Abstract
Plants like other organisms are affected by environmental factors. Cadmium, copper and zinc are considered the most important types of pollutants in the environment. In this study, a comparison of growth and biochemical parameters between the crop wild relative (CWR) Solanum nigrum versus its cultivated relative Solanum lycopersicum to different levels of Cu, Zn and Cd stress were investigated. The presence of ZnSO4 and CuSO4 in Murashige and Skoog medium affected severely many growth parameters (shoot length, number of roots and leaves, and fresh weight) of both S. nigrum and S. lycopersicum at high levels. On the other hand, CdCl2 significantly reduced most of the studied growth parameters for both species. S. nigrum exhibited higher tolerance than S. lycopersicum for all types of stress. In addition, results show that as stress level increased in the growing medium, proline content of both S. nigrum and S. lycopersicum increased. A significant difference was observed between the two species in proline accumulation as a result of stress. In addition, a higher accumulation rate was observed in the crop wild relative (S. nigrum) than in cultivated S. lycopersicum. Changes in Inter-simple sequence repeat (ISSR) pattern of CuSO4 treated S. nigrum and S. lycopersicum plants were also observed. In conclusion, based on growth and biochemical analysis, S. nigrum showed higher level of metals tolerance than S. lycopersicum which indicates the possibility of using it as a crop wild relative for S. lycopersicum.
Keywords: Solanum nigrum L, Crop wild relatives, In vitro, ISSR, Proline
Introduction
Soil contamination with metals is a widespread problem that affects the environment. The presence of these metals in the soil leads to several problems to humans, animals and plants (Lepp 1981). The major sources of metals contamination are rock erosion and man-made effects by using pesticides, industries, mining, smelting and other human activities that are now rapidly increasing (Sherameti and Varma 2010). Since soil is the main growing medium for plants, the quality of soil is an important issue that must be taken seriously (Sherameti and Varma 2010).
Metals are considered the most important source of environmental pollution that is deposited from natural and human activity (Gaur and Adholeya 2004). Cadmium (Cd), copper (Cu) and zinc (Zn) are examples of metals that are found naturally at low quantities. Zn and Cu are micronutrients of growth medium that are needed by plants for growth and various biochemical and physiological pathways (Narula et al. 2005). High levels of these elements are toxic to sensitive plants (Das et al. 1997). Metal concentration toxicity leads to chlorosis, necrosis and root system damage (Atanassova and Zapryanova 2009), photosynthesis inhibition, plasma membrane permeability damage (Narula et al. 2005).
Cadmium is a non essential element; it is considered a significant soil pollutant because of its toxicity and greater solubility in water (Das et al. 1997; Jain et al. 2007). The main sources of Cd are mining, phosphate fertilizers, pesticides and industrial waste. Cd affects nitrogen metabolism, membrane function and chlorophyll biosynthesis (Jain et al. 2007).
Genetic variation has decreased in many crops because of domestication processes. As a result they become more susceptible to various biotic and abiotic stresses than wild plants. On the other hand, wild plants show great genetic variations that include having genes responsible for various stress resistance. These wild plant species that have a close relation to cultivated ones are called crop wild relatives (CWR) (Jarvis et al. 2008).
Due to their phytotoxic effect, metals affect arable land and cause retardation in crop growth. A huge variation in metals tolerance was observed between different species. This variation could be used for crop improvement by identifying the tolerant species and then studying the mechanisms of tolerance and the possibility of identifying the responsible genes (Hall 2002; Yruela 2009; Fatima et al. 2011). Recently, researchers reported Cd tolerant plant species S. cathayana and L. plyneura that can accumulate high concentrations of cadmium in certain parts such as the root system (Das et al. 1997; Yanqun et al. 2004). Plants that are capable to survive and grow in soil contaminated with these metals are called “metallophytes” (Ashraf and Harris 2005).
Proline is an osmoticum; a preserving agent of enzyme and cellular structure, and a storage compound of decreasing nitrogen for rapid regrowth after stress is removed. It plays a significant role in stress tolerance. It acts as a molecular chaperone capable to defend protein integrity and improve the activities of different enzymes (Szabados and Savoure 2009). Proline accumulation is important for the tolerance of many adverse environmental conditions. It has been shown (Szabados and Savoure 2009) that genotypes accumulating higher levels of proline are more stress tolerant than others. This indicates that proline accumulation could be used as selection criteria for stress-tolerant genotypes (Van Rensburg et al. 1993).
Plant biotechnology is wildly used to improve plants stress tolerance by making physiological and biological changes. In vitro selection has many advantages, including shortening the selection time needed to get disease or abiotic tolerant lines with minimal environmental reaction and low cost laboratory set-up (Rai et al. 2011). In addition, many DNA fingerprinting techniques have been used (PCR-based fingerprinting) to study the effect of different stresses on various plant species (Deshmukh et al. 2012; Kӧrpe and Aras 2011; Enan 2006). Słomka et al. (2011) studied the effect of Zn, Pb, and Cd on genetic diversity of seven populations of Viola tricolor L. using Inter-simple sequence repeat (ISSR) techniques. They found that the population of polluted site had the highest genetic diversity and polymorphism compared to the non-polluted site (control). Recently, different molecular markers have been used to detect plant DNA damage that is caused by various types of pollutants (Al-Qurainy 2010; Enan 2006). Random amplified polymorphic DNA (RAPD) analysis, which is a PCR-based fingerprinting tool, showed high efficiency in detecting various types of DNA damage and mutations throughout the genome in Arabidopsis (Liu et al. 2012). Another study used ISSR to assess the effect of Zn, Pb, and Cd on Eruca sativa (L.) DNA damage (Al-Qurainy 2010).
Solanaceae is a cosmopolitan family that grows in many parts of the world. It includes 96 genera and more than 2800 species. Economically, it is considered the third most important crop family and in terms of vegetable crops, it is considered the most valuable (Foolad 2007). The genus Solanum belongs to this family; it contains many species that are considered as essential fruits and vegetables. It also plays an important role as a model plant. Potato (Solanum tuberosum L.), tomato (Solanum lycopersicum L.) (Poczai and Hyvönen 2011; Edmonds and Chweya 1997), and eggplant (Solanum melongena L.) are examples of this family. Tomato is the most popular and commonly cultivated seasonal vegetable crop worldwide. It has been previously shown that tomato is a Cd, (Rehman et al. 2011), Cu (Mami et al. 2011), and Zn (Muschitz et al. 2009) sensitive crop.
Solanum nigrum L. or black nightshade is one of the largest species of the Solanum genus (Edmonds and Chweya 1997). It is an annual herb with a wide range of medicinal uses (Sridhar and Naidu 2011).
The aim of this project is to study the effect of Cu, Cd and Zn on growth, proline content, and DNA stability of Solanum nigrum L. and the cultivated relative tomato (Solanum lycopersicum L.).
