Abstract
A field study was carried out to investigate the effect of three Zn levels 0, 20 kg ZnSO4 ha−1 and 20 kg ZnSO4 ha−1+ foliar spray of 0.5 % ZnSO4 on superoxide dismutase activity, acid phosphatase activity and grain yield and a pot experiment to study the effect of zinc deficient and sufficient conditions on organic acid exudation. Increasing Zn levels was established as beneficial in improving the enzyme activities of genotypes. Combined foliar and soil application of Zn proved to be superior of all the treatments. Zinc application resulted in a maximum increment limit of 96.8 % in superoxide dismutase activity, 75.76 % in acid phosphatase activity, and a decrement limit of 88.57 % in oxalic acid exudation irrespective of stages and year of study. The increased enzyme activities had a positive impact on grain yield. As an average of all genotypes an improvement of 19.88 % in 2009 and 21.29 % in 2010 due to soil application while of 16.45 % in 2009 and 13.01 % in 2010 due to combined application was calculated for grain yield. There existed a variation among genotypes in showing responses towards zinc application and the genotypes UP 2584 and PBW 550 were found to be more responsive.
Keywords: SOD activity, Acid phosphatase activity, Oxalic acid, Zinc, Wheat
Introduction
Zinc is a ubiquitous micronutrient required as a structural and functional component of many enzymes and proteins. It is a cofactor of about 300 enzymes (Coleman 1998) and plays a vital role in transcriptional and post transcriptional processes, protein degradation and protein-protein interactions (Marschner 1995). In this way it participates in plant metabolism, growth and development. Unfortunately the uptake limits from the soil to the plants are seldom met as 50 % of the soil samples around the world are found to be zinc deficient (Sillanpaa 1990). Zinc deficiency in soil is mainly due to decreased availability of zinc and its low content in soil. The low availability of zinc in soil is influenced by properties like high pH, moisture, organic matter, temperature etc. (Alloway 2008). Its inadequacy in plants may disturb the essential photosynthetic activity, carbon assimilation and thus hamper several growth parameters. Not only this, it also makes the plants susceptible to several kinds of stresses such as drought, heat, chilling, high light intensity etc. (Graham and McDonald 2001, Wang and Jin 2005). With the ever changing global climate, the impact of stress is also becoming intense. It is well documented that in plants reactive (ROS) oxygen species are produced due to leakage of electrons from electron transport chain to molecular O2 which are afterward removed by the plant anti-oxidative system (Cakmak 2000). Under various kinds of stress their formation and removal lacks balance resulting in excess of ROS which can cause an oxidation of bio molecules like lipids, proteins, chlorophyll, and nucleic acids. In this regard the enzyme superoxide dismutase is very important acting as the first line of defence to scavenge ROS (Alscher et al. 2002). This enzyme is a cupro-zinc protein and thus it becomes highly important to maintain zinc homeostasis in plant cells as to protect them from oxidative stress. In an effort to maintain physiologically sufficient zinc, a decline in the level of phosphate pool may occur owing to competition between zinc and phosphorus during their absorption through root cells. Inorganic phosphate is a structural component of many important bio molecules and also plays a role in energy transfer. It therefore becomes essential to assess an important enzyme such as acid phosphatase which catalyses the removal of inorganic phosphate from organic phosphate esters and thus helps in maintaining inorganic phosphate metabolism (Asmar et al. 1995). Under zinc deficiency, plants may undergo a spectrum of physiological changes in order to maintain cellular metabolism and to evade any sudden changes in the process of development. One such phenomenon in graminaceous plants is the secretion of organic acids. Whenever there is a zinc inadequacy low molecular weight organic acids are released through their roots. They are involved in lowering the pH of rhizosphere and thus increasing the solubilisation of zinc (Pérez-Esteban et al. 2013). It is of no surprise to know why members of this family e.g. wheat are adapted to exhibit this phenomenon as they are inherently low in zinc. While cereals provide 56 % of the food energy on earth it becomes important to maintain a homeostasis of zinc concentration in them (Stoskopf 1985). Zinc deficiency may adversely affect the wheat production which is important for mankind and livestock. In this respect the most reasonable approach is to fertilize the soils with zinc fertilizers. Other methods like breeding genotypes tolerant to soil Zn deficiency may take a long period of time while selection of the genotypes with high Zn uptake efficiency may be limited by soil Zn availability. If possible, zinc should also be supplied through foliar application. To select such genotypes that are more responsive to the zinc application will help in resolving the problem. The work presented in this paper has been designed with this objective.
