Abstract
The current study analyses few important biochemical parameters and microRNA expression in two closely related species (wild but tolerant Ipomoea campanulata L. and cultivated but sensitive Jacquemontia pentantha Jacq.G.Don) exposed to water deficit conditions naturally occurring in the field. Under soil water deficit, both the species showed reduction in their leaf area and SLA as compared to well-watered condition. A greater decrease in chlorophyll was noticed in J. pentantha (~50 %) as compared to I. campanulata (20 %) under stress. By contrast, anthocyanin and MDA accumulation was greater in J. pentantha as compared to I. campanulata. Multiple isoforms of superoxide dismutases (SODs) with differing activities were observed under stress in these two plant species. CuZnSOD isoforms showed comparatively higher induction (~10–40 %) in I. campanulata than J. pentantha. MicroRNAs, miR398, miR319, miR395 miR172, and miR408 showed opposing expression under water deficit in these two plant species. Expression of miR156, miR168, miR171, miR172, miR393, miR319, miR396, miR397 and miR408 from either I. campanulata or J. pentantha or both demonstrated opposite pattern of expression to that of drought stressed Arabidopsis. The better tolerance of the wild species (I. campanulata) to water deficit could be attributed to lesser variations in chlorophyll and anthocyanin levels; and relatively higher levels of SODs than J. pentantha. miRNA expression was different in I. campanulata than J. pentantha.
Keywords: Ipomoea campanulata, Jacquemontia pentantha, Water deficit, Lipid peroxidation, SOD, miRNAs
Introduction
Plants are persistently exposed to human driven climate change that has exceeded the bounds of natural variability resulting in extremes of temperature and precipitation, decreases in seasonal and perennial snow and sea level rise (Karl and Trenberth 2003). Global warming due to changes in the concentrations of green house gases (GHGs) of the atmosphere causes extreme and erratic precipitation. It leads to drought/land surface drying due to decrease in precipitation and flooding due to heavy precipitation (where mean precipitation amounts are not increasing) (Solomon et al. 2007). Significant drying has been observed in the Sahel, the Mediterranean, southern Africa and parts of southern Asia (Alley et al. 2007). These kinds of land surface drying affects plant available water leading to drought stress, which affects plant growth and productivity of major crop plants (Rampino et al. 2006; Reddy et al. 2004; Bartels and Sunkar 2005). Water deficit triggers a cascade of physiological, biochemical and molecular alterations in plants resulting in adaptive responses (Urano et al. 2010; Reddy et al. 2004). At physiological level, drought affects CO2 assimilation rates and synthesis of photosynthetic pigments (Jaleel et al. 2009; Reddy et al. 2004), induces production of reactive oxygen species (ROS)leading to oxidative damage which can be measured by the level of lipid peroxidation(Cruz de Carvalho 2008).
In plants, stress-induced changes in gene expression operate at multiple levels; transcriptional, post-transcriptional and post-translational regulations (Seo et al. 2009; Sakuma et al. 2006; Li et al. 2008; Bartles and Sunkar 2005; Sunkar et al. 2012). Stress-induced transcriptional regulation has been extensively studied over the past couple of decades (Yamaguchi-Shinozaki and Shinozaki 2006). On the other hand, miRNA-dependent post transcriptional gene regulation has emerged in recent years (Sunkar et al. 2007; Sunkar et al. 2012). MicroRNAs (20-22nt in length) originate from non protein coding sequences and regulate target gene expression at the mRNA level (Sunkar and Zhu 2007). The versatile feature of miRNAs is that they can spontaneously regulate the existing pool of mRNA targets without de novo synthesis (Leung and Sharp 2007). Approximately two-dozen miRNA families are highly conserved in diverse plant species indicating their essential roles in plant growth and development as well as other processes (Axtell and Bartel 2005; Sunkar and Jagadeeswaran 2008). Several conserved and species-specific miRNAs responsive to drought have been identified in Arabidopsis thaliana, Oryza sativa, Triticum dicoccoide, Medicago truncatula, Phaseolous vulagris and Populus trichocarpa (Sunkar and Zhu 2004; Liu et al. 2008; Zhao et al. 2007; Zhou et al. 2010; Kantar et al. 2011; Trindade et al. 2010; Arenas-Huertero et al. 2009; Lu et al. 2008).
