Abstract
Apolipoprotein CIII (ApoCIII) not only serves as an inhibitor of triglyceride hydrolysis but also participates in diabetes-related pathological events such as hyperactivation of voltage-gated Ca2+ (CaV) channels in the pancreatic β cell. However, nothing is known about the molecular mechanisms whereby ApoCIII hyperactivates β cell CaV channels. We now demonstrate that ApoCIII increased CaV1 channel open probability and density. ApoCIII enhanced whole-cell Ca2+ currents and the CaV1 channel blocker nimodipine completely abrogated this enhancement. The effect of ApoCIII was not influenced by individual inhibition of PKA, PKC, or Src. However, combined inhibition of PKA, PKC, and Src counteracted the effect of ApoCIII, similar results obtained by coinhibition of PKA and Src. Moreover, knockdown of β1 integrin or scavenger receptor class B type I (SR-BI) prevented ApoCIII from hyperactivating β cell CaV channels. These data reveal that ApoCIII hyperactivates β cell CaV1 channels through SR-BI/β1 integrin-dependent coactivation of PKA and Src.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-013-1442-x) contains supplementary material, which is available to authorized users.
Keywords: Ca2+ channel, Integrin, Pancreatic β cell, Protein kinase, Scavenger receptor
Introduction
Voltage-gated calcium (CaV) channels are critical in pancreatic β cell physiology and pathophysiology [1, 2]. They not only take center stage in the regulation of insulin secretion but are also involved in β cell development, survival, and growth through the regulation of protein phosphorylation, gene expression, and the cell cycle [1, 2]. The function and density of β cell CaV channels are regulated by a wide range of mechanisms either shared by other cell types or specific to β cells, e.g., channel phosphorylation, interaction with other molecules, and glucose metabolism-derived signaling [1–3]. Dysfunctional CaV channels cause β cell malfunction and even death as manifested in the most common metabolic disorder diabetes mellitus [1, 2]. Indeed, a T-lymphocyte-mediated autoimmune attack plays a crucial role in β cell death in type 1 diabetes. In addition, exposure to type 1 diabetic serum results in unphysiological amounts of Ca2+ in the pancreatic β cell and consequent Ca2+-dependent apoptosis. This is, at least in part, due to excessive Ca2+ influx through hyperactivated CaV channels [4, 5]. Undoubtedly, this process aggravates the disease development on top of the autoimmune attack [1, 2].
It has been demonstrated that elevated apolipoprotein CIII (ApoCIII) acts as a diabetogenic serum factor to drive β cell destruction via hyperactivation of β cell CaV channels [5, 6]. Moreover, we have recently shown that in vivo suppression of ApoCIII delays the onset of diabetes in the BioBreeding rat, a rat model for human type 1 diabetes [7]. Normally, ApoCIII is a blood plasma component. It is synthesized predominantly in the liver and to a minor extent in the intestine. Liver and intestinal cells release this apolipoprotein into the blood where it is situated on the surface of chylomicrons, very-low-density lipoproteins (VLDLs) and high-density lipoproteins (HDLs) [8, 9]. ApoCIII is composed of 79 amino acid residues that form six amphiphilic α-helixes, each containing about ten residues. The three-dimensional NMR structure and dynamics of ApoCIII have been resolved when it complexes with sodium dodecyl sulfate micelles, mimicking its natural lipid-bound state. The six amphiphilic α-helixes assemble into a necklace-like chain wrapping around the sodium dodecyl sulfate micelle surface [8]. Dogmatically, ApoCIII serves as an effective inhibitor of triglyceride hydrolysis by inhibiting lipoprotein lipase and through interference with triglyceride-rich lipoproteins binding to the negatively charged cell surface where lipoprotein lipases and lipoprotein receptors reside [8, 9]. It impedes the selective uptake of cholesteryl esters from LDL and HDL by binding to the scavenger receptor class B type I (SR-BI), and hampers the endocytosis of cholesterol-rich LDL by prevention of apolipoprotein B binding to LDL receptors [10–12]. Elevated plasma ApoCIII concentration is a feature of dyslipidemia in obesity and observed in both type 1 and 2 diabetes [5, 13, 14], whereas a group of Ashkenazi Jews with reduced plasma ApoCIII concentration maintains cardiovascular health and greater insulin sensitivity with age and reaches exceptional longevity [15].
In addition to the dogmatic roles in lipid metabolism, ApoCIII is also a multifaceted player in cell signaling. It can bind to distinct cell surface receptors including SR-BI and uncharacterized binding sites relaying corresponding signals to their downstream effectors, e.g., β1 integrin, pertussis toxin-sensitive G proteins, NF-κB, and protein kinases [10, 16–18]. However, nothing is known about the molecular mechanisms whereby ApoCIII hyperactivates β cell CaV channels. In the present study, we demonstrate that ApoCIII upregulates β cell CaV1 channels through SR-BI/β1 integrin-dependent coactivation of PKA and Src kinase.
Materials and methods
Cell culture and treatments
Islets of Langerhans were isolated from adult male and female mice and dispersed into single islet cells [19]. RINm5F cells at about 70 % confluency were trypsinized. The resultant suspension of cells was seeded into Petri dishes or 12-well plates. The cells were cultivated in RPMI 1640 medium supplemented with 10 % fetal bovine serum, 2 mM l-glutamine, and 100 U/100 μg/ml penicillin/streptomycin (Invitrogen, Carlsbad, CA, USA) and maintained at 37 °C in a humidified 5 % CO2 incubator [19]. They were grown overnight and then subjected to siRNA transfection. For patch-clamp analysis, cells underwent overnight treatment with ApoCIII, the PKA inhibitors H-89 (Calbiochem, La Jolla, CA, USA) and myristoylated PKI (14–22) (PKI, Sigma–Aldrich, St. Louis, MO, USA), the PKC inhibitor calphostin C (Calbiochem), the Src kinase inhibitor PP2 (Calbiochem) and the CaV1 channel blocker nimodipine (Calbiochem) in RPMI medium at final concentrations of 20 μg/ml, 0.5, 1, 0.1, 0.1, and 5 μM, respectively. ApoCIII was dissolved in 0.1 % trifluoroacetic acid (TFA) to make a stock solution of 1 mg/ml, PKI was dissolved in water to prepare a stock solution of 0.5 mM, whereas H-89, calphostin C, PP2, and nimodipine were dissolved in dimethyl sulfoxide (DMSO) to form stock solutions of 5, 1, 1, and 10 mM, respectively. As vehicle controls, 0.002 % TFA and/or 0.03 % DMSO were used.
siRNA design and transfection
Two pairs of 21-mer siRNA duplexes targeting the rat β1 integrin (β1 integrin siRNA #1, ID127971 and β1 integrin siRNA #2, ID127972) and SR-BI (ID128929) were designed and chemically synthesized by Applied Biosystems/Ambion (Austin, TX, USA). Their sequences were subjected to BLAST search to ensure their specificity. Silencer Select Negative Control siRNA (4390843), not targeting any gene product, and Silencer Select GAPDH Positive Control siRNA (4390849), efficiently silencing GAPDH in human, mouse, and rat cells, were purchased from Applied Biosystems/Ambion (Austin, TX, USA). RINm5F cells were reversely transfected with Lipofectamine RNAiMAX. Briefly, negative control siRNA, β1 integrin siRNA #1, β1 integrin siRNA #2 or SR-BI siRNA was mixed with Lipofectamine RNAiMAX followed by 20-min incubation at room temperature. Subsequently, cells were added to the siRNA/Lipofectamine RNAiMAX mixtures followed by gentle agitation and kept at 37 °C in a humidified 5 % CO2 incubator. After 72 h, the transfected cells were grown to about 70 % confluency and subjected to different treatments followed by immunoblot assay or electrophysiological analysis.
Semiquantitative RT-PCR
Total RNA was isolated from RINm5F cells using the RNeasy Micro Kit as recommended by the manufacturer (Qiagen, Valencia, CA, USA). RT-PCR primer pairs were synthesized by Sigma–Aldrich. The SR-BI primer pair consisted of the forward primer 5′-CAAGAAGCCAAGCTGTAGGG-3′ and the reverse primer 5′-CCCAACAGGCTCTACTCAGC-3′. The GAPDH primer pair comprised the forward primer 5′-TAGACAAGATGGTGAAGG-3′ and the reverse primer 5′-TCCTTGGAGGCCATGTAG-3′; 500 ng of total RNA was reverse transcribed with SuperScript II Reverse Transcriptase (Invitrogen) and Oligo(dT)12–18 Primer (Invitrogen). Polymerase chain reaction was carried out using the Platinum Taq DNA Polymerase (Invitrogen). It underwent 90 s at 94 °C for completely denaturing templates and activating the Taq DNA Polymerase, followed by 29 cycles of denaturing at 94 °C for 30 s, annealing at 55 °C for 30 s and extension at 72 °C for 30 s, and ending with a final extension at 72 °C for 5 min. The amplified PCR products were detected by agarose gel electrophoresis and ethidium bromide staining.