Material and methods
Plant material and field experimentation
The field study was conducted at the Norman E. Borlaug Crop Research Centre, Govind Ballabh Pant University of Agriculture and Technology, Pantnagar, Udham Singh Nagar (Uttarakhand) during the winter-summer seasons of 2009–2010 and 2010–2011. Geographically, the site lies in Tarai plains about 30 km southwards of foothills of Shivalik range of the Himalayas at 29° N latitude, 79° 29′ E longitude and at an altitude of 243.8 meter above the mean sea level. The experimental plot (typic haplndoll) had a loam texture, 7.0 pH, 0.278 dSm−1 E.C. at 25 º C, 10.3 g organic C kg−1 and 0.42 mg DTPA extractable Zn mg kg−1 soil. Ten wheat genotypes namely UP 262, UP 2338, UP 2382, UP 2572, UP 2554, UP 2584, PBW 343, PBW 550, PBW 175 and PBW 590 were used for the given experiment. Three treatments were given namely 0 kg ZnSO4 ha−1 (Zn0 or T1), 20 kg ZnSO4 ha−1 (Zn20 or T2) and 20 kg ZnSO4 ha−1 along with foliar spray of 0.5 % solution of ZnSO4 (Zn20+F or T3). Foliar spray was given at maximum tillering and one week after flowering. Each treatment was replicated thrice. Foliar spray solution was made by 100 g ZnSO4 dissolved in 10 litre distilled water and 50 g lime in 10 litres distilled water, separately. The two solutions were mixed after filtering them through muslin cloth. The plots received irrigation from time to time as per their need. N, P and K were applied as per recommendation as urea, triple super phosphate and muriate of potash, respectively in all treatments, through broadcast.
Experimental design
The field experiment was laid out in split plot design with three replications during both years.
Parameters recorded
-
Superoxide dismutase (SOD) activity
Superoxide dismutase activity in leaves was estimated at three growth stages i.e. maximum tillering (S1), flowering (S2) and grain filling (S3). It was estimated according to Giannopolitis and Ries 1977 by measuring its ability to inhibit the photochemical reduction of nitroblue tetrazolium (NBT). The extraction was carried out in phosphate buffer. Reaction was started with adding 2 μM riboflavin in a mixture (1.5 ml) containing 50 mM phosphate buffer (pH 7.8), 13 mM methionine, 75 μM NBT, 2 μM riboflavin, 50 μl enzyme extract, 0.1 μM EDTA. The reaction was carried for 15 min under illumination with 20 W fluorescent tubes. Absorbance of the reaction mixture was recorded at 560 nm.
-
Acid phosphatase (AcPh) activity
Acid Phosphatase was also determined in fresh leaves at three growth stages (maximum tillering, flowering and grain filling). The enzyme was assayed by measuring its ability to hydrolyse p-nitrophenyl phosphate into p-nitrophenol (Sadasivam and Manickam 1991). Three ml of substrate solution was incubated at 37 °C for 5 min. To it 0.5 ml enzyme extract was added and mixed well. After this 0.05 ml of solution was removed immediately and mixed with 9.5 ml of sodium hydroxide 0.085 N. This corresponds to zero time assay (blank).The remaining solution (substrate + enzyme) was incubated for 5 min at 37 °C. 0.5 ml of the sample was drawn and mixed with 9.5 ml sodium hydroxide solution. The absorbance of blank and incubated tubes was recorded at 405 nm. For the preparation of working standards 0.2 to 1.0 ml (4 to 20 mM) of the standard was taken and diluted to 10.0 ml with NaOH solution. Thereafter, absorbance at the same wavelength was recorded and final calculations were done after drawing a standard curve.
-
Root exudates (oxalic acid)
A pot experiment was conducted for the estimation of root exudates (oxalic acid). Two Hoagland solutions (− Zn, + Zn) were given to the plants every alternate days to maintain Zn-deficient and Zn-sufficient conditions respectively. Root exudates were collected at the maximum tillering stage. The samples were prepared and analysed using a modification in the methodology described by Wang et al. 2007. Exudates were filtered with Wattman no.-1 and passed through a cation exchange column filled with Amberlite IR-120C resin followed by an anion exchange column filled with Dowex resin. The organic acids held back on anion exchange resin were eluted by 1 M HCl, and elute was concentrated to dryness. The residue was redissolved in dilute HClO4 solution (pH 2.1) to 1.0 ml. The concentration of organic acids was then analysed by High Pressure Liquid Chromatography (HPLC). Authentic compound namely oxalic acid was used for HPLC analysis. HPLC grade solvents and water were used for the analysis. KH2PO4 (pH adjusted to 2.5 with H3PO4 (125 mM) was used as a mobile phase. Prior to analysis the samples were filtered through 0.45 μm membrane filters (Millipore) before injecting into the HPLC column. 20.0 μl samples were injected into HPLC column for analysis using a micro Hamilton syringe. Absorbance was recorded at 210 nm with UV detector using C18 reverse column at a flow rate of 0.5 ml/min.
-
Grain Yield
Wheat plants from one square meter area from each plot were harvested at maturity. After threshing and winnowing, total weight of grains (g/m2) was recorded from each net plot. Grains obtained from each net plot were weighed and finally grain yield was expressed in tonnes per hectare.