Variations in the expression of miRNA (either up or down-regulation) depend on the nature/type and severity of stress. Upregulation of miRNAs in response to water deficit has been reported in several plant species (Kantar et al. 2011; Trindade et al. 2010; Arenas-Huertero et al. 2009). miR169 was observed to respond to only Polyethanol Glycol (PEG)- mediated water deficit in rice while several miRNAs were observed to respond (both up and downregulated) to field like water deficit (Zhao et al. 2007; Zhou et al. 2010). miRNA expression also varies between closely related species differing in their ability to withstand stress (Kulcheski et al. 2011). Differential miRNA expression in tolerant versus sensitive species implies an important role for miRNAs in stress responses in plants. This knowledge can provide scope for incorporating miRNA-mediated stress tolerance in sensitive species.
Wild plant species generally display greater tolerance to stress than their cultivated relatives, because cultivated species are selected for higher yield while wild species are often subjected to stress conditions in the field, which aids in developing stress tolerance (Mayrose et al. 2011). Drought tolerant species possess adaptive traits like decrease in leaf area along with thickening, sunken stomata, increase in root length and reduction in size of flowers (Carroll et al. 2001; Maroco et al. 2000; Turner 1994). Current study analyzes morphological, biochemical and molecular variations in two related plant species with contrasting stress sensitivities to water deficit. The wild although tolerant species, I. campanulata has wide spread distribution and is known for its vigorous growth across climatic regions with variable water availability. J. pentantha is a cultivated but sensitive species to water stress. The present study attempts to evaluate changes in some of the relevant parameters reflecting the response of plants towards water stress and how variation in miRNA expression is likely to assist wild species in its tolerance.
Material and methods
Plant material and study area
Ipomoea campanulata L. and Jacquemontia pentantha (Jacq.)G. Don belongs to the family Convolvulaceae. Both of these species are perennials and clonally propagated. I. campanulata, commonly known as morning glory, is spread across different geographic regions. It is seen growing naturally in water deficit as well as water logged areas. Its wide distribution across different environmental conditions is indicative of its adaptability. J. pentantha, commonly referred as sky blue cluster vine, is a cultivated species and is sensitive to water deficit and not observed growing under flooding conditions. Experiments were conducted at two nearby sites located near Vadodara, Gujarat, India in the years 2010–2011. Annual rainfall recorded was 900 mm. Both the species were exposed to different water regimes prevalent at the two nearby sites. Rain gauge measurements carried out to measure precipitation at these two sites for 15 days showed a difference of 20 %. Based on these details, the site showing higher rainfall measurements was treated as control and the one with lesser value (of rainfall) was considered as experimental. Maximum and minimum mean temperatures recorded during the study period by local metrological observatory are 36 °C and 19 °C, respectively. All other characteristics were similar at both the sites. Plant material was collected from individuals showing similarity in physical characteristics (such as height and spread of the plant). Mature leaves (sixth from the top/first leaf) at both the sites were collected, immediately frozen in liquid Nitrogen and were brought to the laboratory for further analysis. These samples were used to determine the changes in chlorophyll, anthocyanin, lipid peroxidation, superoxide dismutases and miRNAs.
Determination of soil water content and leaf traits
Soils at both the sites are light brown colored and loamy. Determination of soil water content was carried out in accordance to Granier et al. (2006). Briefly, 50 g of soil samples were collected from three different depths (surface, 20–30 cm and 30–40 cm) of the soil at control and experimental site. These soil samples were weighed before and after drying (4d at 180 °C) to determine the soil water content. Ten samples were collected at each depth for the measurements. These values were averaged subsequently. Differences in the soil water content (at each depth) between the two sites were analysed for the entire study duration. Leaf area was determined by Leaf area meter (CI-203, CID, Inc.). The specific leaf area (SLA) was calculated using the formula SLA = Leaf area (cm2)/ Leaf weight (g).
Chlorophyll analysis
Leaf chlorophyll was determined using a chlorophyll meter (SPAD-502, Minolta, Japan). The Standard curve for quantification of chlorophyll content was prepared as reported previously (Li et al. 2006). Six fully matured leaves from both the species growing at control and experimental sites were used for chlorophyll analysis.