SDS-PAGE and immunoblot analysis
RINm5F cells following different treatments were lysed in a lysis buffer (pH 7.5) consisting of 50 mM HEPES, 150 mM NaCl, 1 mM EGTA, 1 mM EDTA, 10 % glycerol, 1 % triton X-100, 1 mM PMSF and a protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany). The lysate was centrifuged at 800 × g for 10 min at 4 °C to remove cell debris and nuclei. The protein concentration of the resulting samples was determined with Bio-Rad protein assay reagent (Bio-Rad, Hercules, CA, USA). The samples were denatured by heating at 96 °C for 3 min in SDS sample buffer and then underwent sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblot analysis. Briefly, 50, 90, or 180 μg of protein were separated in discontinuous gels consisting of a 4 % acrylamide stacking gel (pH 6.8) and an 8 % acrylamide separating gel (pH 8.8). The separated proteins were then electroblotted to hydrophobic polyvinylidene difluoride membrane (Hybond-P; GE Healthcare, Uppsala, Sweden). The blots were blocked by incubation for 1 h with 5 % non-fat milk powder in a washing buffer, containing 50 mM Tris(hydroxymethyl)aminomethane, 150 mM NaCl and 0.05 % Tween 20 (pH 7.5). They were then incubated overnight at 4 °C with affinity-purified rabbit polyclonal antibodies to β1 integrin (1:500; Millipore, Billerica, MA, USA), SR-BI (1:2,500; Novus, Cambridge, UK), CaV1.2 (1:200) and CaV1.3 (1:200), respectively, and for 1 h at room temperature with mouse monoclonal antibody to glyceraldehyde-3-phosphate dehydrogenase (GAPDH, 1:4000; Applied Biosystems/Ambion, Austin, TX, USA), respectively. After rinsing with the washing buffer, the blots were incubated with the secondary antibodies (either horseradish peroxidase-conjugated goat anti-rabbit IgG or horseradish peroxidase-conjugated goat anti-mouse IgG; 1:50,000; Bio-Rad) at room temperature for 45 min. The immunoreactive bands were visualized with the ECL plus Western blotting detection system (GE Healthcare, Uppsala, Sweden).
Electrophysiology
Mouse islet cells and RINm5F cells following different treatments were subjected to single-channel and whole-cell patch-clamp measurements [20]. Cell-attached and perforated whole-cell patch-clamp configurations were employed [20]. Electrodes were made from borosilicate glass capillaries, fire-polished and coated with Sylgard close to their tips. Some of them were filled with a solution containing (in mM) 110 BaCl2, 10 TEA-Cl, and 5 HEPES [pH 7.4 with Ba(OH)2] for single-channel measurements. Others were filled with a solution composed of (in mM) 76 Cs2SO4, 1 MgCl2, 10 KCl, 10 NaCl, and 5 HEPES (pH 7.35 with CsOH), as well as amphotericin B (0.24 mg/ml) for whole-cell current recordings. Electrode resistance ranged between 4 and 6 MΩ when they were filled with electrode solutions and immersed in bath solutions. The electrode offset potential was corrected in bath solutions prior to gigaseal formation. Single-channel recordings were performed with cells bathed in a depolarizing external recording solution, containing (in mM) 125 KCl, 30 KOH, 10 EGTA, 2 CaCl2, 1 MgCl2, and 5 HEPES–KOH (pH 7.15). This solution was used to bring the intracellular potential to 0 mV. For perforated whole-cell current measurements, the cells were bathed in a solution containing (in mM) 138 NaCl, 5.6 KCl, 1.2 MgCl2, 10 CaCl2, 5 HEPES (pH 7.4). Single-channel and whole-cell currents were recorded with an Axopatch 200B amplifier (Molecular Devices, Foster City, CA, USA) and an EPC-9 patch clamp amplifier (HEKA Elektronik, Lambrecht/Pfalz, Germany), respectively, at room temperature (about 22 °C). Acquisition and analysis of single channel and whole-cell current data were done using the software program pCLAMP 10 (Axon Instruments) and the software program PatchMaster/FitMaster (HEKA), respectively. To guarantee elimination of rapid transient Na+ currents appearing at the initial period of depolarization during whole-cell Ca2+ current recordings [21], we measured peak whole-cell Ca2+ currents within a time window from 30 to 100 ms after the start point of depolarization. The amplitude of whole-cell currents was normalized by the cell capacitance.
Statistical analysis
All data are presented as mean ± SEM. Statistical significance was determined by one-way ANOVA, followed by least significant difference (LSD) test. When two groups were compared, unpaired Student’s t test or Mann–Whitney U test was employed. The significance level was set to 0.05 or 0.01.
Results
Apolipoprotein CIII increases CaV1 channel density and conductivity in the β cell
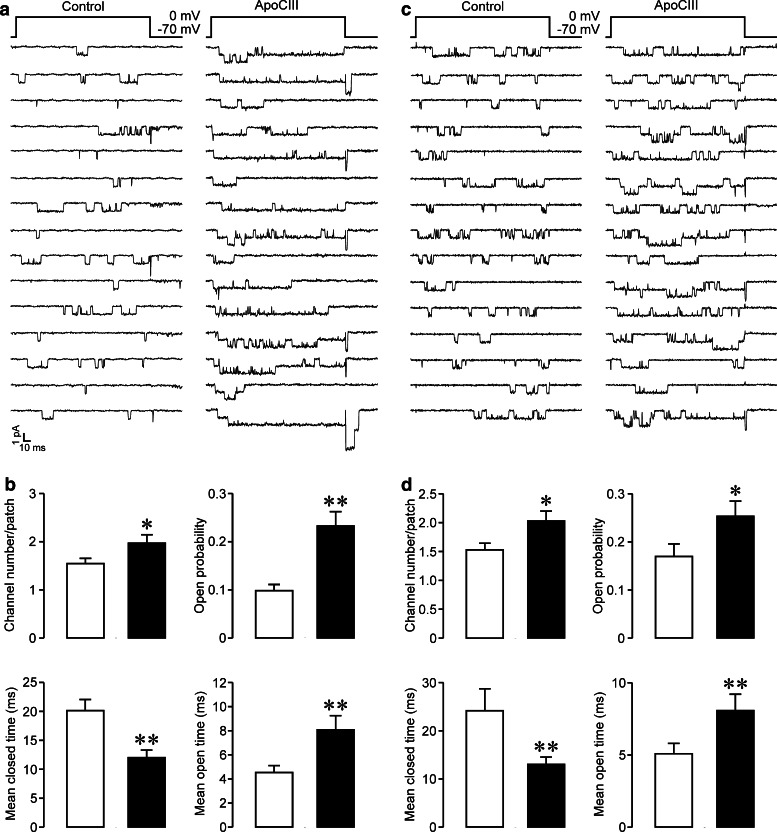
Our previous work reveals that ApoCIII incubation significantly enhances whole-cell Ca2+ currents in the mouse islet β cell [5]. To clarify what type of β cell CaV channels and whether the density or conductivity was affected, we analyzed unitary CaV1 channel currents, characterized by a large unitary Ba2+ conductance with long-lasting openings, in mouse islet β cells (Fig. 1a) and RINm5F cells (Fig. 1c), following ApoCIII incubation. In experiments with mouse islet β cells, we observed more CaV1 channels, reflected by more layers of unitary Ba2+ currents, in plasma membrane patches of ApoCIII-treated cells than in those of control cells (Fig. 1a). The average number, open probability, and mean open time of unitary CaV1 channels in ApoCIII-treated cells (n = 32) were significantly greater than those in cells exposed to control vehicle (n = 33); (Fig. 1b). The mean closed time of unitary CaV1 channels recorded in patches of ApoCIII-incubated cells was significantly shorter than that in control patches (Fig. 1b). There is no significant difference in the unitary slope conductance of CaV1 channels between control and ApoCIII treated groups (23.72 ± 1.53 versus 23.87 ± 1.42 pS, p > 0.05). Likewise, similar effects of ApoCIII occurred on CaV1 channels in insulin-secreting RINm5F cells. Plasma membrane patches of ApoCIII-incubated cells accommodated more CaV1 channels in comparison with those of vehicle-treated cells (Fig. 1c). CaV1 channels in the former opened more frequently than those in the latter (Fig. 1c). ApoCIII incubation (n = 35) significantly increased channel number, elevated open probability, prolonged mean open time and shortened mean closed time of CaV1 channels as compared with incubation with vehicle solution (n = 34) (Fig. 1d). However, it did not alter the unitary slope conductance of CaV1 channels (control group: 23.81 ± 1.42 pS versus ApoCIII group: 24.15 ± 1.07 pS, p > 0.05). Obviously, the data reveal that ApoCIII increases both density and conductivity of β cell CaV1 channels.
Fig. 1.
Apolipoprotein CIII incubation increases both the density and conductivity of CaV1 channels in β cells. a Examples of unitary CaV1 channel currents detected in plasma membrane patches of mouse islet β cells incubated with either vehicle solution as control or apolipoprotein CIII (ApoCIII). b Average number, open probability, mean closed time, and mean open time of unitary CaV1 channels measured in plasma membrane patches attached to mouse islet β cells exposed to either control vehicle (open columns, n = 33) or ApoCIII (filled columns, n = 32). c Examples of unitary CaV1 channel currents recorded in plasma membrane patches attached to either a control RINm5F cell or a cell treated with ApoCIII. d Average number, open probability, mean closed time, and mean open time of unitary CaV1 channels detected in plasma membrane patches of control RINm5F cells (open columns, n = 34) or cells incubated with ApoCIII (filled columns, n = 35). *p < 0.05 and **p < 0.01 versus control
Pharmacological ablation of CaV1 channels prevents apolipoprotein CIII-induced hyperactivation of β cell CaV channels
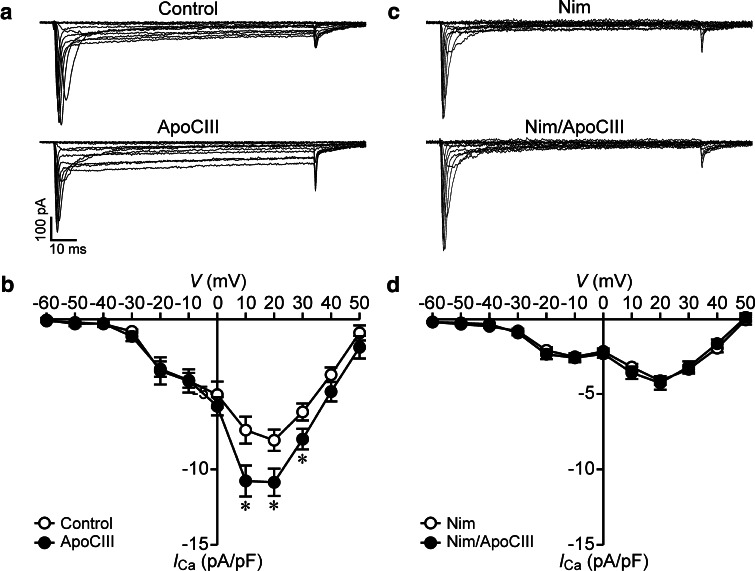
The verification of the effects of ApoCIII on CaV1 channels by single-channel analysis does not necessarily mean that ApoCIII only affects CaV1 channels. To examine if the effects also occur on other types of CaV channels, we analyzed whole-cell Ca2+ currents in RINm5F cells following ApoCIII incubation in the absence and presence of the CaV1 channel blocker nimodipine. Whole-cell Ca2+ currents in cells incubated with ApoCIII were larger than those in cells treated with vehicle solution (Fig. 2a). Whole-cell Ca2+ current densities observed in the voltage range from 10 to 30 mV in the ApoCIII group were significantly higher than those in the control group (Fig. 2b). In striking contrast, whole-cell Ca2+ currents were similar between control cells and cells incubated with ApoCIII in the presence of nimodipine (Fig. 2c). There was no significant difference in the whole-cell Ca2+ current density between the two treatments (Fig. 2d). The data confirm that ApoCIII solely impinges on β cell CaV1 channels.