Statistical analysis
The statistical analysis of data for all the parameters was carried out with analysis of variance for split plot design. Standard error of mean (SEM±) and critical difference (CD) was evaluated at 5 % level of significance. The means were tested at P > 0.05 using a STPR software designed at Department of Mathematics, Statistics and Computer Science, CBSH, G.B. Pant Univ. of Agri. & Tech, Pantnagar, India. SPSS software program was also used for Duncan’s test which was applied for comparison of mean and their interactive effect.
Results
Superoxide dismutase activity
Evaluating the data concerning superoxide dismutase activity revealed its significant dependence on different levels of zinc nutrition. The enzyme activity enhanced increasingly on increasing zinc levels for majority of the genotypes with Zn20+F proving to be the best application. As an average of all genotypes the increment due to soil application with respect to control (Zn0) was 12.8 % at S1, 9.6 % at S2, 21 % at S3 during 2009 and 12.6 % at S1, 9.0 % at S2, 22.3 % at S3 during 2010 while combined application (Zn20+F) brought an additional increment of 6.5 % at S1, 5.1 % at S2 during 2009 relative to soil application. It is of surprise that at S3 the enhancement in activity was greater due to Zn20 by 4 %. Similarly in 2010 it brought about an additional increment of 4.7 % at S1, 9.2 % at S2, and a marked increment of 35 % at S3. Overall Zn20 + F enhanced the activity considerably by an average of 20 % at S1, 14.8 % at S2 and 16.6 % at S3 during the first crop season while 17.2 % at S1, 18.8 % at S2, and 61.8 % at S3 during the second crop season. The maximum limit by which the activity was observed to fasten on zinc application was 65.2 % in 2009–10 and 96.8 % in 2010–11. Irrespective of the application given and the stage of growth, during 2009–10 the genotypes PBW 175, PBW 550, UP 2584 showed the maximum increments of 65.2 %, 48.2 % and 23.2 % respectively while during 2010–11 the genotypes PBW 590, PBW 550, UP 2584 exhibited the maximum increments of 121.8 %, 96.8 % and 86.6 % respectively (Table 1).
Table 1.
Effect of different Zn levels on superoxide dismutase activity (units g−1) in different genotypes of wheat (2009–2010 and 2010–2011)
| Maximum tillering | Flowering | Grain filling | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genotypes | 2009 | 2010 | 2009 | 2010 | 2009 | 2010 | ||||||||||||
| T1 | T2 | T3 | T1 | T2 | T3 | T1 | T2 | T3 | T1 | T2 | T3 | T1 | T2 | T3 | T1 | T2 | T3 | |
| UP 262 | 508.5 | 626.0 | 676.5 | 410.4 | 641.6 | 563.5 | 616.6 | 640.5 | 714.2 | 603.5 | 619.0 | 627.7 | 637.1 | 670.3 | 709.6 | 448.1 | 589.3 | 584.1 |
| UP2338 | 582.5 | 585.7 | 590.0 | 512.7 | 549.9 | 541.1 | 622.8 | 651.2 | 722.7 | 495.7 | 623.1 | 679.5 | 587.8 | 713.0 | 695.1 | 355.6 | 409.9 | 577.3 |
| UP2382 | 479.1 | 522.1 | 579.7 | 513.6 | 536.8 | 522.9 | 623.6 | 659.7 | 701.7 | 554.1 | 525.0 | 667.0 | 611.3 | 710.6 | 655.4 | 453.9 | 539.6 | 591.9 |
