship AR056937.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi E, Hayashi T. In vitro formation of hybrid fibrils of type V collagen and type I collagen. Limited growth of type I collagen into thick fibrils by type V collagen. Connect Tissue Res. 1986;14:257–66. doi: 10.3109/03008208609017469. [DOI] [PubMed] [Google Scholar]
- Banos CC, Thomas AH, Kuo CK. Collagen fibrillogenesis in tendon development: current models and regulation of fibril assembly. Birth Defects Res C Embryo Today. 2008;84:228–44. doi: 10.1002/bdrc.20130. [DOI] [PubMed] [Google Scholar]
- Bateman JF, Cole WG, Pillow JJ, Ramshaw JA. Induction of procollagen processing in fibroblast cultures by neutral polymers. J Biol Chem. 1986;261:4198–203. [PubMed] [Google Scholar]
- Birk D, Bruckner P. Collagens, suprastructures and collagen fibril assembly. In: MRP, editor. The Extracellular Matrix: an Overview. Vol. 1. Springer; New York: 2011. In Press. [Google Scholar]
- Birk DE. Type V collagen: heterotypic type I/V collagen interactions in the regulation of fibril assembly. Micron. 2001;32:223–37. doi: 10.1016/s0968-4328(00)00043-3. [DOI] [PubMed] [Google Scholar]
- Birk DE, Fitch JM, Babiarz JP, Linsenmayer TF. Collagen type I and type V are present in the same fibril in the avian corneal stroma. J Cell Biol. 1988;106:999–1008. doi: 10.1083/jcb.106.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birk DE, Fitch JM, Linsenmayer TF. Organization of collagen types I and V in the embryonic chicken cornea. Invest Ophthalmol Vis Sci. 1986;27:1470–7. [PubMed] [Google Scholar]
- Birk DE, LT . Collagen fibril assembly, deposition, and organization into tissue specific matrices. In: Yurchenco BDPD, Mecham RP, editors. Extracellular Matrix Assembly and Structure. Academic Press; New York: 1994. pp. 91–128. [Google Scholar]
- Birk DE, Nurminskaya MV, Zycband EI. Collagen fibrillogenesis in situ: fibril segments undergo post-depositional modifications resulting in linear and lateral growth during matrix development. Dev Dyn. 1995;202:229–43. doi: 10.1002/aja.1002020303. [DOI] [PubMed] [Google Scholar]
- Birk DE, Trelstad RL. Extracellular compartments in matrix morphogenesis: collagen fibril, bundle, and lamellar formation by corneal fibroblasts. J Cell Biol. 1984;99:2024–33. doi: 10.1083/jcb.99.6.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birk DE, Trelstad RL. Extracellular compartments in tendon morphogenesis: collagen fibril, bundle, and macroaggregate formation. J Cell Biol. 1986;103:231–40. doi: 10.1083/jcb.103.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birk DE, Zycband E. Assembly of the tendon extracellular matrix during development. J Anat. 1994;184(Pt 3):457–63. [PMC free article] [PubMed] [Google Scholar]
- Blaschke UK, Eikenberry EF, Hulmes DJ, Galla HJ, Bruckner P. Collagen XI nucleates self-assembly and limits lateral growth of cartilage fibrils. J Biol Chem. 2000;275:10370–8. doi: 10.1074/jbc.275.14.10370. [DOI] [PubMed] [Google Scholar]
- Canty EG, Lu Y, Meadows RS, Shaw MK, Holmes DF, Kadler KE. Coalignment of plasma membrane channels and protrusions (fibripositors) specifies the parallelism of tendon. J Cell Biol. 2004;165:553–63. doi: 10.1083/jcb.200312071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan D, Lamande SR, Cole WG, Bateman JF. Regulation of procollagen synthesis and processing during ascorbate-induced extracellular matrix accumulation in vitro. Biochem J. 1990;269:175–81. doi: 10.1042/bj2690175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessler LI, Robinson WJ, Fessler JH. Biosynthesis of procollagen [(pro alpha 1 V)2 (pro alpha 2 V)] by chick tendon fibroblasts and procollagen (pro alpha 1 V)3 by hamster lung cell cultures. J Biol Chem. 1981;256:9646–51. [PubMed] [Google Scholar]