Material and methods
Plant materials
Ripe fruits of Solanum nigrum L. were collected in May 2011 from the northern area of the Jordan valley, Jordan. The fruit were collected from mother plants and allowed to dry in the lab. Then, seeds were extracted from the fruit and left in the lab until completely dry. After, the seeds were collected and stored for further experiments. The seeds of tomato (Solanum lycopersicum L.) were bought from the local market in Irbid, Jordan.
Establishement of in vitro mother stock cultures
The seeds of S. lycipersicum and S. nigrum were sterilized by washing under running tap water and detergent for 20 min. Then, they were immersed in 70 % (v/v) ethanol under laminar air–flow cabinet for 30 s followed by washing with 20 % (v/v) Clorox for 15 min. Next, they were washed with 70 % (v/v) ethanol for 30 s. At the end, the seeds were washed with sterile distilled water for three times (5 min each). The sterilized seeds were inoculated on germination media (half strength Murashige and Skoog medium (MS), 7.0 g/L plant agar, 15.0 g/L sucrose), pH was adjusted to 5.8 using 0.1 N NaOH or 0.1 N HCl, then autoclaved at 121 °C and 1.15 kg/cm pressure for 15 min. After that, cultures were incubated in the growth chamber at 24 °C (± 1) ˚ under 16/8 h light/dark photo-period, respectively.
Cd, Zn and Cu stress
S. lycopersicum and S. nigrum seedlings were sub-cultured into full MS medium containing 7 g/L plant agar, 30 g/L sucrose and supplemented with various concentrations of different metals (cadmium, zinc and copper). For Cadmium stress, 100, 200 and 400 μM of CdCl2 were used, for Zinc stress, 0.25, 0.5, 0.75 and 1.0 mM of ZnSO4 were used, and for Copper stress, 50, 100, 150, and 200 μM of CuSO4 were used in addition to the control media. Cultures were incubated at 24 °C (±1) under 16/8 h light/dark photoperiod, respectively. Data were taken after 5 weeks for shoot height, number of roots and leaves, and fresh weight.
Proline content determination
Proline was measured as described by Bates et al. (1973). 0.5 g of frozen plant materials were homogenized in 10 ml of 3 % sulphosalicylic acids, then the mixture was filtered through filter paper, 2 ml of the extract were added to 2 ml acid ninhydrin and 2 ml glacial acetic acid in a test tube for 1 h at 100 °C, . The reaction then was stopped in an ice bath. Next, the chromophore was extracted by adding 4 ml of toluene and mixed vigorously for 15–20 s, then tubes were left until the two phases separated. After that, the chromophore that contained toluene was transferred to another test tube. Absorbance was taken at 520 nm. Proline concentration was determined by using a standard curve in the range of 20–100 mg\ml and it was calculated on a fresh weight basis.
Genomic DNA extraction
S. nigrum and S. lycopersicum microshoots were grown on a medium supplemented with different levels of CuSO4 as a representative for metal stress. Genomic DNA was extracted according to the Wizard genomic DNA purification kit (Promega). Forty milligram leaf tissues were grinded in liquid nitrogen, and then 600 μL of nuclei lysis solution was added and incubated for 15 min at 65 °C. After that, 3 μL of RNase solution was added and incubated for 15 min at 37 °C. The sample was cooled at room temperature for 15 min followed by addition of 200 μL of protein precipitation solution and vortex, then the sample was centrifuged at 16,000 × g for 3 min, then 600 μL of room temperature isopropanol was added and mixed gently by inversion and centrifuge for 1 min at 16,000 × g. After that, supernatant was thrown away and 600 μL of 70 % ethanol was added then centrifuged for 1 min at 16,000 × g. After that, ethanol was aspirated, and 100 μL of DNA rehydration solution was added. The sample was then incubated for 1 h at 65 °C and stored at 4 °C for further use.
ISSR-PCR and gel elctrophoresis
Fourteen ISSR primers (Table 1) were used to amplify the genomic DNA extracted from each concentration of CuSO4. Each PCR reaction was carried out in total volume 25 μL consisting of 12.5 μL master mix, 2 μL DNA, 1.5 μL primer and 9 μL DNase free water. DNA amplification was then carried out in PCR machine under the following conditions: initial denaturation at 94 °C for 5 min followed by 45 cycle of 55 s at 94 °C, annealing step at 49 °C for 59 s, extension at 72 °C for 59 s and final extension after at 72 °C for 5 min then stored at 12 °C. Seven μL of each PCR product were mixed with 4 μL of bromophenol blue as loading dye and loaded on 1.5 % agarose gel. 1× Tris borate EDTA buffer was used. Gel was run at 90 V for 110 min. Finally, the gel was examined under UV light.
Table 1.
List of ISSR primers used
| UBC No. | Sequence (5′-3′) |
|---|---|
| 807 | AGAGAGAGAGAGAGAGT |
| 810 | GAGAGAGAGAGAGAGAT |
| 812 | GAGAGAGAGAGAGAGAA |
| 815 | CTCTCTCTCTCTCTCTG |
| 826 | ACACACACACACACACC |
| 828 | TGTGTG TGTGTGTGTGA |
| 834 | AGAGAGAGAGAGAGAC YT |
| 835 | AGAGAGAGAGAGAGAG YC |
| 841 | GAGAGAGAGAGAGAGA YC |
| 843 | CTCTCTCTCTCTCTCT RA |
| 848 | CACACACACACACACA RG |
| 876 | GATAGATAGACAGACA |
| 899 | ACTTCCCCACAGGTTAACACA |
| 900 | ACTTCCCCACAGGTTAACACA |
Statistical analysis
Six replicates were used for each of the treatments and each experiment was repeated at least twice. Completely Randomized Design (CRD) was used. The data were analyzed by analysis of variance (Two-way ANOVA) followed by Tukey’s test for mean comparison using SPSS version 16.0 (SPSS Inc., USA).
Results
In this study, various concentrations of CuSO4, ZnSO4, CdCl2 were used. Analysis of Variance (ANOVA) showed significant effect (P ≤ 0.05) for metal levels and species for shoot length, number of roots and leaves, fresh weight and biochemical analysis. Data are presented as relative percentage to the control for each species due to species differences in absolute numbers.
Growth parameters
Results showed that fresh weight (Fig. 1), number of leaves (Fig. 2), number of roots (Fig. 3), and shoot length (Fig. 4) decreased in response to increasing Cu, Cd and Zn for both plant species.