| UP2572 | 570.8 | 581.1 | 585.7 | 485.0 | 501.7 | 526.4 | 493.9 | 703.9 | 692.6 | 592.1 | 582.7 | 618.7 | 612.8 | 707.6 | 682.8 | 407.8 | 620.1 | 493.1 |
| UP2554 | 488.4 | 558.6 | 566.6 | 487.7 | 504.7 | 556.1 | 655.6 | 671.5 | 673.7 | 591.4 | 661.5 | 612.9 | 558.6 | 687.1 | 550.2 | 325.8 | 419.9 | 608.1 |
| UP2584 | 419.7 | 598.4 | 615.2 | 456.4 | 512.9 | 500.1 | 618.4 | 673.7 | 681.4 | 471.5 | 517.8 | 674.3 | 624.4 | 696.8 | 673.9 | 318.4 | 335.7 | 594.1 |
| PBW343 | 444.1 | 485.6 | 592.6 | 455.7 | 493.9 | 530.6 | 635.0 | 704.6 | 703.5 | 539.6 | 602.8 | 575.1 | 616.7 | 695.8 | 606.6 | 555.3 | 440.8 | 625.0 |
| PBW550 | 491.7 | 566.55 | 592.3 | 425.1 | 498.6 | 632.3 | 619.2 | 626.9 | 735.2 | 439.4 | 479.5 | 492.2 | 463.8 | 680.3 | 687.3 | 306.4 | 406.3 | 603.1 |
| PBW175 | 574.0 | 578.7 | 665.7 | 466.9 | 482.3 | 532.2 | 630.2 | 664.5 | 688.0 | 505.4 | 589.6 | 694.3 | 413.7 | 629.9 | 683.4 | 360.3 | 359.2 | 609.9 |
| PBW590 | 521.2 | 579.7 | 573.6 | 486.6 | 534.5 | 565.8 | 651.6 | 715.3 | 727.8 | 520.2 | 567.2 | 622.1 | 666.3 | 703.1 | 665.8 | 264.6 | 421.4 | 586.9 |
| SEM± | CD | SEM± | CD | SEM± | CD | SEM± | CD | SEM± | CD | SEM± | CD | |||||||
| T | 5.7 | 22.4 | 5.2 | 20.4 | 12.9 | 50.3 | 8.4 | 32.8 | 5.2 | 20.2 | 9.6 | 37.7 | ||||||
| V | 13.3 | 37.8 | 11.7 | 33.1 | 11.7 | 33.2 | 9.5 | 26.8 | 5.6 | 15.8 | 14.3 | 40.6 | ||||||
| V withinT | 65.4 | 57.4 | 57.5 | 46.4 | 27.4 | 70.3 | ||||||||||||
| V across T | 65.7 | 57.9 | 73.3 | 54.4 | 32.5 | 76.0 | ||||||||||||
The values were analysed with analysis of variance (ANOVA); SEM± represents standard error of mean; CD represents the critical difference value to test the level of significance between means (P > 0.05)
Acid phosphatase activity
Interpreting the data relating to acid phosphatase activity revealed that it showed a significant enhancement on the application of different regimes of zinc. Like superoxide dismutase activity it showed an increasing pattern on application of different zinc levels for majority of genotypes showing highest activity at Zn20+F. As an average of all genotypes the activity was increased due to Zn20 by 14.92 % at S1, 15.47 % at S2, 22.69 % at S3 during 2009 while by 22.08 % at S1, 26.09 % at S2, 29.77 % at S3. Combined application brought about a further increment of 22.90 % at S1, 12.36 % at S2, 18.93 % at S3 in 2009 while of 19.16 % at S1, 10.25 % at S2, 21.52 % at S3 in 2010 with respect to soil application. Overall Zn20 + F brought about a considerable enhancement of 40.3 % at S1, 29.51 % at S2 and 44.96 % at S3 in 2009 and 42.69 % at S1, 38.68 % at S2 and 55.49 % at S3 in 2010. The activity exhibited a maximum increment limit of 68.46 % during first crop season and 75.76 % during the second crop season on zinc application (Table 2).
Table 2.
Effect of different Zn levels on acid phosphatase activity (units g−1) in different genotypes of wheat (2009–2010 and 2010–2011)
| Maximum tillering | Flowering | Grain filling | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genotypes | 2009 | 2010 | 2009 | 2010 | 2009 | 2010 | ||||||||||||