Fig. 2.
Apolipoprotein CIII incubation increases whole-cell Ca2+ currents and coincubation with the CaV1 channel blocker nimodipine abrogates the effect of apolipoprotein CIII incubation in RINm5F cells. a Sample whole-cell Ca2+ current traces from a cell incubated with vehicle solution as control (cell capacitance: 10.1 pF) and apolipoprotein CIII (ApoCIII)-treated cell (cell capacitance: 11.1 pF). b Average Ca2+ current density–voltage relationships in control cells (open circles, n = 26) and cells treated with ApoCIII (filled circles, n = 26). *p < 0.05 versus control. c Sample whole-cell Ca2+ current traces from a nimodipine (Nim)-incubated cell (cell capacitance: 10 pF) and a cell exposed to Nim together with ApoCIII (Nim/ApoCIII) (cell capacitance: 11.9 pF). d Average Ca2+ current density–voltage relationships in Nim-treated cells (open circles, n = 20) and cells incubated with Nim/ApoCIII (filled circles, n = 21)
Apolipoprotein CIII hyperactivates β cell CaV channels via coactivation of PKA and Src kinase
The increase in open probability of β cell CaV1 channels by ApoCIII and the mediating role of protein kinases in ApoCIII signaling suggest that ApoCIII may signal upstream of some protein kinases to hyperactivate β cell CaV channels [16, 22–25]. Therefore, we explored the involvement of PKA, PKC, and Src kinase in ApoCIII-induced hyperactivation of β cell CaV channels.
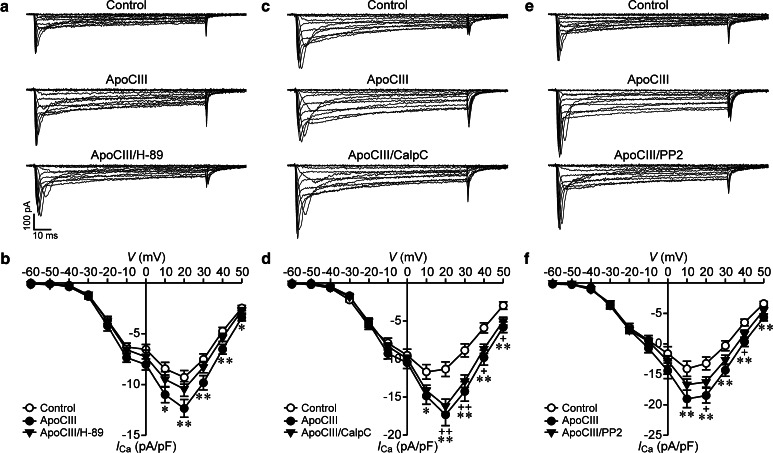
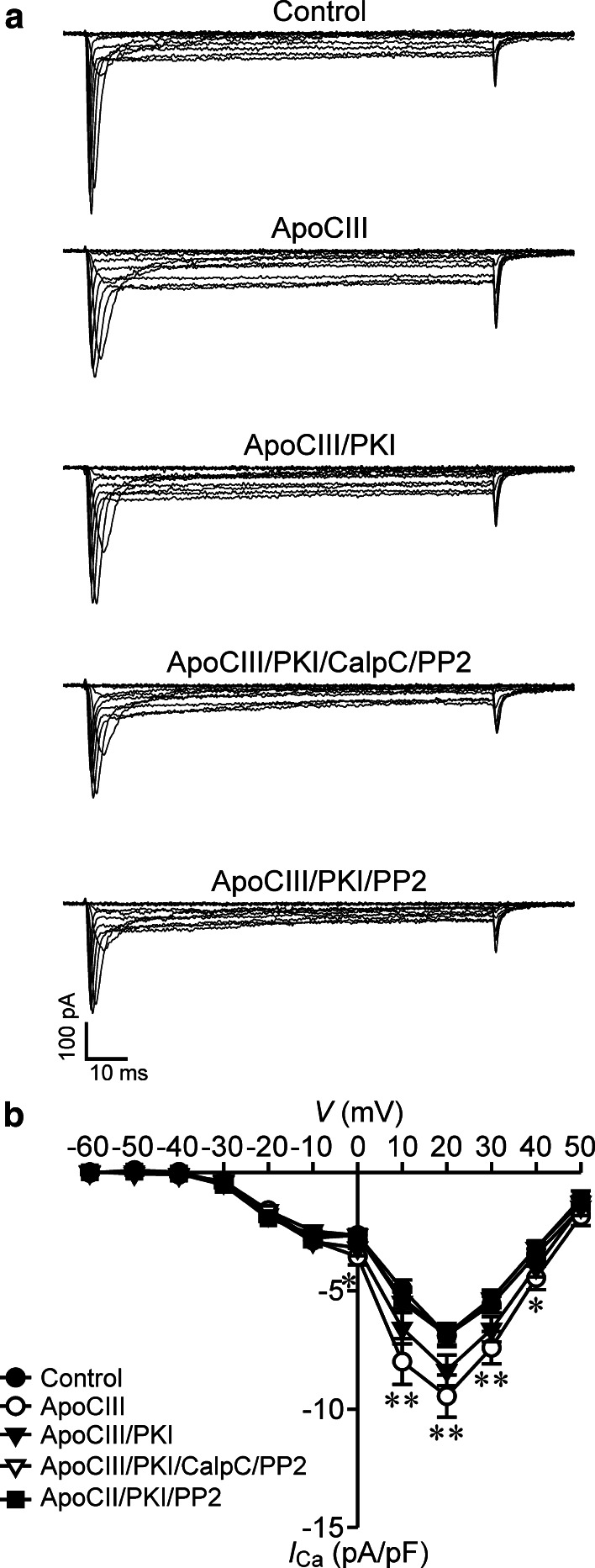
First, we examined the effect of the PKA inhibitor H-89 on ApoCIII-induced hyperactivation of β cell CaV channels in RINm5F cells. Whole-cell Ca2+ currents registered in control cells were smaller than those in cells treated with ApoCIII, whereas whole-cell Ca2+ currents recorded in cells incubated with ApoCIII plus H-89 sized in between (Fig. 3a). Average Ca2+ current densities measured in ApoCIII-treated cells (filled circles, n = 36) were significantly higher than those in vehicle-treated control cells (open circles, n = 37) at voltages ranging from 10 to 50 mV (Fig. 3b). However, cells following cotreatment of ApoCIII and H-89 (filled triangles, n = 36) did not significantly differ from either cells treated with ApoCIII or control cells in terms of Ca2+ current density (Fig. 3b). Moreover, H-89 treatment did not significantly influence Ca2+ current densities under basal conditions, i.e., in the absence of ApoCIII (Supplementary Fig. S1A and B). In addition, we also evaluated the effect of the more specific PKA inhibitor myristoylated PKI on ApoCIII-induced hyperactivation of β cell CaV channels since H-89 might produce non-specific effects. Figure 4 shows that whole-cell Ca2+ currents observed in cells exposed to ApoCIII (open circles, n = 30) were significantly greater than those in cells treated with vehicle solution (filled circles, n = 30). Whole-cell Ca2+ currents recorded in ApoCIII-treated cells in the presence of PKI (filled triangles, n = 30) fell between the former and the latter (Fig. 4). Furthermore, PKI treatment had no effect on whole-cell Ca2+ currents in the absence of ApoCIII (Supplementary Fig. S3). The results indicate that PKA inhibition marginally reduces ApoCIII-induced hyperactivation of β cell CaV channels.
Fig. 3.