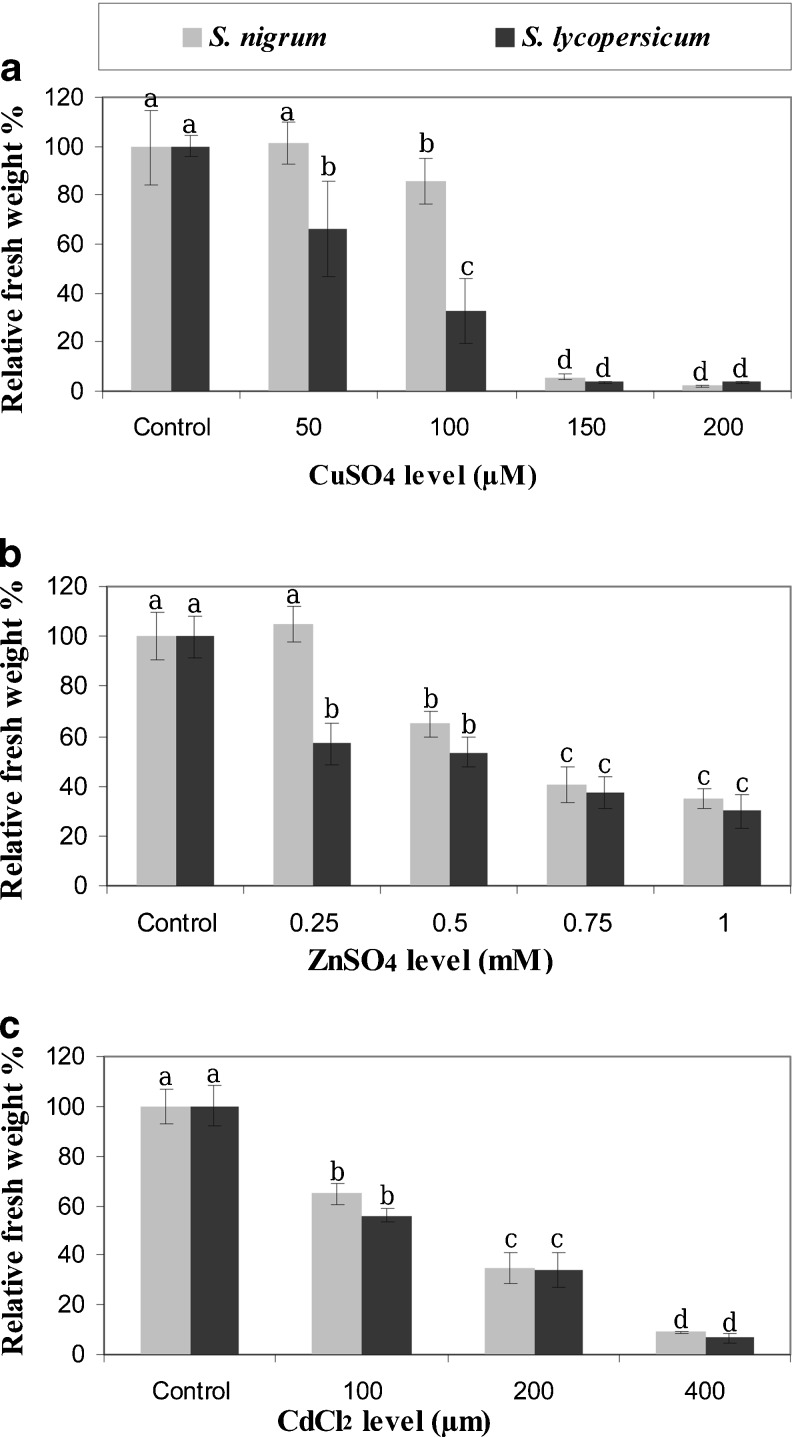
Fig. 1.
Relative fresh weight of S. lycopersicum and S. nigrum grown in vitro under metals stress. Error bars represent standard error. Means with the same letter are not statistically different at p ≤ 0.05
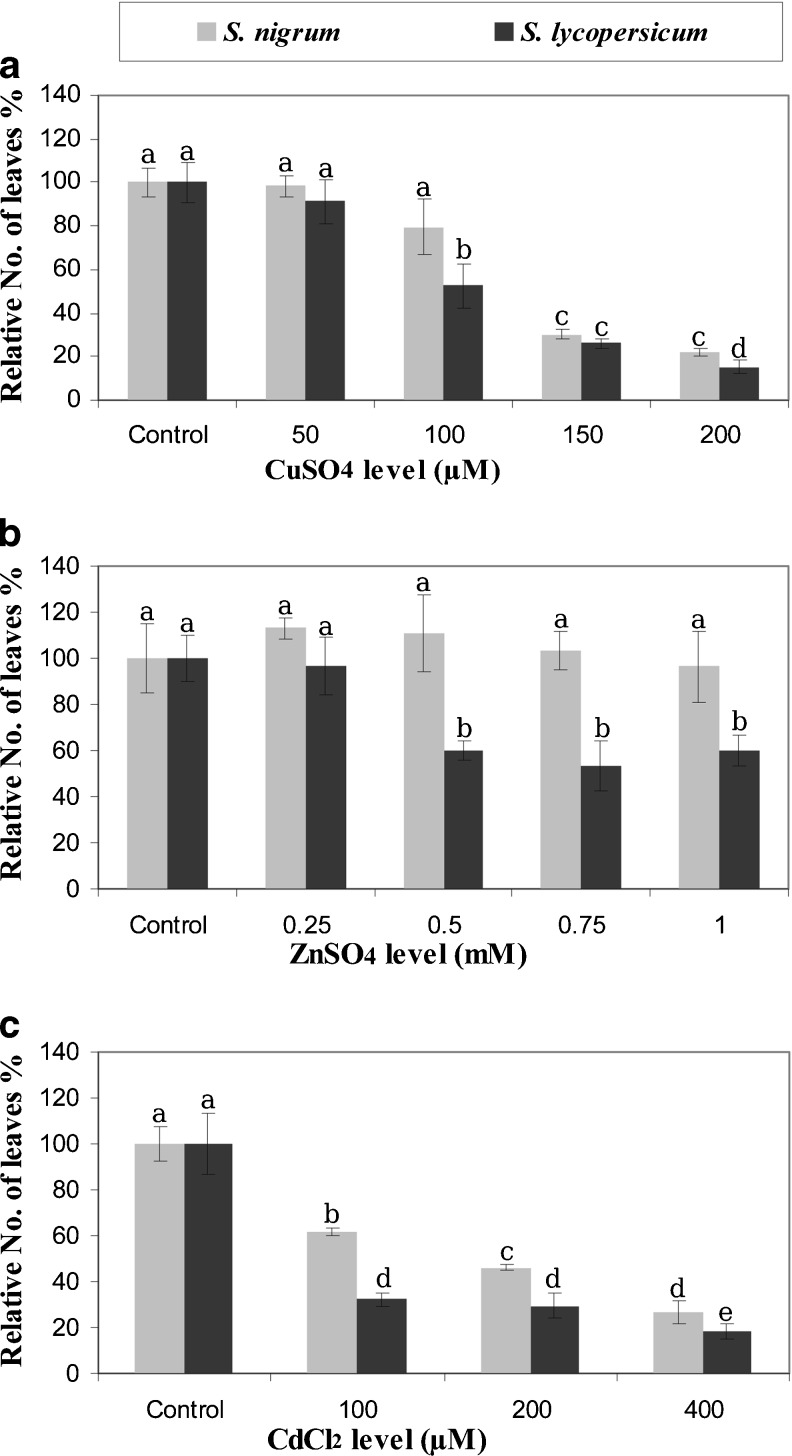
Fig. 2.