| T1 | T2 | T3 | T1 | T2 | T3 | T1 | T2 | T3 | T1 | T2 | T3 | T1 | T2 | T3 | T1 | T2 | T3 | |
| UP 262 | 1574.7 | 2077.7 | 1779.6 | 1404.7 | 1977.1 | 2010.8 | 1697.6 | 2043.8 | 2409.0 | 1659.8 | 2021.7 | 2375.3 | 1400.5 | 1736.5 | 1997.9 | 1049.0 | 1403.6 | 1666.0 |
| UP2338 | 1506.3 | 1719.4 | 2118.7 | 1645.3 | 2011.3 | 2275.3 | 1703.9 | 2001.0 | 2373.3 | 1768.2 | 2197.5 | 2316.7 | 1402.6 | 1774.2 | 2362.8 | 1003.3 | 1383.4 | 1763.5 |
| UP2382 | 1373.0 | 1655.6 | 2161.2 | 1714.2 | 1660.3 | 2348.4 | 1724.6 | 1980.2 | 2253.5 | 1705.9 | 1949.1 | 2077.2 | 1426.4 | 1983.3 | 1718.4 | 979.0 | 1627.6 | 1707.5 |
| UP2572 | 1443.6 | 1702.3 | 2167.9 | 1616.2 | 1415.0 | 2298.6 | 1705.4 | 1985.9 | 2169.0 | 1758.3 | 1950.7 | 2360.3 | 1441.0 | 1733.3 | 2048.7 | 1191.6 | 1413.5 | 1749.0 |
| UP2554 | 1351.3 | 1769.2 | 2225.5 | 1640.1 | 2011.9 | 2508.1 | 1991.1 | 2081.3 | 2311.0 | 1701.3 | 2299.1 | 2440.7 | 1613.1 | 2124.4 | 2371.7 | 1073.3 | 1607.9 | 1772.3 |
| UP2584 | 1530.2 | 1741.2 | 2182.9 | 1633.9 | 2034.7 | 2391.9 | 1919.0 | 2011.3 | 2261.8 | 1368.9 | 1984.9 | 1976.6 | 1646.0 | 1708.0 | 2375.5 | 1332.1 | 1089.9 | 1648.9 |
| PBW343 | 1549.3 | 1668.2 | 2195.9 | 1662.9 | 2023.8 | 2034.7 | 1974.5 | 2037.6 | 2361.3 | 1407.8 | 2095.3 | 2347.3 | 1484.0 | 1753.6 | 2074.2 | 1420.7 | 1436.3 | 1695.6 |
| PBW550 | 1548.3 | 1754.7 | 2113.5 | 1450.8 | 2038.3 | 2348.9 | 1799.8 | 1996.8 | 2340.6 | 1669.6 | 2033.6 | 2411.6 | 1424.4 | 1719.6 | 2280.6 | 1053.6 | 1479.3 | 1711.1 |
| PBW175 | 1673.8 | 1539.0 | 2138.4 | 1493.9 | 2056.4 | 2044.0 | 1544.7 | 2124.9 | 2286.2 | 1742.7 | 2076.2 | 2134.2 | 1444.0 | 1764.9 | 1999.9 | 1073.3 | 1419.7 | 1752.6 |
| PBW590 | 1642.2 | 1756.7 | 2108.3 | 1574.7 | 1989.0 | 2297.0 | 1768.7 | 2204.2 | 2202.2 | 1735.0 | 2077.2 | 2344.7 | 1403.1 | 1690.9 | 2050.4 | 1069.7 | 1451.3 | 1767.1 |
| SEM± | CD | SEM± | CD | SEM± | CD | SEM± | CD | SEM± | CD | SEM± | CD | |||||||
| T | 7.8 | 30.3 | 9.8 | 38.8 | 9.9 | 38.9 | 11.3 | 44.3 | 12.5 | 48.9 | 7.4 | 28.9 | ||||||
| V | 42.8 | 121.2 | 25.2 | 71.4 | 23.3 | 66.2 | 18.7 | 53.0 | 17.3 | 48.9 | 19.2 | 54.6 | ||||||
| V withinT | 210.0 | 123.7 | 114.6 | 91.8 | 84.8 | 94.5 | ||||||||||||
| V across T | 201.3 | 123.0 | 115.0 | 97.1 | 93.3 | 93.9 | ||||||||||||
The values were analysed with analysis of variance (ANOVA); SEM± represents standard error of mean; CD represents the critical difference value to test the level of significance between means (P > 0.05)
Oxalic acid
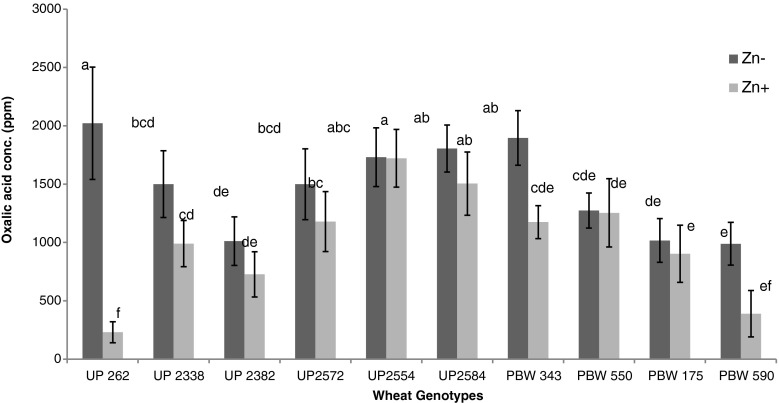
The data regarding oxalic acid (root exudates) reveals that its secretion was enhanced under zinc deficient conditions whilst reduced under zinc sufficient conditions. As an average of all genotypes a 30 % decrement was observed under zinc supplementation. Some genotypes like UP 262 and PBW 590 showed a highly enhanced secretion of oxalic acid under zinc deficiency conditions and exhibiting a reduction of 88.57 % and 60 % respectively when supplemented with zinc (Fig. 1).
Fig. 1.