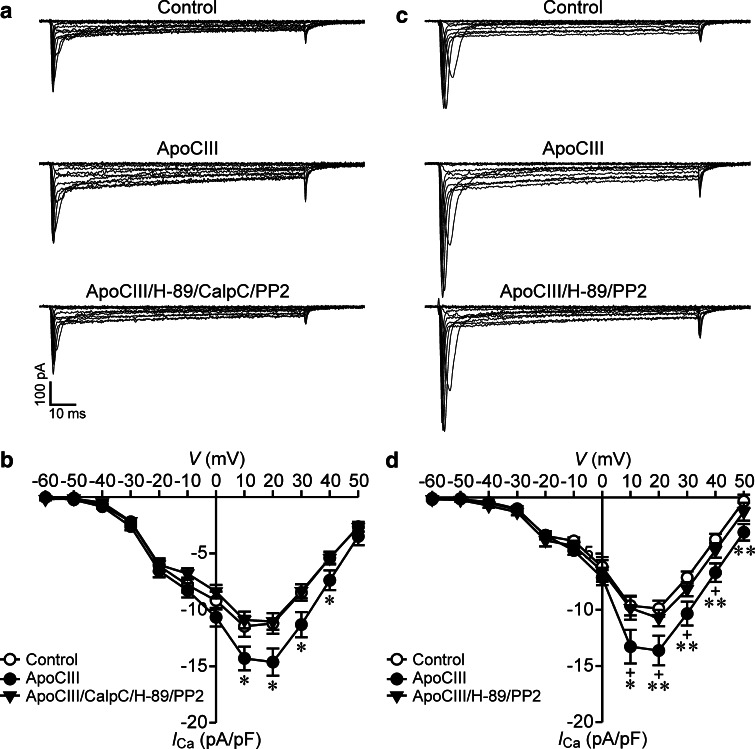
PKA or Src kinase inhibition marginally reduces but PKC inhibition does not affect apolipoprotein CIII-induced enhancement of whole-cell Ca2+ currents in RINm5F cells. a Sample whole-cell Ca2+ current traces from a cell incubated with vehicle solution as control (cell capacitance: 8.5 pF), an apolipoprotein CIII (ApoCIII)-treated cell (cell capacitance: 8.2 pF) and a cell exposed to ApoCIII plus the PKA inhibitor H-89 (ApoCIII/H-89, cell capacitance: 8.4 pF). b Average Ca2+ current density–voltage relationships in control cells (open circles, n = 37) and cells treated with ApoCIII (filled circles, n = 36) or ApoCIII/H-89 (filled triangles, n = 36). *p < 0.05 and **p < 0.01 versus control. c Sample whole-cell Ca2+ current traces registered in a control cell (cell capacitance: 12.5 pF), an ApoCIII-incubated cell (cell capacitance: 12.0 pF) and a cell subjected to cotreatment with ApoCIII and the PKC inhibitor calphostin C (ApoCIII/CalpC, cell capacitance: 12.1 pF). d Average Ca2+ current density–voltage relationships in control cells (open circles, n = 33), ApoCIII-treated cells (filled circles, n = 33) and cells exposed to ApoCIII/CalpC (filled triangles, n = 33). *p < 0.05 and **p < 0.01 ApoCIII versus control. +p < 0.05 and ++p < 0.01 ApoCIII/CalpC versus control. e Sample whole-cell Ca2+ current traces acquired in a control cell (cell capacitance: 9.5 pF), an ApoCIII-incubated cell (cell capacitance: 9.2 pF) and a cell exposed to ApoCIII together with the Src kinase inhibitor PP2 (ApoCIII/PP2, cell capacitance: 10.0 pF). f Average Ca2+ current density–voltage relationships in control cells (open circles, n = 40) and cells incubated with ApoCIII (filled circles, n = 40) or ApoCIII/PP2 (filled triangles, n = 40). **p < 0.01 ApoCIII versus control. +p < 0.05 ApoCIII/PP2 versus control
Fig. 4.
PKI alone marginally diminishes, but PKI in combination with either dual inhibition of PKC and Src kinase or singular inhibition of Src kinase ablates apolipoprotein CIII-induced elevation of whole-cell Ca2+ currents in RINm5F cells. a Sample whole-cell Ca2+ current traces acquired in a vehicle-incubated cell (control, cell capacitance: 12.03 pF), a cell treated with apolipoprotein CIII (ApoCIII, cell capacitance: 12.08 pF), a cell subsequent to co-treatment with ApoCIII and PKI (ApoCIII/PKI, cell capacitance: 12.5 pF), a cell exposed to ApoCIII in the presence of the protein kinase inhibitor cocktail of PKI, calphostin C and PP2 (ApoCIII/PKI/CalpC/PP2, cell capacitance: 12.27 pF) and a cell incubated with ApoCIII together with PKI and PP2 (ApoCIII/PKI/PP2, cell capacitance: 12.6 pF). b Average Ca2+ current density–voltage relationships in control cells (filled circles, n = 30), cells treated with ApoCIII (open circles, n = 30), cells incubated with ApoCIII/PKI (filled triangles, n = 30) and cells exposed to ApoCIII/PKI/CalpC/PP2 (open triangles, n = 30), or to ApoCIII/PKI/PP2 (filled squares, n = 30). *p < 0.05 and **p < 0.01 versus control, ApoCIII/PKI/CalpC/PP2 and ApoCIII/PKI/PP2
Second, we tested the effect of the PKC inhibitor calphostin C (CalpC) on ApoCIII-induced hyperactivation of β cell CaV channels in RINm5F cells. We observed that cells incubated with ApoCIII and ApoCIII/CalpC-cotreated cells displayed similar whole-cell Ca2+ currents, which were larger than those acquired in vehicle-treated cells (Fig. 3c). Mean Ca2+ current densities in ApoCIII-treated cells (filled circles, n = 33) at the voltage range of 10–50 mV and cells exposed to ApoCIII/CalpC (filled triangles, n = 33) at a voltage range from 20 to 50 mV increased significantly in comparison with vehicle-treated control cells (open circles, n = 33) (Fig. 3d). There is no difference between ApoCIII-treated cells and ApoCIII/CalpC-cotreated cells with regard to the Ca2+ current density (Fig. 3d). Furthermore, cells exposed to control vehicle were similar to CalpC-treated cells in terms of Ca2+ current density (Supplementary Fig. S1C and D). These data demonstrate that PKC inhibition does not affect ApoCIII-induced hyperactivation of β cell CaV channels.
Third, we evaluated the effect of the Src kinase inhibitor PP2 on ApoCIII-induced hyperactivation of β cell CaV channels in RINm5F cells. We found smaller and larger whole-cell Ca2+ currents in cells following incubation with vehicle solution and ApoCIII-incubated cells, respectively (Fig. 3e). Cells exposed to ApoCIII and PP2 fell between vehicle control cells and cells treated with ApoCIII with regard to whole-cell Ca2+ currents (Fig. 3e). Whole-cell Ca2+ current densities quantified in cells treated with ApoCIII (filled circles, n = 40) at the voltage range 10–50 mV were significantly elevated as compared with those determined in vehicle control cells (open circles, n = 40); (Fig. 3f). Cells subjected to cotreatment of ApoCIII and PP2 (filled triangles, n = 40) showed significantly larger Ca2+ currents at the voltage range 20–40 mV compared to vehicle-treated control cells (open circles, n = 40). However, the difference in the Ca2+ current density between ApoCIII/PP2-cotreated cells and cells incubated with vehicle solution is less prominent than that between cells treated with ApoCIII and vehicle-treated control cells (Fig. 3f). Moreover, vehicle-treated cells (open circles, n = 20) and cells incubated with PP2 (filled circles, n = 19) exhibited similar Ca2+ current densities (Supplementary Fig. S1E and F). The results suggest that Src kinase inhibition has a tendency to decrease ApoCIII-induced hyperactivation of β cell CaV channels.
The marginal and null effects of PKA, PKC or Src kinase inhibitors on ApoCIII-induced hyperactivation of β cell CaV channels made us wonder what happens if a more complex inhibition of all these kinases is applied. To address this question, we characterized the effect of the protein kinase inhibitor cocktail H-89, CalpC and PP2 on ApoCIII-induced hyperactivation of β cell CaV channels in RINm5F cells. Larger whole-cell Ca2+ currents appeared in an ApoCIII-treated cells, whereas smaller whole-cell Ca2+ currents occurred in vehicle-treated control cells and cells treated with ApoCIII in the presence of H-89, CalpC and PP2 (Fig. 5a). ApoCIII treatment (filled circles, n = 35) significantly increased Ca2+ current densities at the voltage range 10–40 mV as compared with vehicle-treated control cells (open circles, n = 35) and treatment with ApoCIII together with H-89, CalpC and PP2 (filled triangles, n = 34). The profile of Ca2+ current densities in cells exposed to ApoCIII in the presence of H-89, CalpC and PP2 resembled that in vehicle-treated control cells (Fig. 5b). Furthermore, treatment of control cells with the protein kinase inhibitor cocktail H-89, CalpC and PP2 had no significant effect on whole-cell Ca2+ currents under basal conditions, i.e., in the absence of ApoCIII (Supplementary Fig. S2A and B). H-89 might produce non-specific effects. To exclude this, we performed another set of experiments where PKI substituted for H-89. The data obtained with PKI were similar to those with H-89 (Fig. 4 and Supplementary Fig. S3). The results demonstrate that combined inhibition of PKA, PKC and Src kinase effectively ablates ApoCIII-induced hyperactivation of β cell CaV channels.
Fig. 5.