Estimation of anthocyanin content
Anthocyanin levels were measured as described previously (Rabino and Mancinelli 1986; Sunkar et al. 2006). Briefly, 0.2 g of leaf sample was extracted with 5 ml of 99:1 methanol: HCl (v/v) at 4 °C and OD530 and OD657 were measured. Relative anthocyanin content was determined by using the equation (0.25 × OD657) × extraction volume (mL) × 1/weight of tissue sample (g) (Sunkar et al. 2006).
Lipid peroxidation
Lipid peroxidation assay was carried out by thiobarbituric acid (TBA) method, wherein thiobarbituric acid reacting substances (TBARS) act as an indicator of membrane lipid peroxidation which was measured in terms of malondialdehyde (MDA) concentration (Heath and Packer 1968; Fazeli et al. 2007). 0.2 g leaf samples from both the sites were weighed and homogenized in 4 ml of 0.1 % trichloroacetic acid (TCA) solution. Samples were then centrifuged at 11,000 g for 10 min, and the supernatant was collected. One ml of 20 % TCA containing 0.5 % TBA was added to 0.5 ml of supernatant. Samples were shaken thoroughly and placed in boiling water bath for 30 min. They were removed and cooled in an ice bath. These, samples were again centrifuged at 11,000 g for 15 min and supernatants were collected. Their absorbance was measured at OD532 andOD600. MDA concentration was calculated by using extinction coefficient 155 mM−1 cm−1.
SOD enzyme extraction
Leaves collected were weighed (0.5 g) and kept in liquid nitrogen. Crude extract was made by grinding each sample with 5 ml of 75 mM Tris–HCl buffer, pH 7.5 containing 5 % glycerol (w/v), 5 % PVP-40 (w/v), 14 mM mercaptoethanol (0.1 % v/v), 50 mM Na-salt, 10 mM dithiothreitol (DTT) and 0.1 % bovine serum albumin (w/v) (Wendel and Weeden 1989). The homogenate was centrifuged at 10, 000 g for 20 min at 4 °C and the supernatant was used for identification of different isoforms of SOD.
Native polyacrylamide gel electrophoresis (native PAGE) and SOD activity staining
Native PAGE of SOD was performed on a 10 % resolving gel at constant supply of current at 100 V and 4 °C. Subsequently the activity staining for SOD isoenzymes was performed as reported by Beauchamp and Fridovich (1971); and Fazeli et al. (2007). For activity staining, the gel was incubated in two solutions consecutively. The gel was first kept in 2.5 mM nitro-blue tetrazolium (NBT) for 30 min and then washed thoroughly. Later it was kept in 50 mM K-phosphate buffer (pH 7.8) containing 28 mM riboflavin in darkness for 30 min. They were washed thoroughly again and exposed to light for 30 min. Enzyme isoforms appeared as colorless bands in a purple background. For identification and characterization of isoenzymes, before activity staining the gel was treated with 50 mM K-phosphate buffer (pH 7.8) containing either 3 mM KCN or 5 mM H2O2 for 20–30 min. It aids in the identification and characterization of isoenzymes such as CuZnSOD, FeSOD and MnSOD bands showing differential sensitivity to KCN and H2O2.CuZnSOD bands are sensitive to both KCN and H2O2.MnSOD bands are resistant to both KCN and H2O2. FeSOD bands are inhibited by H2O2 but are resistant to KCN.
Protein extraction and detection by immunoblot for CuZnSOD
Protein extracts were prepared from leaf samples using trichloroacetic acid (TCA) extraction buffer (Isaacson et al. 2006). Isolated proteins were separated on 10 % SDS-PAGE (Laemmli 1970) and electrotransferred onto polyvinylidenedifluoride (PVDF) membrane (Bio-Rad). Membrane was blocked using 5 % non fat dry milk in TBS for 2 h at room temperature and then incubated with antiserum against CuZnSOD (1:2000 dilution) for 2 h at RT (Kliebenstein et al. 1998). After washing the membrane with TBST, it was incubated with HRP conjugated secondary antibody (Thermo Scientific). Immunoblot was detected using Pierce ECL2 Western blotting kit.