Effect of zinc deficient and zinc sufficient conditions on oxalic acid secretion through roots of different genotypes of wheat (vertical bars indicates ± SD) (Means with same letter are not significantly different)
Grain yield and its correlation with SOD & acid phosphatase activity
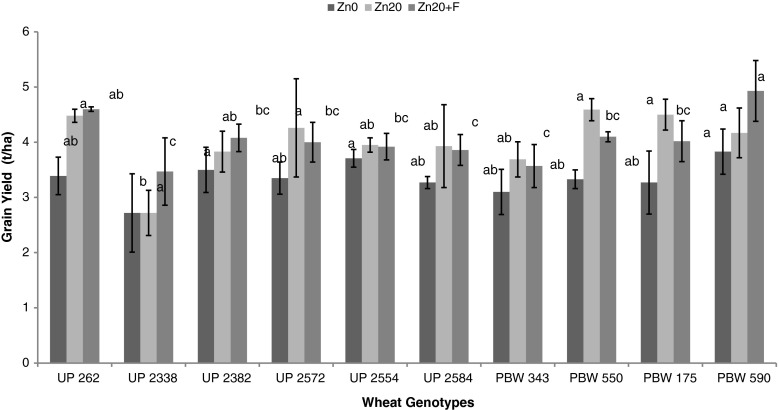
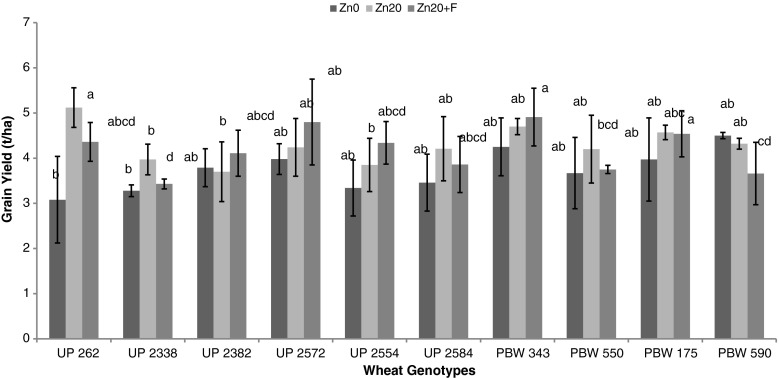
Evaluation of data regarding grain yield revealed its positive relationship with the zinc application in both the years (Figs. 2 & 3). An increment in also the two enzyme activities on application of zinc exhibits their positive impact on the grain yield. During 2009 an enhancement of 12.4 % in SOD activity and 17.7 % in AcPh activity (average across all genotypes and three stages) led to an average increment of 19.88 % in grain yield due to soil application while of 17.1 % in SOD activity and 38.56 % in AcPh due to combined application gave an increment of 16.45 % in the same. During 2010 an increment of 14.6 % in SOD activity and 25.98 % in AcPh exhibited an increment of 21.29 % due to soil application while of 32.6 % in SOD activity and 45.01 % in AcPh because of combined application improved the yield by an average of 13.01 %.
Fig. 2.
Effect of different zinc levels on grain yield in different genotypes of wheat where; Zn0 represents 0 kg ZnSO4 ha−1, Zn20 represents 20 kg ZnSO4 ha−1 and Zn20+F 20 kg ZnSO4 ha−1 along with foliar spray of 0.5 % solution of ZnSO4 during 2009–2010 (vertical bars indicates ± SD) (Means with same letter are not significantly different)
Fig. 3.
Effect of different zinc levels on grain yield in different genotypes of wheat where; Zn0 represents 0 kg ZnSO4 ha−1, Zn20 represents 20 kg ZnSO4 ha−1 and Zn20+F 20 kg ZnSO4 ha−1 along with foliar spray of 0.5 % solution of ZnSO4 during 2010–2011 (vertical bars indicates ± SD) (Means with same letter are not significantly different)
Discussion
In the present piece of work a positive relationship was established between zinc applications, yield and the activity of two enzymes. The reason for enhanced yield may be the proper functioning of the enzymes under zinc sufficient conditions. The effectiveness of combined application of soil and foliar was observed to be superior over soil application which indicates that foliar zinc application might have compensated the zinc absorption problem through soil and also its translocation. The timing of foliar zinc application also seemed to have significance in increasing the enzyme activity. In the present investigation, an increment has been observed in the superoxide dismutase enzyme activity with the progressive increment of zinc level. It was observed in all the three stages. The reason for this is that Zn is required as a cofactor in the functioning of SOD (Cu-Zn SOD). Due to this reason a drop could be noticed under deficit conditions (Zn0) and improvement with its supply (Zn20 and Zn20+F). Though Zn20+F was the best supplementation but in certain genotypes Zn20 has shown more increment which might be due to improper utilization of zinc at cellular or tissue level.
The present piece of work derives support from the work of earlier researchers who reported an increased SOD activity (Frei et al. 2010; Singh and Singh 2011). Cu/Zn SOD could play a direct role in tolerance to zinc (Hacisalihoglu and Kochian 2003). Therefore in the present investigation this parameter has been chosen as the criteria to screen out most tolerant genotype under zinc application conditions. In this respect PBW 550, UP 2584 were found to be most responsive. The response of these two genotypes towards grain yield has also been found to be satisfactory. These two genotypes are also important due to their certain characteristics.The genotype PBW 550 has been found to be resistant to yellow and brown rusts. It is an early maturing genotype with a good grain quality. Similarly the genotype UP 2584 is also resistant to various diseases, powdery mildew and karnal bunt.