Combined inhibition of PKA, PKC, and Src kinase counteracts apolipoprotein CIII-induced augmentation of whole-cell Ca2+ currents in RINm5F cells and coinhibition of PKA and Src kinase is sufficient to obtain this counteraction. a Sample whole-cell Ca2+ current traces registered in a vehicle-incubated cell (Control, cell capacitance: 7.9 pF), a cell subsequent to apolipoprotein CIII (ApoCIII) treatment (cell capacitance: 7.0 pF) and a cell exposed to ApoCIII in the presence of the protein kinase inhibitor cocktail of H-89, calphostin C and PP2 (ApoCIII/H-89/CalpC/PP2, cell capacitance: 7.2 pF). b Average Ca2+ current density–voltage relationships in control cells (open circles, n = 35) and cells exposed to ApoCIII (filled circles, n = 34) or to ApoCIII/H-89/CalpC/PP2 (filled triangles, n = 35). *p < 0.05 versus control and apoCIII/H-89/CalpC/PP2. c Sample whole-cell Ca2+ current traces from a control cell (cell capacitance: 8.5 pF), a cell subsequent to ApoCIII treatment (cell capacitance: 8.2 pF) and a cell exposed to ApoCIII in the presence of the protein kinase inhibitors H-89 and PP2 (ApoCIII/H-89/PP2, cell capacitance: 8.7 pF). d Average Ca2+ current density–voltage relationships in control cells (open circles, n = 26) and cells subjected to ApoCIII (filled circles, n = 26) or to ApoCIII/H-89/PP2 (filled triangles, n = 27). *p < 0.05 and **p < 0.01 versus control; +p < 0.05 versus ApoCIII/H-89/PP2
The marginal effect of PKA or Src kinase inhibitors alone on whole-cell Ca2+ currents inevitably raised the question if coinhibition of PKA and Src kinase is sufficient to prevent ApoCIII-induced hyperactivation of β cell CaV channels. We answered the question by analyzing whole-cell Ca2+ currents in RINm5F cells following cotreatment with H-89 and PP2. We observed that whole-cell Ca2+ currents in ApoCIII-treated cells were larger than those in control cells or cells subjected to treatment with ApoCIII in the presence of H-89 and PP2 (Fig. 5c). Significantly higher densities of whole-cell Ca2+ currents appeared in the ApoCIII group (filled circles, n = 26) in comparison with control group (open circles, n = 26) or the group subjected to incubation with ApoCIII in the presence of H-89 and PP2 (filled triangles, n = 27); (Fig. 5d). Moreover, whole-cell Ca2+ currents in control cells resembled those observed in cells treated with H-89 and PP2 (Supplementary Fig. S2C and D). To rule out the possibility of non-specific effects of H-89, we replaced H-89 with PKI. In fact, PKI produced similar results to those above-described in the presence of H-89 (Fig. 4; Supplementary Fig. S3). These data reveal that ApoCIII enhances whole-cell Ca2+ currents via coactivation of PKA and Src Kinase.
Apolipoprotein CIII does not influence β cell CaV1 channel expression
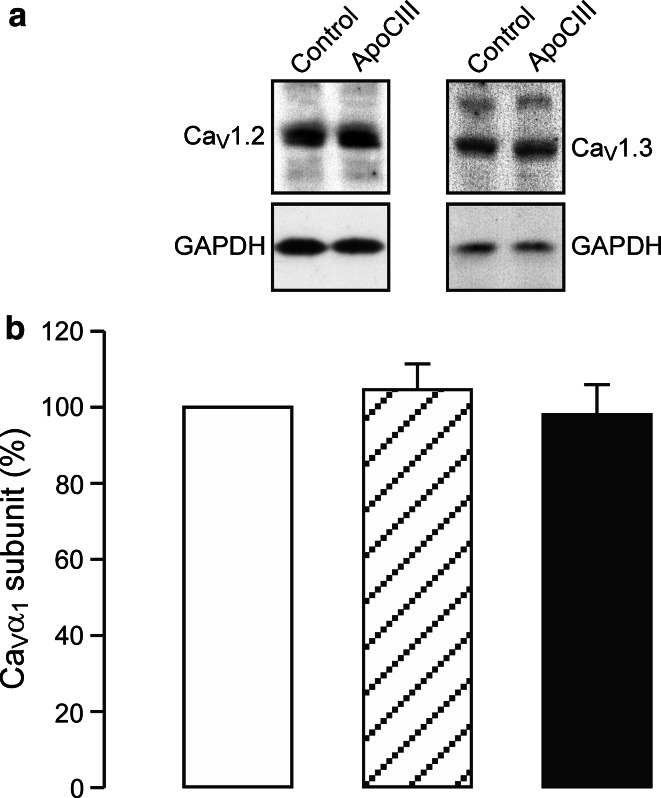
Overnight incubation with ApoCIII may influence β cell CaV1 channel expression. To test for this possibility, we analyzed β cell CaV1 channel expression in RINm5F cells following ApoCIII incubation. We found that anti-CaV1.2, anti-CaV1.3 and anti-GAPDH antibodies detected clear CaV1.2, CaV1.3 and GAPDH immunoreactive bands, respectively. Control and ApoCIII-treated samples gave similar intensities of CaV1.2, CaV1.3 and GAPDH immunoreactivities (Fig. 6a). Figure 6b shows that there was no significant difference in the relative abundance of CaV1.2 (hatched column, n = 6) and CaV1.3 subunits (filled column, n = 6) in RINm5F cell homogenates subjected to ApoCIII incubation in comparison with vehicle incubation (open column, n = 6) (p > 0.05). The data reveal that ApoCIII incubation does not alter β cell CaV1 channel expression at the protein level.
Fig. 6.
Apolipoprotein CIII incubation does not alter β cell CaV1 channel expression. a Representative immunoblots of RINm5F cell homogenates, subjected to incubation with vehicle as control or apolipoprotein CIII (ApoCIII), probed with anti-CaV1.2, anti-CaV1.3 and anti-GAPDH antibodies, respectively. b Immunoblot quantification of the relative abundance of CaV1.2 (hatched column, n = 6) and CaV1.3 subunits (filled column, n = 6) in RINm5F cell homogenates subjected to ApoCIII incubation in comparison with control (open column, n = 6). There was no significant difference in the relative abundance of total CaV1.2 and CaV1.3 subunits between control cells and cells incubated with ApoCIII (p > 0.05)
Apolipoprotein CIII upregulates β cell CaV channels via β1 integrin
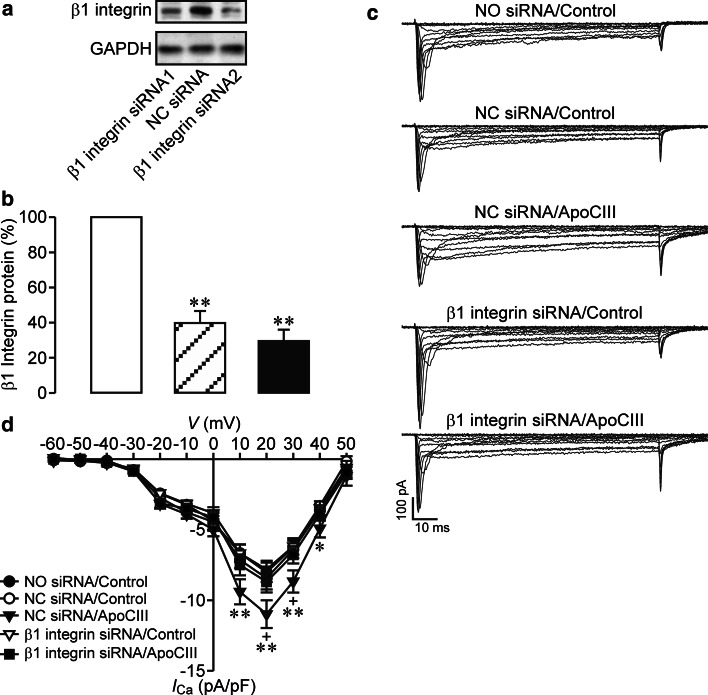
β1 integrin has been verified to serve as a mediator between ApoCIII and a certain number of protein kinases including PKA and Src kinase [16, 22–25]. This together with our results that ApoCIII hyperactivated β cell CaV channels via coactivation of PKA and Src kinase raises the possibility that β1 integrin mediates ApoCIII-induced hyperactivation of β cell CaV channels. We investigated this possibility by implementing RNA interference in combination with whole-cell Ca2+ current analysis in RINm5F cells. It turned out that transfection with two β1 integrin siRNAs significantly decreased β1 integrin expression at the protein level (Fig. 7a, b). Importantly, β1 integrin siRNA pretransfection effectively prevented ApoCIII-induced hyperactivation of β cell CaV channels (Fig. 7c, d). Whole-cell Ca2+ currents in β1 integrin siRNA-pretransfected cells incubated with ApoCIII (β1 integrin siRNA/ApoCIII) were significantly smaller than those in negative control siRNA-pretransfected cells exposed to ApoCIII (NC siRNA/apoCIII), but similar to those in three sets of control cells (Fig. 7c). These control cells were subjected to mock (NO siRNA/Control), negative control siRNA (NC siRNA/Control) and β1 integrin siRNA pretransfection (β1 integrin siRNA/Control), respectively, followed by control vehicle incubation (Fig. 7c). Significantly-reduced Ca2+ current density was observed in cells subsequent to β1 integrin siRNA/ApoCIII (n = 29) in comparison with those to NC siRNA/apoCIII (filled triangles, n = 28); (Fig. 7d). The former displayed similar Ca2+ current density, but the latter exhibited larger Ca2+ current density compared with those subjected to NO siRNA/Control (n = 29), NC siRNA/Control (n = 28) or β1 integrin siRNA/Control (n = 29); (Fig. 7d). Taken together, the results demonstrate that ApoCIII critically relies on β1 integrin to hyperactivate β cell CaV channels.
Fig. 7.