RNA extraction and small RNA blot analysis
Total RNA was isolated from both the plants species at control and experimental site (water deficit) using Trizol Reagent. Forty micrograms of total RNA was resolved on a denaturing 15 % polyacrylamide gel, and transferred electrophoretically to Hybond-N+ membranes. Membranes were UV cross-linked and baked for 2 h at 80 °C. DNA oligonucleotides complementary to conserved miRNA sequences of Arabidopsis were end labeled with γ-32P-ATP using T4 polynucleotide kinase (New England Biolabs). Membranes were prehybridized for at least 1 h and hybridized overnight using perfect hybridization buffer (Sigma) at 38 °C. Blots were washed three times (two times with 2 × SSC + 1 % SDS and one time with 1 × SSC + 0.5 % SDS) at 50 °C. The membranes were briefly air dried and then exposed to phosphorscreen and images were acquired by scanning the films with a Typhoon scanner.
Statistical analysis
Values of the measured parameters are averages coming from triplicate samples (excepting for soil water content). ANOVA has been carried out to test whether the differences seen in the measured values are statistically significant or not.
Results
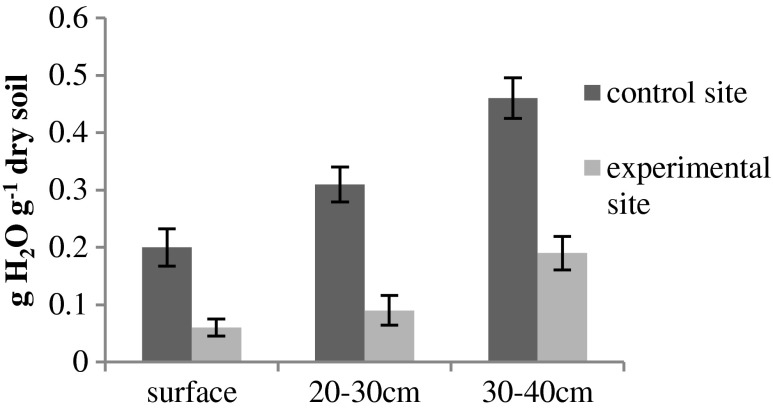
The soil water content analysis at three major soil depths (surface, 20–30 cm and 30–40 cm) for both the control and experimental sites revealed ~50 % lower average soil water content at experimental site as compared to control (Fig. 1). These analyses were carried out several times during the entire study duration in order to ensure that the difference in soil water content has not been deviated throughout the experimental cycle. Leaf area and specific leaf area (SLA) of both the species were greater at control site than compared to experimental site. Soil water deficit at experimental site has reduced growth of both the species. It is reflected in the measured leaf traits such as leaf area and SLA. Reduction in leaf area in I. campanulata and J. pentantha was ~12 % and ~20 % respectively as compared to control (Table 1). The reduction in SLA was comparatively higher in J. pentantha than I. campanulata (Table 1).
Fig. 1.
Soil water content analysed at different depths. Data are means ±SD (n = 10)
Table 1.
Variations observed in leaf area. Data are means ±SD (n = 3)
| Plant species | Control site | Experimental site | ||
|---|---|---|---|---|
| I. campanulata | J. pentantha | I. campanulata | J. pentantha | |
| Leaf area (cm2) | 98 ± 4.0 | 24 ± 6.5 | 85 ± 2.6 | 19 ± 1.9 |
| SLA(cm2/g) | 274.93 | 283.26 | 143.31 | 115.21 |
Chlorophyll and anthocyanin levels were analysed in both the species growing at control and experimental site. Chlorophyll content was reduced in both the species in response to water deficit. Reduction was higher in J. pentantha (by 50 %) as compared to I. campanulata (by 20 %) (Table 2). Anthocyanin levels increased in both the species under stress. The degree of increase varied between the two species, nearly two-fold increase was seen in I. campanulata and three-fold increase was recorded in J. pentantha (Table 2).
Table 2.