- Gay S, Martin GR, Muller PK, Timpl R, Kuhn K. Simultaneous synthesis of types I and III collagen by fibroblasts in culture. Proc Natl Acad Sci U S A. 1976;73:4037–40. doi: 10.1073/pnas.73.11.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay S, Rhodes RK, Gay RE, Miller EJ. Collagen molecules comprised of alpha 1(V)-chains (B-chains): an apparent localization in the exocytoskeleton. Coll Relat Res. 1981;1:53–8. doi: 10.1016/s0174-173x(80)80007-0. [DOI] [PubMed] [Google Scholar]
- Govindraj P, West L, Koob TJ, Neame P, Doege K, Hassell JR. Isolation and identification of the major heparan sulfate proteoglycans in the developing bovine rib growth plate. J Biol Chem. 2002;277:19461–9. doi: 10.1074/jbc.M200786200. [DOI] [PubMed] [Google Scholar]
- Greenspan DS, Cheng W, Hoffman GG. The pro-alpha 1(V) collagen chain. Complete primary structure, distribution of expression, and comparison with the pro-alpha 1(XI) collagen chain. J Biol Chem. 1991;266:24727–33. [PubMed] [Google Scholar]
- Hassell JR, Kane BP, Etheredge LT, Valkov N, Birk DE. Increased stromal extracellular matrix synthesis and assembly by insulin activated bovine keratocytes cultured under agarose. Exp Eye Res. 2008;87:604–11. doi: 10.1016/j.exer.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes DF, Graham HK, Kadler KE. Collagen fibrils forming in developing tendon show an early and abrupt limitation in diameter at the growing tips. J Mol Biol. 1998;283:1049–58. doi: 10.1006/jmbi.1998.2153. [DOI] [PubMed] [Google Scholar]
- Imamura Y, Steiglitz BM, Greenspan DS. Bone morphogenetic protein-1 processes the NH2-terminal propeptide, and a furin-like proprotein convertase processes the COOH-terminal propeptide of pro-alpha1(V) collagen. J Biol Chem. 1998;273:27511–7. doi: 10.1074/jbc.273.42.27511. [DOI] [PubMed] [Google Scholar]
- Izu Y, Ansorge HL, Zhang G, Soslowsky LJ, Bonaldo P, Chu ML, Birk DE. Dysfunctional tendon collagen fibrillogenesis in collagen VI null mice. Matrix Biol. 2011;30:53–61. doi: 10.1016/j.matbio.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koda JE, Rapraeger A, Bernfield M. Heparan sulfate proteoglycans from mouse mammary epithelial cells. Cell surface proteoglycan as a receptor for interstitial collagens. J Biol Chem. 1985;260:8157–62. [PubMed] [Google Scholar]
- Linsenmayer TF, Fitch JM, Birk DE. Heterotypic collagen fibrils and stabilizing collagens. Controlling elements in corneal morphogenesis? Ann N Y Acad Sci. 1990;580:143–60. doi: 10.1111/j.1749-6632.1990.tb17926.x. [DOI] [PubMed] [Google Scholar]
- Madri JA, Dreyer B, Pitlick FA, Furthmayr H. The collagenous components of the subendothelium. Correlation of structure and function. Lab Invest. 1980;43:303–15. [PubMed] [Google Scholar]
- Madri JA, Furthmayr H. Isolation and tissue localization of type AB2 collagen from normal lung parenchyma. Am J Pathol. 1979;94:323–31. [PMC free article] [PubMed] [Google Scholar]
- Martinez-Hernandez A, Gay S, Miller EJ. Ultrastructural localization of type V collagen in rat kidney. J Cell Biol. 1982;92:343–9. doi: 10.1083/jcb.92.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor RR, Sippola-Thiele M, McKeon J, Berger J, Prockop DJ. Defects in the processing of procollagen to collagen are demonstrable in cultured fibroblasts from patients with the Ehlers-Danlos and osteogenesis imperfecta syndromes. J Biol Chem. 1986;261:10006–14. [PubMed] [Google Scholar]
- Musselmann K, Kane B, Alexandrou B, Hassell JR. Stimulation of collagen synthesis by insulin and proteoglycan accumulation by ascorbate in bovine keratocytes in vitro. Invest Ophthalmol Vis Sci. 2006;47:5260–6. doi: 10.1167/iovs.06-0612. [DOI] [PubMed] [Google Scholar]