Relative leaves number of S. lycopersicum and S. nigrum grown in vitro under metals stress. Error bars represent standard error. Means followed by the same letter are not statistically different at p ≤ 0.05
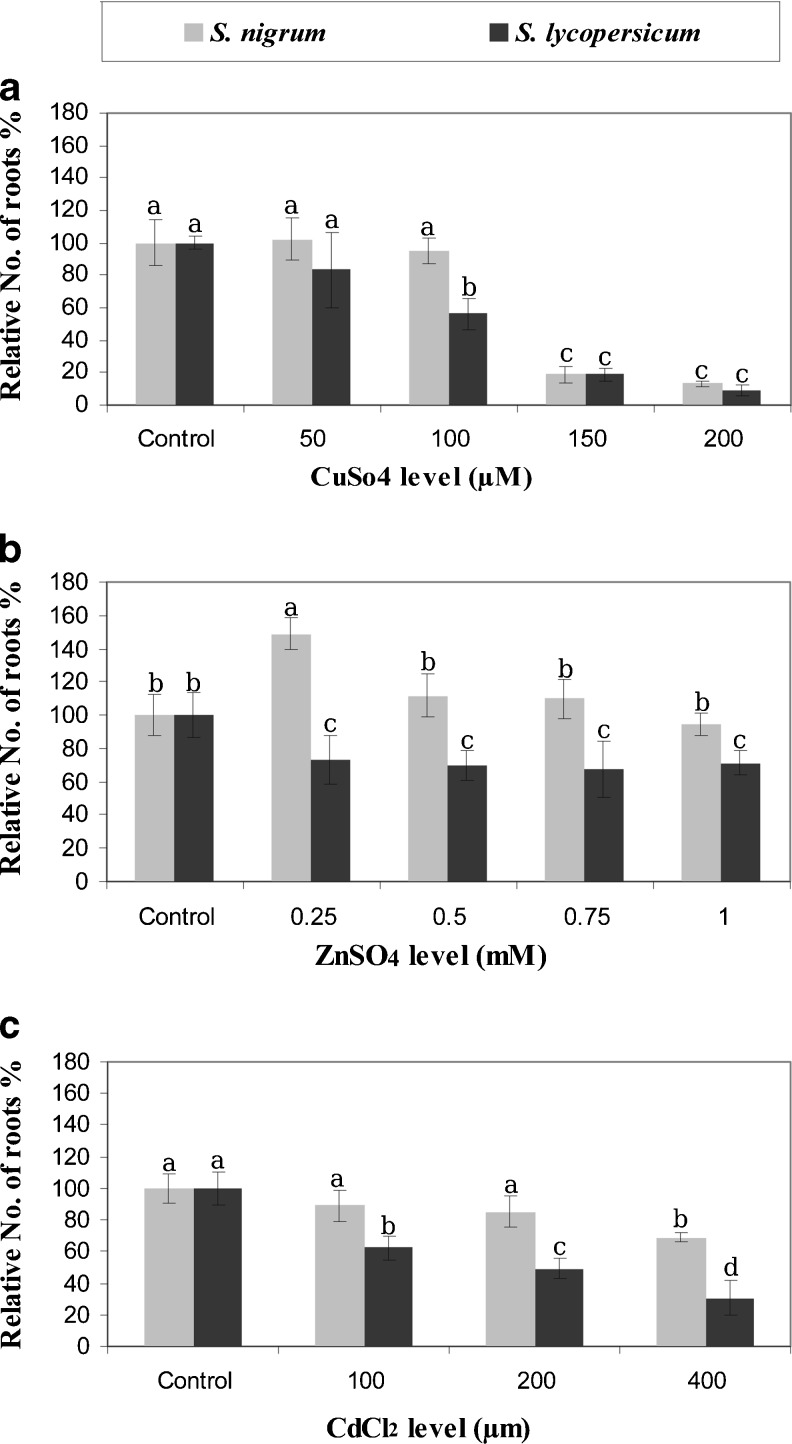
Fig. 3.
Relative roots number of S. lycopersicum and S. nigrum grown in vitro under metals stress. Error bars represent standard error. Means followed by the same letter are not statistically different at p ≤ 0.05
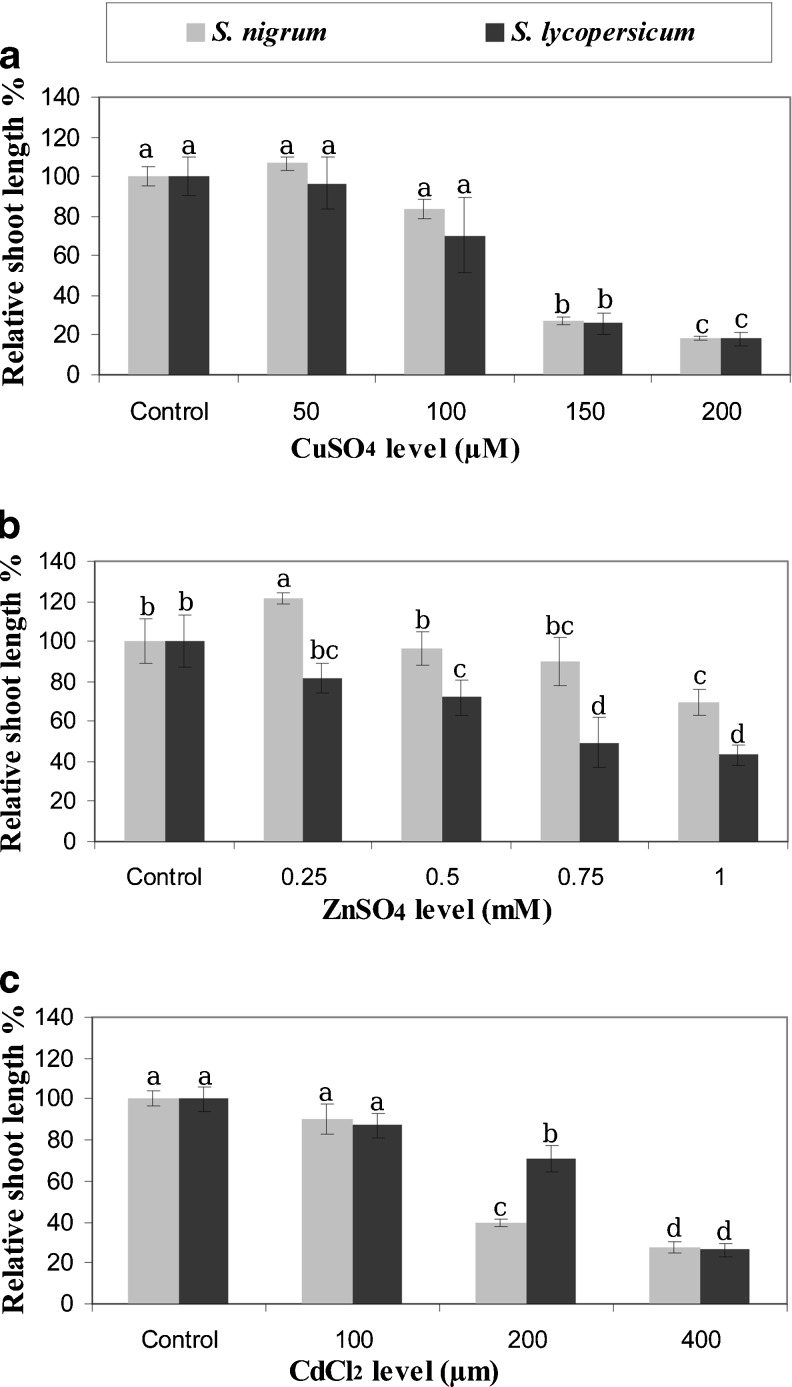
Fig. 4.