Knockdown of β1 integrin abrogates apolipoprotein CIII-induced exaggeration of whole-cell Ca2+ currents in RINm5F cells. a Representative blots of β1 integrin- and GAPDH-immunoreactive bands in β1 integrin siRNA #1-, negative control siRNA (NC siRNA)- and β1 integrin siRNA #2-transfected cells. b Immunoblot quantifications of β1 integrin protein in NC siRNA- (open column, n = 6), β1 integrin siRNA #1- (hatched column, n = 6) and β1 integrin siRNA #2-transfected RINm5F cells (filled column, n = 6). **p < 0.01 versus NC siRNA. c Sample whole-cell Ca2+ current traces registered in individual cells following mock transfection and incubation with control vehicle (NO siRNA/Control, cell capacitance: 12.1 pF), NC siRNA transfection and control vehicle treatment (NC siRNA/Control, cell capacitance: 11.4 pF), NC siRNA transfection and apolipoprotein CIII (ApoCIII) incubation (NC siRNA/ApoCIII, cell capacitance: 12.1 pF), β1 integrin siRNA transfection and exposure to vehicle solution (β1 integrin siRNA/Control, cell capacitance: 11.9 pF) and β1 integrin siRNA transfection and ApoCIII exposure (β1 integrin siRNA/ApoCIII, cell capacitance: 12.4 pF), respectively. d Ca2+ current density–voltage relationships in cells subjected to NO siRNA/Control (filled circles, n = 29), NC siRNA/Control (open circles, n = 28), NC siRNA/apoCIII (filled triangles, n = 28), β1 integrin siRNA/Control (open triangles, n = 29) and β1 integrin siRNA/ApoCIII (filled squares, n = 29). *p < 0.05 and **p < 0.01 versus NO siRNA/Control, NC siRNA/Control and β1 integrin siRNA/Control. +p < 0.05 versus β1 integrin siRNA/ApoCIII
Apolipoprotein CIII hyperactivates β cell CaV channels via SR-BI
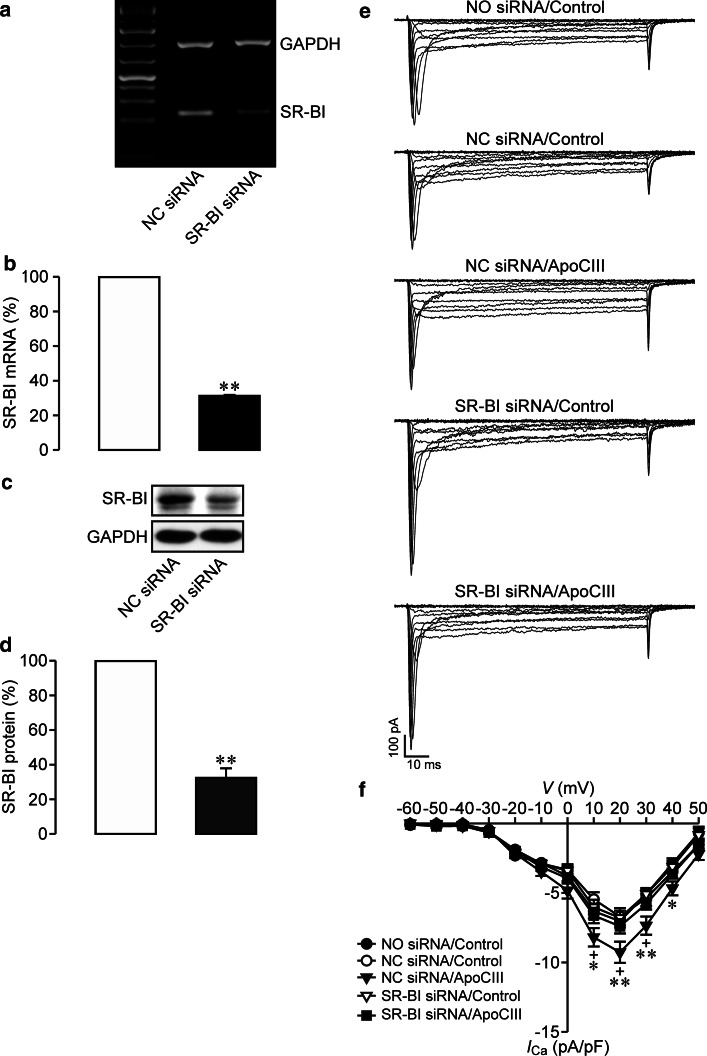
Previous studies have shown that there is no direct interaction of ApoCIII with β1 integrin [16, 18]. In search for a molecular bridge between ApoCIII and β1 integrin we focused our interest to SR-BI since this receptor physically associates with ApoCIII and interacts with β1 integrin [10, 26]. We combined siRNA-mediated gene silencing and whole-cell Ca2+ current analysis to examine if SR-BI can serve as a molecular bridge between ApoCIII and β1 integrin in hyperactivating β cell CaV1 channels. As shown in Fig. 8a–d, SR-BI siRNA transfection significantly lowered SR-BI at both mRNA and protein levels in RINm5F cells. It is important to note that such downregulation sufficiently abolished enhancement of whole-cell Ca2+ currents by ApoCIII (Fig. 8e, f). Figure 8e shows that SR-BI siRNA pretransfected cells incubated with ApoCIII (SR-BI siRNA/ApoCIII) exhibited smaller whole-cell Ca2+ currents as compared with those pretransfected with negative control siRNA followed by ApoCIII exposure (NC siRNA/apoCIII). Whole-cell Ca2+ currents in cells subjected to SR-BI siRNA/ApoCIII did not differ from those in control vehicle-treated cells subjected to mock (NO siRNA/Control), negative control siRNA (NC siRNA/Control) and SR-BI siRNA pretransfection (SR-BI siRNA/Control), respectively (Fig. 8e). In contrast, whole-cell Ca2+ currents in NC siRNA/apoCIII-treated cells were larger than those visualized in the afore-mentioned control cells (Fig. 8e). Ca2+ current density in SR-BI siRNA/ApoCIII group (n = 30) was significantly decreased in comparison with that in NC siRNA/apoCIII group (filled triangles, n = 30); (Fig. 8f). The former is similar to, but the latter is significantly larger than that in NO siRNA/Control (n = 30), NC siRNA/Control (n = 29) or SR-BI siRNA/Control (n = 29); (Fig. 8f). The data verify that ApoCIII employs SR-BI as an indispensable conveyor for signaling from this apolipoprotein to β cell CaV channels.
Fig. 8.
Knockdown of SR-BI prevents apolipoprotein CIII-induced enhancement of whole-cell Ca2+ currents in RINm5F cells. a Representative blots of GAPDH- and SR-BI-mRNA bands in negative control siRNA (NC siRNA)- and SR-BI siRNA-transfected cells. b Quantifications of SR-BI mRNA in NC siRNA- (open column, n = 7) and SR-BI siRNA-transfected RINm5F cells (filled column, n = 7). **p < 0.01 versus NC siRNA. c Sample blots of SR-BI- and GAPDH-immunoreactive bands in NC siRNA- and SR-BI siRNA-transfected cells. d Quantitative immunoblot measurements of SR-BI protein in NC siRNA- (open column, n = 7) and SR-BI siRNA-transfected RINm5F cells (filled column, n = 7). **p < 0.01 versus NC siRNA. e Representative whole-cell Ca2+ current traces from individual cells subsequent to mock transfection and incubation with control vehicle (NO siRNA/Control, cell capacitance: 13.87 pF), NC siRNA transfection and control vehicle treatment (NC siRNA/Control, cell capacitance: 13.18 pF), NC siRNA transfection and apolipoprotein CIII (ApoCIII) incubation (NC siRNA/ApoCIII, cell capacitance: 13.53 pF), SR-BI siRNA transfection and exposure to vehicle solution (SR-BI siRNA/Control, cell capacitance: 12.90 pF) and SR-BI siRNA transfection and ApoCIII exposure (SR-BI siRNA/ApoCIII, cell capacitance: 13.01 pF), respectively. f Ca2+ current density–voltage relationships in cells subjected to NO siRNA/Control (filled circles, n = 30), NC siRNA/Control (open circles, n = 29), NC siRNA/apoCIII (filled triangles, n = 30), SR-BI siRNA/Control (open triangles, n = 29) and SR-BI siRNA/ApoCIII (filled squares, n = 30). *p < 0.05 and **p < 0.01 versus NO siRNA/Control, NC siRNA/Control and SR-BI siRNA/Control. +p < 0.05 versus SR-BI siRNA/ApoCIII
Discussion
The gross conductivity of CaV channels depends on the density and activity of functional channels in the plasma membrane of the cell. Enhancement of whole-cell Ca2+ currents by type 1 diabetic serum and its factor ApoCIII can result from enriched density and/or increased conductivity of functional CaV channels in the β cell plasma membrane [4, 5]. However, all studies [1, 2, 5, 27] except one [4] have so far examined the effect of type 1 diabetic serum on CaV channels only at the whole cell level. In the study by Juntti-Berggren et al. [4] the increase in β cell CaV channel activity by type 1 diabetic serum was characterized at both the single channel and the whole-cell level. However, this work did not analyze whether type 1 diabetic serum could alter the density of functional CaV channels in the β cell plasma membrane [4]. Although we have previously revealed that ApoCIII serves as a type 1 diabetic serum factor, hyperactivating β cell CaV channels, only whole-cell patch-clamp analysis was performed [5]. Undoubtedly, detailed examination of biophysical properties of single CaV channels in ApoCIII-treated cells should be implemented to mechanistically dissect hyperactivation of β cell CaV channels by this apolipoprotein. Interestingly, cell-attached single channel recordings in the present work reveal that incubation with ApoCIII not only augments the activity of individual β cell CaV1 channels but also enriches the number of functional CaV1 channels in the recorded area of the β cell plasma membrane. The augmentation of single CaV1 channel activity is visualized as an increased open probability attributed to the prolonged mean open time and shortened mean closed time. Enrichment of number of functional CaV1 channels is verified by appearance of more levels of single CaV1 channel conductance.