- Olsen BR, Berg RA. Post-translational processing and secretion of procollagen in fibroblasts. Symp Soc Exp Biol. 1979;33:57–78. [PubMed] [Google Scholar]
- Rentz TJ, Poobalarahi F, Bornstein P, Sage EH, Bradshaw AD. SPARC regulates processing of procollagen I and collagen fibrillogenesis in dermal fibroblasts. J Biol Chem. 2007;282:22062–71. doi: 10.1074/jbc.M700167200. [DOI] [PubMed] [Google Scholar]
- San Antonio JD, Karnovsky MJ, Gay S, Sanderson RD, Lander AD. Interactions of syndecan-1 and heparin with human collagens. Glycobiology. 1994;4:327–32. doi: 10.1093/glycob/4.3.327. [DOI] [PubMed] [Google Scholar]
- Smith LT, Holbrook KA, Madri JA. Collagen types I, III, and V in human embryonic and fetal skin. Am J Anat. 1986;175:507–21. doi: 10.1002/aja.1001750409. [DOI] [PubMed] [Google Scholar]
- Smith SM, Birk DE. Focus on Molecules: Collagens V and XI. Exp Eye Res. 2010 doi: 10.1016/j.exer.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Thomas CE, Birk DE. Pericellular proteins of the developing mouse tendon: a proteomic analysis. Connect Tissue Res. 2011;53:2–13. doi: 10.3109/03008207.2011.602766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Chen S, Adams SM, Florer JB, Liu H, Kao WW, Wenstrup RJ, Birk DE. Collagen V is a dominant regulator of collagen fibrillogenesis: dysfunctional regulation of structure and function in a corneal-stroma-specific Col5a1-null mouse model. J Cell Sci. 2011;124:4096–105. doi: 10.1242/jcs.091363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symoens S, Renard M, Bonod-Bidaud C, Syx D, Vaganay E, Malfait F, Ricard-Blum S, Kessler E, Van Laer L, Coucke P, Ruggiero F, De Paepe A. Identification of binding partners interacting with the alpha1-N-propeptide of type V collagen. Biochem J. 2011;433:371–81. doi: 10.1042/BJ20101061. [DOI] [PubMed] [Google Scholar]
- Unsold C, Pappano WN, Imamura Y, Steiglitz BM, Greenspan DS. Biosynthetic processing of the pro-alpha 1(V)2pro-alpha 2(V) collagen heterotrimer by bone morphogenetic protein-1 and furin-like proprotein convertases. J Biol Chem. 2002;277:5596–602. doi: 10.1074/jbc.M110003200. [DOI] [PubMed] [Google Scholar]
- Wenstrup RJ, Florer JB, Brunskill EW, Bell SM, Chervoneva I, Birk DE. Type V collagen controls the initiation of collagen fibril assembly. J Biol Chem. 2004a;279:53331–7. doi: 10.1074/jbc.M409622200. [DOI] [PubMed] [Google Scholar]
- Wenstrup RJ, Florer JB, Cole WG, Willing MC, Birk DE. Reduced type I collagen utilization: a pathogenic mechanism in COL5A1 haplo-insufficient Ehlers-Danlos syndrome. J Cell Biochem. 2004b;92:113–24. doi: 10.1002/jcb.20024. [DOI] [PubMed] [Google Scholar]
- Wenstrup RJ, Florer JB, Davidson JM, Phillips CL, Pfeiffer BJ, Menezes DW, Chervoneva I, Birk DE. Murine model of the Ehlers-Danlos syndrome. col5a1 haploinsufficiency disrupts collagen fibril assembly at multiple stages. J Biol Chem. 2006;281:12888–95. doi: 10.1074/jbc.M511528200. [DOI] [PubMed] [Google Scholar]
- Wenstrup RJ, Smith SM, Florer JB, Zhang G, Beason DP, Seegmiller RE, Soslowsky LJ, Birk DE. Regulation of collagen fibril nucleation and initial fibril assembly involves coordinate interactions with collagens V and XI in developing tendon. J Biol Chem. 2011;286:20455–65. doi: 10.1074/jbc.M111.223693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Young BB, Ezura Y, Favata M, Soslowsky LJ, Chakravarti S, Birk DE. Development of tendon structure and function: regulation of collagen fibrillogenesis. J Musculoskelet Neuronal Interact. 2005;5:5–21. [PubMed] [Google Scholar]