Relative shoot length of S. lycopersicum and S. nigrum grown in vitro under metals stress. Error bars represent standard error. Means followed by the same letter are not statistically different at p ≤ 0.05
A significant reduction (P ≤ 0.05) in fresh weight was observed for S. lycopersicum grown on medium supplemented with 50 and 100 μM CuSO4 (66.05 % and 32.85 % relative to control, respectively) (Fig. 1a). S. lycopersicum microshoots fresh weight reduced significantly at 0.25 mM. (56.97 % relative to control) (Fig. 1b). Also, no significant differences were observed between S. nigrum and S. lycopersicum for fresh weight when grown on medium supplemented with different levels of CdCl2 (Fig. 1c).
Growing S. nigrum and S. lycopersicum microshoots on medium supplemented with 100 μM CuSO4 or higher reduced number of leaves significantly (P ≤ 0.05) (Fig. 2a). S. lycopersicum microshoots showed a huge reduction in leaves number when grown at 0.5, 0.75 and 1.0 mM ZnSO4 (Fig. 2b). S. nigrum microshoots grown on medium containing 100 μM CdCl2 resulted in 61.95 % leaves number relative to control, whereas only 32.24 % for S. lycopersicum (Fig. 2c).
Growing both species on medium supplemented with 200 μM CuSO4 or higher resulted in a sharp reduction in number of roots (P ≤ 0.05) (Fig. 3a). A significant reduction (P ≤ 0.05) in roots number of S. lycopersicum was observed at 0.5, 0.75 and 1.0 mM ZnSO4 (Fig. 3b). Growing S. nigrum microshoots on medium supplemented with 100 and 200 μM CdCl2 did not affect number of roots compared to the control in contrast to S. lycopersicum (Fig. 3c).
A sharp reduction (P ≤ 0.05) in shoot length of both species was observed starting at 150 μM CuSO4 (for S. nigrum 27.19 % relative to control and 25.77 % S. lycopersicum) (Fig. 4a). Under ZnSO4 stress, S. nigrum microshoots showed significantly (P ≤ 0.05) longer shoots than S. lycopersicum under all levels (Fig. 4b). A sharp decline in shoot length was observed in S. nigrum at 200 μM CdCl2 to reach 39.62 % relative to its control (Fig. 4c).
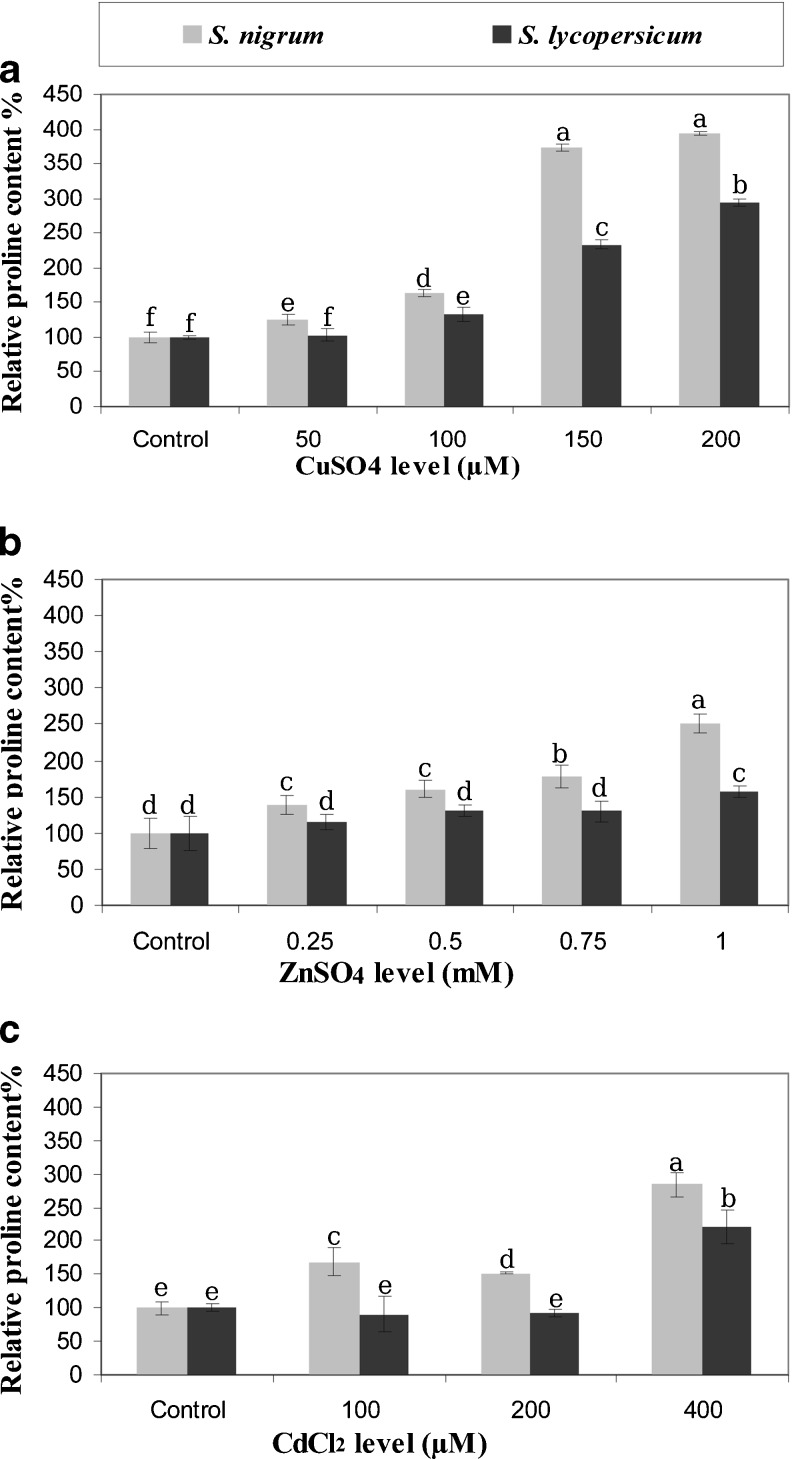
Proline content
Our results show that proline accumulation increased for both species as metal level(s) increased (Fig. 5). Significant differences (P ≤ 0.05) in proline content were observed between S. nigrum and S. lycopersicum microshoots grown under different levels of CuSO4, ZnSO4 and CdCl2 (Fig. 5). Higher proline accumulation rate was found in S. nigrum than in S. lycopersicum which indicates that S. nigrum is more tolerant to CuSO4, ZnSO4 and CdCl2 stresses than S. lycopersicum.