Varaition in (a) chlorophyll, (b) anthocyanin and (c) malondialdehyde (MDA) in I. campanulata and J. pentantha growing under water deficit. Data are means± SD (n = 3)
| Parameters analysed | Control site | Experimental site | ||
|---|---|---|---|---|
| I. campanulata | J. pentantha | I. campanulata | J. pentantha | |
| Chlorophyll mg g−1 fresh weight | 15.2 ± 0.71 | 6.57 ± 0.32 | 12.37 ± 0.47 | 3.06 ± 0.44 |
| Anthocyanin µg g−1 fresh weight | 0.53 ± 0.08 | 1.31 ± 0.06 | 1.05 ± 0.06 | 3.87 ± 0.04 |
| MDA n mol g−1 fresh weight | 14.52 ± 1.54 | 12.92 ± 2.21 | 17.74 ± 1.55 | 20.67 ± 1.15 |
MDA levels were increased approximately by 20 % and 60 % in I. campanulata and J. pentantha respectively, in response to water deficit at experimental site (Table 2). Native PAGE analysis was used to differentiate the SOD isoforms. The in-gel activity assay revealed the presence of multiple SOD isoforms, i.e., two MnSODs, two FeSODs and four CuZnSODs in both the species (Fig. 2a). Intensity analysis of MnSOD isoforms showed significant rise in its activity in both the species (P < 0.05) (Fig. 2b). FeSOD I and II depicted significant rise in their activity in I. campanulata exposed to water deficit (P < 0.05) (Fig. 2c). Contrary to this response J. pentantha