It is extensively reviewed in literature that absorption of zinc and phosphorus by roots is antagonistic. The increased zinc concentration marks the decrement of later. This is why to overcome the deficit of phosphorus acid phosphatase activity increases in leaves. Likewise the enzyme activity was found to be greater at higher concentrations of Zn while lesser at lower concentrations in study of Kaya et al. 2000. It may also seek support from the study of Gunes and Inal 2008 where acid phosphatase activity was decreased in wheat with phosphorus supply which indicates that due to proper inorganic phosphate metabolism there was no necessity of the enzyme activity to breakdown phosphate esters to release inorganic phosphorus.
In the present investigation the yield has also been found to increase with increasing zinc application. Notably a reduction has been observed in many genotypes in combined application with respect to soil application but it is mostly non significant. One reason of increment in grain yield is that zinc acts a cofactor in the enzymes directly involved in the process of photosynthesis (e.g. carbonic anhydrase and rubisco) which may have lead to accumulation of more of photosynthates in the grain and thereby increasing grain yield. The study seeks support from that of Narwal et al. 2011 and Bharti et al. 2013 in which an increase in grain yield was found to be reported on both soil and foliar application of zinc. The other reason of increase in yield could be proper functioning of both the enzymes. It is well known through literature that active oxygen species can cause great damage to membrane integrity and thus to the leaves, the most important organs of photosynthates production. Moreover chloroplasts are one of the most important sites for the production of ROS (Fridivich 1986). In this way formation of assimilates can be severely hampered if ROS will not be scavenged. The proper functioning of SOD helps in scavenging ROS and improvement in yield. The proper inorganic phosphate metabolism would also have facilitated assimilate production due to energy transfer reactions.
While the genotypes were undergoing reduced enzyme activity under zinc deficiency they simultaneously seemed to manage the stress conditions by secreting more of exudates under the zinc deficient conditions, the plant root system acting as a gating system. Phytosiderophores has been considered as the main agents facilitating the chelation and mobilization of zinc in soil. Recently the study of acidification of rhizosphere or lowering of soil pH an alternative mean of assimilating zinc is gaining importance. Though the reason of this root-soil communication is not clearly understood but upto some extent it may be governed by high amount of CaCO3 present in the zinc deficient soil. It may enhance the CO2 fixation or other metabolic processes resulting in an increase in organic acids synthesis. In the present investigation oxalic acid was found to be the major organic acid released from the roots of wheat genotypes. The study finds support from the work in wheat crop in which oxalic acid was reported in much greater amounts (Szmigielska et al. 1996). Similarly, higher rates of organic acid excretion were reported in genotypes tolerant to Zn deficiency (Hoffland et al. 2006). UP 262 and PBW 590 might be considered as the efficient genotype on the basis of their greater secretion of oxalic acid even under the zinc stress conditions to mobilize more of zinc in soil. In general, these two genotypes have been considered as superior genotypes over many other existing genotypes. The PBW 590 has high degree of resistance to yellow and brown rusts, has better appearance and has high protein content. Similarly UP 262 is a genotype that is not only resistant to rusts but also to Alternaria and Helmimthosporium. It is suitable for timely sowing as well as late sowing in Indian states like Uttar Pradesh, Bihar, West Bengal, Orrisa, Assam etc.
In the current study it can be concluded that soil zinc application alongwith foliar spray was most effective in enhancing the enzyme activities in most of the wheat genotypes. The timing and number of foliar applications applied in the study was found to be suitable in improving zinc utilization and in the enhancement of yield. A genotypic variation was observed in genotypes in exhibiting response towards zinc application. Considering the findings of both the growing seasons among all the genotypes PBW 550 and UP 2584 were found to be more responsive to zinc application.
Acknowledgments
The financial assistance provided by NAIP/ ICAR New Delhi, India is duly acknowledged.