Fig. 5.
Relative proline content (μg/ml) of S. nigrum and S. lycopersicum grown in vitro under metals stress. Error bars represent standard error. Means followed by the same letter are not statistically different at p ≤ 0.05
ISSR- PCR analysis
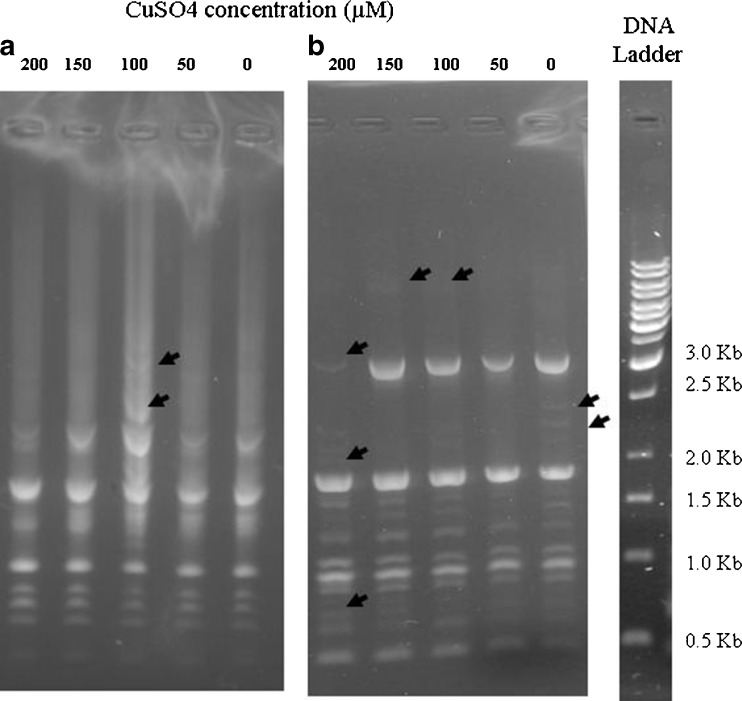
In order to detect the DNA stability of S. nigrum and S. lycopersicum when grown on MS medium supplemented with different levels of CuSO4, ISSR was used as molecular marker in this study. S. nigrum and S. lycopersicum were grown for 5 weeks under different levels of CuSO4. Equal amount of extracted DNA was used for each lane. A total of 14 primers were used to amplify genomic DNA. All the 14 primers resulted in clear bands for both species (only gels of one primer are shown here).
In general, growing S. nigrum and S. lycopersicum microshoots under different levels of CuSO4 did not affect DNA integrity when amplified with many primers (9 out of 14 primers). On the other hand, four primers (out of 14) resulted in differences between different levels of CuSO4. CuSO4 affects DNA stability and cause mutations at low and high levels in S. lycopersicum more than S. nigrum (Fig. 6). Using primer UBC-810, seven changes were observed in S. lycopersicum (five new bands appeared and two bands disappeared) compared to only two bands appearing in S. nigrum when grown under CuSO4 stress. This indicates that CuSO4 has less effect (lower number of mutational events) on S. nigrum genome stability than S. lycopersicum.
Fig. 6.
ISSR fingerprints for S. nigrum (a) and S. lycopersicum (b) treated with various concentrations of CuSO4 (0, 50, 100, 150 and 200 μM). Arrows indicate band appearance or absence after treatment
Discussion
In the present study, S. nigrum and S. lycopersicum were grown in vitro and the effect of different levels of Cu, Cd and Zn were assessed. Results showed that nearly all growth parameters decreased and proline content increased as metal level increased. In addition, results showed clearly that S. nigrum was more tolerant to metals than S. lycopersicum.
Results showed that lower levels of CuSO4 reduced fresh weight, leaves number; roots number and shoot length slightly compared to higher levels for both tested species. Higher levels have toxic effects on all growth parameters with a strong significant reduction in S. lycopersicum compared to S. nigrum. These results were in agreement with Fatima et al. (2011) who studied W. somnifera, another member of the Solanaceae, they found an increase in biomass and shoot length grown in vitro of W. somnifera until 100 μM CuSO4, then a decline in these parameters were observed at higher levels.
High levels of Cu may exert a toxic effect as presented in the current study. Manivasagaperumal et al. (2011) reported that high levels of Cu affect protein formation, interfere with photosynthetic processes, enzyme activity, and change plasma membrane permeability. Also, excess amounts of Zn may prevent plant growth and development by making imbalance between intake and distribution of minerals or by interfering with metabolic processes and antioxidant defense system (Xu et al. 2010).
Hédiji et al. (2010) reported fresh weight reduction of Solanum lycopersicum under high Cd levels. Similarly a sharp reduction in leaf number was observed by Jing et al. (2005) and Rehman et al. (2011). Toxicity of Cu, Zn and Cd on plants depends on type of plant species (sensitivity or tolerance), concentration of the metal and the threshold level that species can tolerate. S. nigrum may have mechanisms to tolerate metals toxicity over S. lycopersicum, by protection of the plasma membrane integrity against metals toxicity in order to decrease metals uptake and thus decrease their toxicity (Hall 2002).