The insulin-secreting RINm5F cell is equipped with CaV1, CaV2 and CaV3 channels [1, 2]. We investigated if ApoCIII selectively hyperactivates CaV1 channels or indiscriminately impacts all these three types of CaV channels in this insulin-secreting cell. It turned out that ApoCIII-induced hyperactivation of β cell CaV channels could no longer take place following pharmacological ablation of CaV1 channels. This means that ApoCIII selectively hyperactivates CaV1 channels, which are the major CaV channel type playing a predominant role over other types of CaV channels in β cell physiology and pathophysiology. Give that ApoCIII acts as the actual factor in type 1 diabetic serum to drive Ca2+-dependent β cell death that is prevented by the CaV1 channel blocker verapamil, the selective hyperactivation of β cell CaV1 channels observed in the present work most likely accounts for the pathophysiological role of this apolipoprotein in Ca2+-dependent β cell [1, 2, 4, 5].
A series of protein kinases, such as PKA and PKC, can effectively phosphorylate CaV channels resulting in increases in the open channel density and activity due to phosphorylation-induced conformational changes in these channels [3, 28, 29]. Increases in the number and open probability of functional CaV channels by ApoCIII might be mediated by protein kinases. ApoCIII has been demonstrated to activate PKC through β1 integrin in monocytic cells [16]. Furthermore, β1 integrin activation can also upregulate CaV1 channels in neurons, ventricular myocytes and vascular smooth muscle cells through stimulation of PKA, PKC and Src kinase [22–25]. All these components are present in β cells [2, 30–33] and may suggest that ApoCIII employs the β1 integrin-PKA/PKC/Src kinase cascade to hyperactivate β cell CaV channels. Indeed, the present work shows that complex inhibition of PKA, PKC and Src kinase effectively abrogates ApoCIII-induced hyperactivation of β cell CaV channels and that coinhibition of PKA and Src kinase is enough for this effect. However, individual inhibition of PKA, PKC or Src kinase only produced, if anything, a marginal effect on ApoCIII-induced hyperactivation of β cell CaV channels. Hence, we conclude that ApoCIII relies on parallel PKA and Src pathways to upregulate β cell CaV channels.
Occurrence of ApoCIII-induced hyperactivation of β cell CaV channels requires overnight incubation. Hence, the effect might be accounted for by an increase in CaV channel expression. Therefore, we quantified immunoreactivities of CaV1.2 and CaV1.3 subunits in RINm5F cells following overnight incubation with ApoCIII. However, the incubation had no influence on β cell CaV1 channel expression. We therefore excluded the possibility that ApoCIII elevates β cell CaV1 channel expression.
The transmembrane receptor β1 integrin is noncovalently associated with other integrins to form a set of heterodimers. They recognize a large number of soluble and surface-bound proteins to mediate cell–cell, cell-extracellular matrix and cell-pathogen interactions [34]. β1 Integrin is situated downstream of ApoCIII and upstream of PKA/PKC/Src kinase in some cell types [16, 22–25]. This made us investigate whether the ApoCIII-β1 integrin-PKA/PKC/Src kinase pathway operates in the β cell as the mechanism whereby this apolipoprotein hyperactivates CaV1 channels. Interestingly, knockdown of β1 integrin does not influence β cell CaV channel activity in the absence of ApoCIII, but significantly abrogates ApoCIII-induced hyperactivation of β cell CaV channels. The results clearly verify that β1 integrin plays a significant role in mediating the action of ApoCIII on β cell CaV1 channel activity.
Although β1 integrin can couple ApoCIII to the corresponding downstream effectors PKA, PKC and Src kinase, β1 integrin is unlikely to directly interact with this apolipoprotein [16, 22–25]. Previous work shows that SR-BI not only physically associates with ApoCIII but also interacts with β1 integrin [10, 26]. This pinpoints the possibility that SR-BI may serve as a molecular bridge between ApoCIII and β1 integrin with regard to β cell CaV channel hyperactivation. Indeed, in the present study we could demonstrate that SR-BI serves as a molecular bridge since SR-BI gene silencing efficiently nullifies ApoCIII-induced hyperactivation of β cell CaV channels. This generates a complete picture of the novel cascade of β cell CaV channel hyperactivation, namely ApoCIII-SR-BI-β1 integrin-PKA/Src.
It is worth noting that ApoCIII-induced hyperactivation of β cell CaV1 channels observed in the present work occurred when cells were depolarized to more positive potentials than +10 mV. It seems that β cell CaV1 channels in vivo never experience such strong depolarization and thus the effect of ApoCIII could hardly happen since the action potential of the β cell peaks at about −20 mV [35–37]. However, the effect of ApoCIII was detected by using the perforated whole-cell patch-clamp recording mode under experimental conditions where 10 mM Ca2+ was added in extracellular solution to obtain optimal Ca2+ currents. Such a high concentration of extracellular Ca2+ (10 mM) in comparison with physiological concentration of extracellular Ca2+ (2.5 mM) can significantly shift the I–V curve to more positive potentials [38–40]. The perforated whole-cell patch-clamp recording mode has a similar effect. Hence, under in vivo conditions ApoCIII is likely to affect β cell CaV1 currents within the physiological membrane potential range [38–40].
In conclusion, our findings demonstrate that ApoCIII selectively hyperactivates β cell CaV1 channels through parallel PKA and Src kinase pathways in a SR-BI/β1 integrin-dependent fashion. ApoCIII-induced hyperactivation of β cell CaV1 channels is characterized by the enriched density and increased activity of functional CaV1 channels in the β cell plasma membrane. Undoubtedly, this novel signal-transduction pathway has a potential to serve as an innovative drug discovery platform for the prevention of Ca2+-dependent β cell death in association with diabetes.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by grants from Berth von Kantzow’s Foundation, Diabetes Research and Wellness Foundation, EuroDia (FP6-518153), the Family Erling-Persson Foundation, Fredrik and Ingrid Thuring’s Foundation, Funds of Karolinska Institutet, the Knut and Alice Wallenberg Foundation, Magn. Bergvall’s Foundation, Novo Nordisk Foundation, Skandia Insurance Company, Ltd., the Stichting af Jochnick Foundation, Strategic Research Program in Diabetes at Karolinska Institutet, the Swedish Alzheimer Association, the Swedish Diabetes Association, the Swedish Foundation for Strategic Research, the Swedish Research Council, the Swedish Society of Medicine, Torsten and Ragnar Söderberg Foundation, VIBRANT (FP7-228933-2) and Åke Wiberg’s Foundation. P.-O. Berggren is founder of the Biotech Company BioCrine AB and is also a member of the board of this company. S.-N.Yang is a consultant to BioCrine AB. BioCrine AB is developing ApoCIII as a novel druggable target for the treatment of diabetes.
Abbreviations
- ApoCIII
Apolipoprotein CIII
- CaV
Voltage-gated Ca2+
- SR-BI
Scavenger receptor class B type I
Footnotes
Yue Shi and Guang Yang have contributed equally to this work.
Contributor Information
Per-Olof Berggren, Phone: +46-85-1775731, FAX: +46-85-1779450, Email: per-olof.berggren@ki.se.
Shao-Nian Yang, Phone: +46-85-1779456, FAX: +46-85-1779450, Email: shao-nian.yang@ki.se.