References
- Alloway BJ. Zinc in soils and crop nutrition. 2. Brussels: IZA; 2008. [Google Scholar]
- Alscher RG, Erturk N, Heath LS. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot. 2002;53(372):1331–1341. doi: 10.1093/jexbot/53.372.1331. [DOI] [PubMed] [Google Scholar]
- Asmar F, Gahoonia T, Nielsen N. Barley genotypes differ in activity of soluble extracellular phosphatase and depletion of organic phosphorous in the rhizosphere soil. Plant Soil. 1995;172:117–122. doi: 10.1007/BF00020865. [DOI] [Google Scholar]
- Bharti K, Pandey N, Shankhdhar D, Srivastava PC, Shankhdhar SC. Evaluation of some promising wheat genotypes (Triticum aestivum L.) at different zinc regimes for crop production. Cereal Res Commun. 2013 [Google Scholar]
- Cakmak I. Role of zinc in protecting plant cells from reactive oxygen species. New Phytol. 2000;146:185–205. doi: 10.1046/j.1469-8137.2000.00630.x. [DOI] [PubMed] [Google Scholar]
- Coleman JE. Zinc enzymes. Curr Opin Chem Biol. 1998;2:222–234. doi: 10.1016/S1367-5931(98)80064-1. [DOI] [PubMed] [Google Scholar]
- Frei M, Tanaka PJ, Wissuwa M. Biochemical factors conferring shoot tolerance to oxidative stress in rice grown in low zinc soil. Funct Plant Biol. 2010;37:74–84. doi: 10.1071/FP09079. [DOI] [Google Scholar]
- Fridivich I. Superoxide dismutases. Adv Enzymol Relat Areas Mol Biol. 1986;58:61–97. doi: 10.1002/9780470123041.ch2. [DOI] [PubMed] [Google Scholar]
- Giannopolitis CN, Ries SK. Superoxide dismutase occurrence in higher plants. Plant Physiol. 1977;59:309–314. doi: 10.1104/pp.59.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AW, McDonald GK (2001) Effect of zinc on photosynthesis and yield of wheat under heat stress. Proceedings of the 10th Australian Agronomy Conference 2001, Australian Society of Agronomy. Hobart, Tasmania, Australia. Available online at http://www.regional.org.au/au/asa/2001/2/c/graham.htm
- Gunes A, Inal A. Significance of intracellular and secreted acid phosphatase enzyme activities, and zinc and calcium interactions, on phosphorus efficiency in wheat, sunflower, chickpea, and lentil cultivars. Aust J Agric Res. 2008;59:339–347. doi: 10.1071/AR07195. [DOI] [Google Scholar]
- Hacisalihoglu G, Kochian LV. How do some plants tolerate low levels of soil zinc? Mechanisms of zinc efficiency in crop plants. New Phyt. 2003;159:341–350. doi: 10.1046/j.1469-8137.2003.00826.x. [DOI] [PubMed] [Google Scholar]
- Hoffland E, Wei C, Matthias W. Organic anion exudation by Lowland Rice ( Oryza sativa L.) at zinc and phosphorus deficiency. Plant Soil. 2006;15(2):283. [Google Scholar]
- Kaya C, David H, Agneta B. Phosphorus and acid phosphatase enzyme activity in leaves of tomato cultivars in relation to zinc supply. Comm Soil Sci Plant Anal. 2000;31(19–20):3239–3248. doi: 10.1080/00103620009370664. [DOI] [Google Scholar]
- Marschner H. Mineral nutrition of higher plants. 2. London: Academic Press; 1995. [Google Scholar]
- Narwal RP, Malik RS, Dahiya RR (2011) Addressing variations in status of a few nutritionally important micronutrients in wheat crop. www.zinccrops2011.org/…/2011_zinccrops2011
- Pérez-Esteban J, Escolástico C, Moliner A, Masaguer A. Chemical speciation and mobilization of copper and zinc in naturally contaminated mine soils with citric and tartaric acids. Chemosphere. 2013;90(2):276–283. doi: 10.1016/j.chemosphere.2012.06.065. [DOI] [PubMed] [Google Scholar]
- Sadasivam S, Manickam A. Biochemical methods. 2. Coimbatore: New Age International (P) Limited; 1991. [Google Scholar]
- Sillanpaa M. Micronutrient assessment at country level: An international study. Rome: FAO; 1990. [Google Scholar]
- Singh B, Singh BK. Effect of reduced seed and applied zinc on zinc efficiency of wheat genotypes under zinc deficiency in nutrient solution culture. J Plant Nutr. 2011;34:449–464. doi: 10.1080/01904167.2011.536884. [DOI] [Google Scholar]
- Stoskopf NC. Cereal grain crops. Reston: Reston Publishing Co., Inc.; 1985. [Google Scholar]
- Szmigielska AM, VanRees KCJ, Cieslinski G, Huang PM. Low molecular weight dicarboxylic acids in rhizosphere soil of durum wheat. J Agric Food Chem. 1996;44:1036–1040. doi: 10.1021/jf950272z. [DOI] [Google Scholar]
- Wang H, Jin JY. Photosynthetic rate, chlorophyll fluorescence parameters, and lipid peroxidation of maize leaves as affected by zinc. Photosynthetica. 2005;43(4):591–596. doi: 10.1007/s11099-005-0092-0. [DOI] [Google Scholar]
- Wang P, Zhou R, Cheng J, Bi S. LC determination of trace short-chain organic acids in wheat root exudates under aluminum stress. Chromatographia. 2007;66:867–872. doi: 10.1365/s10337-007-0418-0. [DOI] [Google Scholar]