Current results showed that proline accumulation increased for both species as Cu, Zn, and Cd levels increased with the highest amount found in S. nigrum. It has been shown previously that proline accumulation in stress tolerance plants is higher than in stress sensitive plants (Ashraf and Foolad 2007). Parlak and Yilmaz (2012) reported that proline content increased under Zn in three tested plants, Lemna gibba, Lemna minor, and Spirodela polyrrhiza L. Same results were obtained by Narula et al. (2005), Aly and Mohamed (2012), and Karimi et al. (2012) who showed that growing plants under Cu stress increased proline content.
Proline prevents membrane damage and had a protective role in lipid proxidation induced by metals (Thounaojam et al. 2012). In our case, accumulation of proline in response to Cu, Cd and Zn was seen in S. nigrum much more than S. lycopersicum, this may be due to decreased activity of enzyme responsible for proline degradation under stress that leads to increase proline accumulation in S. nigrum (Madan et al. 1995) or increase activity of some enzymes that were responsible for proline biosynthesis (Charest and Pan 1990). Further research is needed to identify genes responsible for proline synthesis and stabilization in S. nigrum, in addition to optimize transformation protocol for these genes from the crop wild relative to their cultivated crop.
In this study, the molecular marker ISSR was used to evaluate the effect of Cu on DNA stability of both S. nigrum and S. lycopersicum. Our results showed changes in ISSR pattern of CuSO4 treated S. nigrum and S. lycopersicum plants. Results showed that S. nigrum and S. lycopersicum undergo mutational events at their DNA. This is due to a genotoxic effect and mutagenicity of Cu which induce DNA damage through single and/or double strand breaks, modified bases, and DNA–protein cross-links (Lloyd and Phillips 1999). Kӧrpe and Aras (2011) found that newly amplified bands or complete absence of others were observed in S. melongena exposed to Cu stress compared to control using RAPD molecular markers resulted in DNA damage. Similarly, presence and absence of bands were observed by Enan (2006) in treated plants compared to untreated Phaseolus vulgaris with Cd, Cu, Mn, and Pb using RAPD molecular markers. In another study, unique bands in high and medium levels of Zn, Pb, and Cd were observed using ISSR technique in Eruca sativa (L.) with the most genotoxicity being Cd (Al-Qurainy 2010).
In our case, tomato plants showed higher levels of genomic instability under CuSO4 compared to the crop wild relative S. nigrum. This finding indicates that S. nigrum is more tolerant to Cu toxicity at the molecular level than S. lycopersicum. This could be achieved by having more effective DNA repair mechanisms.
Crop wild relatives are considered as an important source to agricultural crops since they can be used to improve the tolerance and adaptation of cultivated crops to various biotic and abiotic stresses. In the present study, the crop wild relative (S. nigrum) exhibited a higher level of tolerance than the cultivated crop (S. lycopersicum) under all of the three metals tested. Based on biochemical and growth parameters, S. nigrum showed a higher degree of tolerance than S. lycopersicum. Finally, S. nigrum could be used as a gene donor to improve S. lycopersicum ability to tolerate the tested stresses.
Acknowledgments
The authors would like to thank professors J. Lahham and A. El-Oqlah for their help in plant identification and Adel Rababaeh for technical assistant. This project was funded by the deanship of research at Yarmouk University, Project #12/2012.
References
- Al-Qurainy F. Application of inter simple sequence repeat (ISSR marker) to detect genotoxic effect of heavy metals on Eruca sativa (L.) African J Biotechnol. 2010;9:467–474. [Google Scholar]
- Aly AA, Mohamed AA. The impact of copper ion on growth, thiol compounds and lipid peroxidation in two maize cultivars (Zea mays L.) grown in vitro. Aust J Crop Sci. 2012;6:541–549. [Google Scholar]
- Ashraf M, Foolad M. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot. 2007;59:206–216. doi: 10.1016/j.envexpbot.2005.12.006. [DOI] [Google Scholar]
- Ashraf M, Harris PJC. Abiotic stresses : plant resistance through breeding and molecular approaches/Crop science. New York: Food Products Press; 2005. [Google Scholar]
- Atanassova B, Zapryanova N. Influence of heavy metal stress on growth and flowering of Salvia splendens Ker.-Gawl. Inst Ornamental Plants Sofia. 2009;1222:173–176. [Google Scholar]
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- Charest C, Pan T. Cold acclimation of wheat properties of enzymes involved in proline metabolism. Physiol Plant. 1990;80:159–168. doi: 10.1111/j.1399-3054.1990.tb04391.x. [DOI] [Google Scholar]
- Das P, Samantaray S, Rout GR. Studies on cadmium toxicity in plants: a review. Environ Pollut. 1997;98:29–36. doi: 10.1016/S0269-7491(97)00110-3. [DOI] [PubMed] [Google Scholar]
- Deshmukh R, Tomar N, Tripathi N, Tiwari S. Identification of RAPD and ISSR markers for drought tolerance in wheat (Triticum aestivum L.) Physiol Mol Biol Plant. 2012;18(1):101–104. doi: 10.1007/s12298-011-0096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds JM, Chweya JA (1997) Black nightshades, Solanum nigrum L. and related species. Promoting the conservation and use of underutilized and neglected crops, vol 15. International Plant Genetic Resources Institute. Rome, Italy
- Enan MR. Application of random amplified polymorphic DNA (RAPD) to detect the genotoxic effect of heavy metals. Biotechnol Appl Biochem. 2006;43:147–154. doi: 10.1042/BA20050172. [DOI] [PubMed] [Google Scholar]
- Fatima N, Ahmad N, Anis M. Enhanced in vitro regeneration and change in photosynthetic pigments, biomass and proline content in Withania somnifera L. (Dunal) induced by copper and zinc ions. Plant Physiol Biochem. 2011;49:1465–1471. doi: 10.1016/j.plaphy.2011.08.011. [DOI] [PubMed] [Google Scholar]
- Foolad MR (2007) Genome Mapping and Molecular Breeding of Tomato. Int J Plant Genomics 2007:1–52 [DOI] [PMC free article] [PubMed]
- Gaur A, Adholeya A (2004) Prospects of arbuscular mycorrhizal fungi in phytoremediation of heavy metal contaminated soils. Curr Sci 86(4):528–534