References
- 1.Yang SN, Berggren PO. β-cell CaV channel regulation in physiology and pathophysiology. Am J Physiol. 2005;288:E16–E28. doi: 10.1152/ajpendo.00042.2004. [DOI] [PubMed] [Google Scholar]
- 2.Yang SN, Berggren PO. The role of voltage-gated calcium channels in pancreatic β-cell physiology and pathophysiology. Endocr Rev. 2006;27:621–676. doi: 10.1210/er.2005-0888. [DOI] [PubMed] [Google Scholar]
- 3.Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 4.Juntti-Berggren L, Larsson O, Rorsman P, Ammala C, Bokvist K, Wahlander K, Nicotera P, Dypbukt J, Orrenius S, Hallberg A, Berggren PO. Increased activity of L-type Ca2+ channels exposed to serum from patients with type I diabetes. Science. 1993;261:86–90. doi: 10.1126/science.7686306. [DOI] [PubMed] [Google Scholar]
- 5.Juntti-Berggren L, Refai E, Appelskog I, Andersson M, Imreh G, Dekki N, Uhles S, Yu L, Griffiths WJ, Zaitsev S, Leibiger I, Yang SN, Olivecrona G, Jornvall H, Berggren PO. Apolipoprotein CIII promotes Ca2+-dependent β cell death in type 1 diabetes. Proc Natl Acad Sci USA. 2004;101:10090–10094. doi: 10.1073/pnas.0403551101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sol EM, Sundsten T, Bergsten P. Role of MAPK in apolipoprotein CIII-induced apoptosis in INS-1E cells. Lipids Heal Dis. 2009;8:3. doi: 10.1186/1476-511X-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmberg R, Refai E, Höög A, Crooke RM, Graham M, Olivecrona G, Berggren PO, Juntti-Berggren L. Lowering apolipoprotein CIII delays onset of type 1 diabetes. Proc Natl Acad Sci USA. 2011;108:10685–10689. doi: 10.1073/pnas.1019553108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gangabadage CS, Zdunek J, Tessari M, Nilsson S, Olivecrona G, Wijmenga SS. Structure and dynamics of human apolipoprotein CIII. J Biol Chem. 2008;283:17416–17427. doi: 10.1074/jbc.M800756200. [DOI] [PubMed] [Google Scholar]
- 9.Jong MC, Hofker MH, Havekes LM. Role of ApoCs in lipoprotein metabolism: functional differences between ApoC1, ApoC2, and ApoC3. Arterioscler Thromb Vasc Biol. 1999;19:472–484. doi: 10.1161/01.ATV.19.3.472. [DOI] [PubMed] [Google Scholar]
- 10.Xu S, Laccotripe M, Huang X, Rigotti A, Zannis VI, Krieger M. Apolipoproteins of HDL can directly mediate binding to the scavenger receptor SR-BI, an HDL receptor that mediates selective lipid uptake. J Lipid Res. 1997;38:1289–1298. [PubMed] [Google Scholar]
- 11.Clavey V, Lestavel-Delattre S, Copin C, Bard JM, Fruchart JC. Modulation of lipoprotein B binding to the LDL receptor by exogenous lipids and apolipoproteins CI, CII, CIII, and E. Arterioscler Thromb Vasc Biol. 1995;15:963–971. doi: 10.1161/01.ATV.15.7.963. [DOI] [PubMed] [Google Scholar]
- 12.Huard K, Bourgeois P, Rhainds D, Falstrault L, Cohn JS, Brissette L. Apolipoproteins C-II and C-III inhibit selective uptake of low- and high-density lipoprotein cholesteryl esters in HepG2 cells. Int J Biochem Cell Biol. 2005;37:1308–1318. doi: 10.1016/j.biocel.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Chan DC, Watts GF, Redgrave TG, Mori TA, Barrett PH. Apolipoprotein B-100 kinetics in visceral obesity: associations with plasma apolipoprotein C-III concentration. Metabolism. 2002;51:1041–1046. doi: 10.1053/meta.2002.33339. [DOI] [PubMed] [Google Scholar]
- 14.Sundsten T, Ostenson CG, Bergsten P. Serum protein patterns in newly diagnosed type 2 diabetes mellitus––influence of diabetic environment and family history of diabetes. Diabet Metab Res Rev. 2008;24:148–154. doi: 10.1002/dmrr.789. [DOI] [PubMed] [Google Scholar]
- 15.Atzmon G, Rincon M, Schechter CB, Shuldiner AR, Lipton RB, Bergman A, Barzilai N. Lipoprotein genotype and conserved pathway for exceptional longevity in humans. PLoS Biol. 2006;4:e113. doi: 10.1371/journal.pbio.0040113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawakami A, Aikawa M, Libby P, Alcaide P, Luscinskas FW, Sacks FM. Apolipoprotein CIII in apolipoprotein B lipoproteins enhances the adhesion of human monocytic cells to endothelial cells. Circulation. 2006;113:691–700. doi: 10.1161/CIRCULATIONAHA.105.591743. [DOI] [PubMed] [Google Scholar]
- 17.Fang DZ, Liu BW. Apolipoprotein C-III can specifically bind to hepatic plasma membranes. Mol Cell Biochem. 2000;207:57–64. doi: 10.1023/A:1007038129481. [DOI] [PubMed] [Google Scholar]
- 18.Kawakami A, Aikawa M, Nitta N, Yoshida M, Libby P, Sacks FM. Apolipoprotein CIII-induced THP-1 cell adhesion to endothelial cells involves pertussis toxin-sensitive G protein- and protein kinase Cα-mediated nuclear factor-κB activation. Arterioscler Thromb Vasc Biol. 2007;27:219–225. doi: 10.1161/01.ATV.0000249620.68705.0d. [DOI] [PubMed] [Google Scholar]
- 19.Yang SN, Wenna ND, Yu J, Yang G, Qiu H, Yu L, Juntti-Berggren L, Kohler M, Berggren PO. Glucose recruits KATP channels via non-insulin-containing dense-core granules. Cell Metab. 2007;6:217–228. doi: 10.1016/j.cmet.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Berggren PO, Yang SN, Murakami M, Efanov AM, Uhles S, Kohler M, Moede T, Fernstrom A, Appelskog IB, Aspinwall CA, Zaitsev SV, Larsson O, Moitoso de Vargas L, Fecher-Trost C, Weissgerber P, Ludwig A, Leibiger B, Juntti-Berggren L, Barker CJ, Gromada J, Freichel M, Leibiger IB, Flockerzi V. Removal of Ca2+ channel β3 subunit enhances Ca2+ oscillation frequency and insulin exocytosis. Cell. 2004;119:273–284. doi: 10.1016/j.cell.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 21.Rorsman P, Arkhammar P, Berggren PO. Voltage-activated Na+ currents and their suppression by phorbol ester in clonal insulin-producing RINm5F cells. Am J Physiol. 1986;251:C912–C919. doi: 10.1152/ajpcell.1986.251.6.C912. [DOI] [PubMed] [Google Scholar]
- 22.Rueckschloss U, Isenberg G. Contraction augments L-type Ca2+ currents in adherent guinea-pig cardiomyocytes. J Physiol. 2004;560:403–411. doi: 10.1113/jphysiol.2004.062604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waitkus-Edwards KR, Martinez-Lemus LA, Wu X, Trzeciakowski JP, Davis MJ, Davis GE, Meininger GA. α4β1 Integrin activation of L-type calcium channels in vascular smooth muscle causes arteriole vasoconstriction. Circ Res. 2002;90:473–480. doi: 10.1161/hh0402.105899. [DOI] [PubMed] [Google Scholar]
- 24.Wu X, Davis GE, Meininger GA, Wilson E, Davis MJ. Regulation of the L-type calcium channel by α5β1 integrin requires signaling between focal adhesion proteins. J Biol Chem. 2001;276:30285–30292. doi: 10.1074/jbc.M102436200. [DOI] [PubMed] [Google Scholar]
- 25.Gui P, Wu X, Ling S, Stotz SC, Winkfein RJ, Wilson E, Davis GE, Braun AP, Zamponi GW, Davis MJ. Integrin receptor activation triggers converging regulation of Cav1.2 calcium channels by c-Src and protein kinase A pathways. J Biol Chem. 2006;281:14015–14025. doi: 10.1074/jbc.M600433200. [DOI] [PubMed] [Google Scholar]
- 26.Bamberger ME, Harris ME, McDonald DR, Husemann J, Landreth GE. A cell surface receptor complex for fibrillar beta-amyloid mediates microglial activation. J Neurosci. 2003;23:2665–2674. doi: 10.1523/JNEUROSCI.23-07-02665.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ristic H, Srinivasan S, Hall KE, Sima AA, Wiley JW. Serum from diabetic BB/W rats enhances calcium currents in primary sensory neurons. J Neurophysiol. 1998;80:1236–1244. doi: 10.1152/jn.1998.80.3.1236. [DOI] [PubMed] [Google Scholar]
- 28.Kavalali ET, Hwang KS, Plummer MR. cAMP-dependent enhancement of dihydropyridine-sensitive calcium channel availability in hippocampal neurons. J Neurosci. 1997;17:5334–5348. doi: 10.1523/JNEUROSCI.17-14-05334.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang J, Tsien RW. Enhancement of N- and L-type calcium channel currents by protein kinase C in frog sympathetic neurons. Neuron. 1993;10:127–136. doi: 10.1016/0896-6273(93)90305-B. [DOI] [PubMed] [Google Scholar]
- 30.Mukai E, Fujimoto S, Sato H, Oneyama C, Kominato R, Sato Y, Sasaki M, Nishi Y, Okada M, Inagaki N. Exendin-4 suppresses Src activation and reactive oxygen species production in diabetic Goto-Kakizaki rat islets in an Epac-dependent manner. Diabetes. 2011;60:218–226. doi: 10.2337/db10-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kantengwa S, Baetens D, Sadoul K, Buck CA, Halban PA, Rouiller DG. Identification and characterization of α3β1 integrin on primary and transformed rat islet cells. Exp Cell Res. 1997;237:394–402. doi: 10.1006/excr.1997.3803. [DOI] [PubMed] [Google Scholar]
- 32.Bosco D, Meda P, Halban PA, Rouiller DG. Importance of cell-matrix interactions in rat islet β-cell secretion in vitro: role of α6β1 integrin. Diabetes. 2000;49:233–243. doi: 10.2337/diabetes.49.2.233. [DOI] [PubMed] [Google Scholar]
- 33.Nikolova G, Jabs N, Konstantinova I, Domogatskaya A, Tryggvason K, Sorokin L, Fassler R, Gu G, Gerber HP, Ferrara N, Melton DA, Lammert E. The vascular basement membrane: a niche for insulin gene expression and β cell proliferation. Dev Cell. 2006;10:397–405. doi: 10.1016/j.devcel.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 34.Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007;25:619–647. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ashcroft FM, Rorsman P. Electrophysiology of the pancreatic β-cell. Prog Biophys Mol Biol. 1989;54:87–143. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- 36.Refai E, Dekki N, Yang SN, Imreh G, Cabrera O, Yu L, Yang G, Norgren S, Rossner SM, Inverardi L, Ricordi C, Olivecrona G, Andersson M, Jornvall H, Berggren PO, Juntti-Berggren L. Transthyretin constitutes a functional component in pancreatic beta-cell stimulus-secretion coupling. Proc Natl Acad Sci USA. 2005;102:17020–17025. doi: 10.1073/pnas.0503219102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoon JC, Xu G, Deeney JT, Yang SN, Rhee J, Puigserver P, Levens AR, Yang R, Zhang CY, Lowell BB, Berggren PO, Newgard CB, Bonner-Weir S, Weir G, Spiegelman BM. Suppression of beta cell energy metabolism and insulin release by PGC-1alpha. Dev Cell. 2003;5:73–83. doi: 10.1016/S1534-5807(03)00170-9. [DOI] [PubMed] [Google Scholar]
- 38.Byerly L, Chase PB, Stimers JR. Permeation and interaction of divalent cations in calcium channels of snail neurons. J Gen Physiol. 1985;85:491–518. doi: 10.1085/jgp.85.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Green WN, Andersen OS. Surface charges and ion channel function. Annu Rev Physiol. 1991;53:341–359. doi: 10.1146/annurev.ph.53.030191.002013. [DOI] [PubMed] [Google Scholar]
- 40.Ganitkevich V, Shuba MF, Smirnov SV. Saturation of calcium channels in single isolated smooth muscle cells of guinea-pig taenia caeci. J Physiol. 1988;399:419–436. doi: 10.1113/jphysiol.1988.sp017089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.