- Hall JL. Cellular mechanisms for heavy metal detoxification and tolerance. J Exp Bot. 2002;53:1–11. doi: 10.1093/jexbot/53.366.1. [DOI] [PubMed] [Google Scholar]
- Hédiji H, Djebali W, Cabasson C, Maucourt M, Baldet P, Pertrand A, Zoghlami LB, Deborde C, Moing A, Brouquisse R, Chaibi W, Gallusci P. Effects of long-term cadmium exposure on growth and metabolomic profile of tomato plants. Ecotoxicol Environ Saf. 2010;73:1965–1974. doi: 10.1016/j.ecoenv.2010.08.014. [DOI] [PubMed] [Google Scholar]
- Jain M, Pal M, Gupta P, Gadre R. Effect of cadmium on chlorophyll biosynthesis and enzymes of nitrogen assimilation in greening maize leaf segments: role of 2-oxoglutarate. Indian J Exp Biol. 2007;45:385–389. [PubMed] [Google Scholar]
- Jarvis A, Lane A, Hijmans RJ. The effect of climate change on crop wild relatives. Agric Ecosyst Environ. 2008;126:13–23. doi: 10.1016/j.agee.2008.01.013. [DOI] [Google Scholar]
- Jing D, Fei-bo W, Guo-ping Z. Effect of cadmium on growth and photosynthesis of tomato seedlings. J Zhejiang Univ (Sci) 2005;6(10):974–980. doi: 10.1631/jzus.2005.B0974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi P, Khavari-Nejad R, Niknam V, Ghahremaninejad F, Najafi F. The effects of excess copper on Antioxidative enzymes, lipid peroxidation, proline, chlorophyll, and concentration of Mn, Fe, and Cu in Astragalus neo-mobayenii. Sci World J. 2012;2012:1–6. doi: 10.1100/2012/615670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körpe DA, Aras SM. Evaluation of copper-induced stress on eggplant (Solanum melongena L.) seedlings at the molecular and population levels by use of various biomarkers. Mutat Res Genet Toxicol Environ Mutagen. 2011;719:29–34. doi: 10.1016/j.mrgentox.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Lepp NW. Effect of Heavy Metal Pollution on Plants/Pollution monitoring series. London Englewood: Applied Science Publishers; 1981. [Google Scholar]
- Liu W, Sun L, Zhong M, Zhou Q, Gong Z, Li P, Tai P, Li Z. Cadmium-induced DNA damage and mutations in Arabidopsis plantlet shoots identified by DNA fingerprinting. Chemosphere. 2012;89:1048–1055. doi: 10.1016/j.chemosphere.2012.05.068. [DOI] [PubMed] [Google Scholar]
- Lloyd DR, Phillips DH. Oxidative DNA damage mediated by copper(II), iron(II) and nickel(II) Fenton reactions: evidence for site-specific mechanisms in the formation of double-strand breaks, 8-hydroxydeoxyguanosine and putative intrastrand cross-links. Mutat Res Fundam Mol Mech Mutagen. 1999;424:23–36. doi: 10.1016/S0027-5107(99)00005-6. [DOI] [PubMed] [Google Scholar]
- Madan S, Nainawatee H, Jain R, Chowdhury J (1995) Proline and proline metabolising enzymes in in-vitro selected NaCl-tolerant Brassica juncea L. under salt stress. Ann Bot 76:51–57
- Mami Y, Ahmadi G, Shahmoradi M, Ghorbani H. Influence of different concentration of heavy metals on the seed germination and growth of tomato. Afr J Environ Sci Technol. 2011;5(6):420–426. [Google Scholar]
- Manivasagaperumal R, Vijayarengan P, Balamurugan S, Thiyagarajan G. Effect of copper on growth, dry matter yield and nutrient content of Vigna radiata (l.) Wilczek. J Phytol. 2011;3(3):53–62. [Google Scholar]
- Muschitz A, Faugeron C, Morvan H. Response of cultured tomato cells subjected to excess zinc: role of cell wall in zinc compartmentation. Acta Physiol Plant. 2009;31:1197–1204. doi: 10.1007/s11738-009-0354-8. [DOI] [Google Scholar]
- Narula A, Kumar S, Srivastava PS. Abiotic metal stress enhances diosgenin yield in Dioscorea bulbifera L. cultures. Plant Cell Rep. 2005;24:250–254. doi: 10.1007/s00299-005-0945-9. [DOI] [PubMed] [Google Scholar]
- Parlak KU, Yilmaz DD. Response of antioxidant defences to Zn stress in three duckweed species. Ecotoxicol Environ Saf. 2012;85:52–58. doi: 10.1016/j.ecoenv.2012.08.023. [DOI] [PubMed] [Google Scholar]
- Poczai P, Hyvönen J. On the origin of Solanum nigrum: can networks help? Mol Biol Rep. 2011;38:1171–1185. doi: 10.1007/s11033-010-0215-y. [DOI] [PubMed] [Google Scholar]
- Rai MK, Kalia RK, Singh R, Gangola MP, Dhawan AK. Developing stress tolerant plants through in vitro selection—an overview of the recent progress. Environ Exp Bot. 2011;71:89–98. doi: 10.1016/j.envexpbot.2010.10.021. [DOI] [Google Scholar]
- Rehman F, Khan F, Varshney D, Naushin F, Rastogi J. Effect of cadmium on the growth of tomato. Biol Med. 2011;3:187–190. [Google Scholar]
- Sherameti I, A Varma (2010) Definition of “Heavy Metals” and their role in biological systems. Soil Heavy Metals, vol. 19. Springer. Soil Biology
- Słomka A, Sutkowska A, Szczepaniak M, Malec P, Mitka J, Kuta E (2011) Increased genetic diversity of Viola tricolor L. (Violaceae) in metal-polluted environments. Chemosphere 83:435–442 [DOI] [PubMed]
- Sridhar TM, Naidu CV. An efficient callus induction and plant regeneration of Solanum nigrum (L.)—an important antiulcer medicinal plant. J Phytol. 2011;3(5):23–28. [Google Scholar]
- Szabados L, Savoure A. Proline: a multifunctional amino acid. Trends Plant Sci. 2009;15(2):89–98. doi: 10.1016/j.tplants.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Thounaojam TC, Panda P, Mazumdar P, Kumar D, Sharma GD, Sahoo L, Panda SK. Excess copper induced oxidative stress and response of antioxidants in rice. Plant Physiol Biochem. 2012;53:33–39. doi: 10.1016/j.plaphy.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Van Rensburg L, Krüger G, Krüger H. Proline accumulation as drought-tolerance selection criterion: its relationship to membrane integrity and chloroplast ultrastructure in Nicotiana tabacum L. J Plant Physiol. 1993;141:188–194. doi: 10.1016/S0176-1617(11)80758-3. [DOI] [Google Scholar]
- Xu J, Yin H, Li Y, Liu X. Nitric oxide is associated with long-term zinc tolerance in Solanum nigrum. Plant Physiol. 2010;154:319–1334. doi: 10.1104/pp.110.162982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanqun Z, Yuan L, Schvartz C, Langlade L, Fan L. Accumulation of Pb, Cd, Cu and Zn in plants and hyperaccumulator choice in Lanping lead–zinc mine area, China. Environ Int. 2004;30(4):567–576. doi: 10.1016/j.envint.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Yruela I. Copper in plants: acquisition, transport and interactions. Funct Plant Biol. 2009;36:409–430. doi: 10.1071/FP08288. [DOI] [PubMed] [Google Scholar]