showed significant rise in the activity for FeSODII (P < 0.05), but not for FeSOD I (P > 0.05) (Fig. 2c). CuZnSOD isoforms were induced significantly in both the species in response to water deficit (P < 0.05); however the increase was 10–40 % higher in I. campanulata compared to J. pentantha (Fig. 2d). To further validate the differential accumulation of CuZnSOD (as evident from in-gel activity assay), immunoblot analyses were carried out using polyclonal anti-CSD2 antiserum (Kliebenstein et al. 1998). The immunoblot results confirmed a prominent induction in I. campanulata and transient/less induction in J. pentantha under water deficit (Fig. 2e), similar to the in-gel activity observed for CuZnSOD isoforms.
Fig. 2.
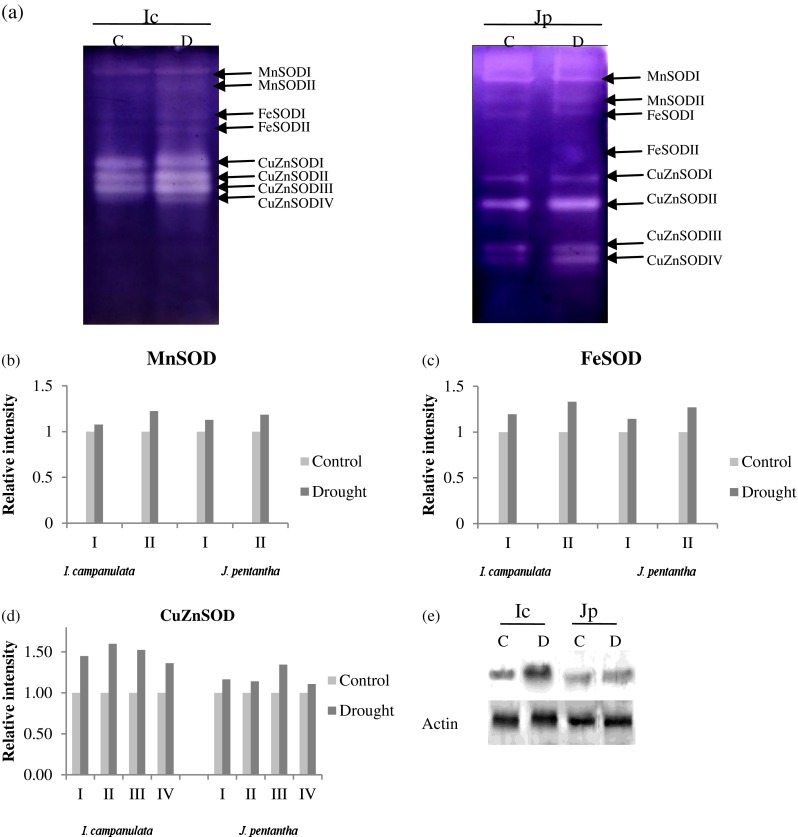
SOD isoforms of I.campanulata (Ic) and J. pentantha (Jp) growing under control (C) and water deficit conditions (D) as anlysed by a activity staining of native PAGE. Relative intensity of b MnSOD, c FeSOD and d CuZnSOD isoforms in I. campanulata and J. pentantha exposed to water deficit than compared to control. e Immunoblot showing level of CuZnSOD in I. campanulata and J.pentantha growing under water deficit. Actin was used as loading control
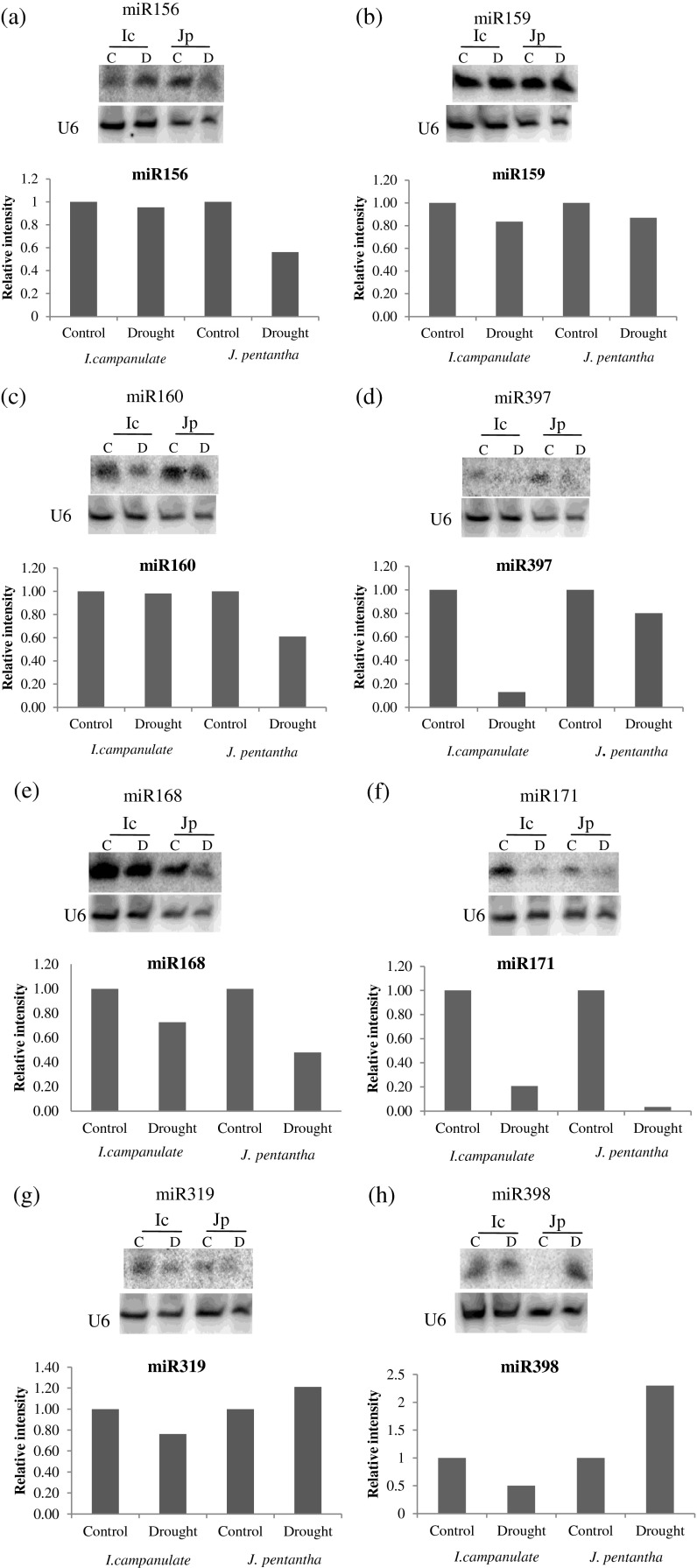
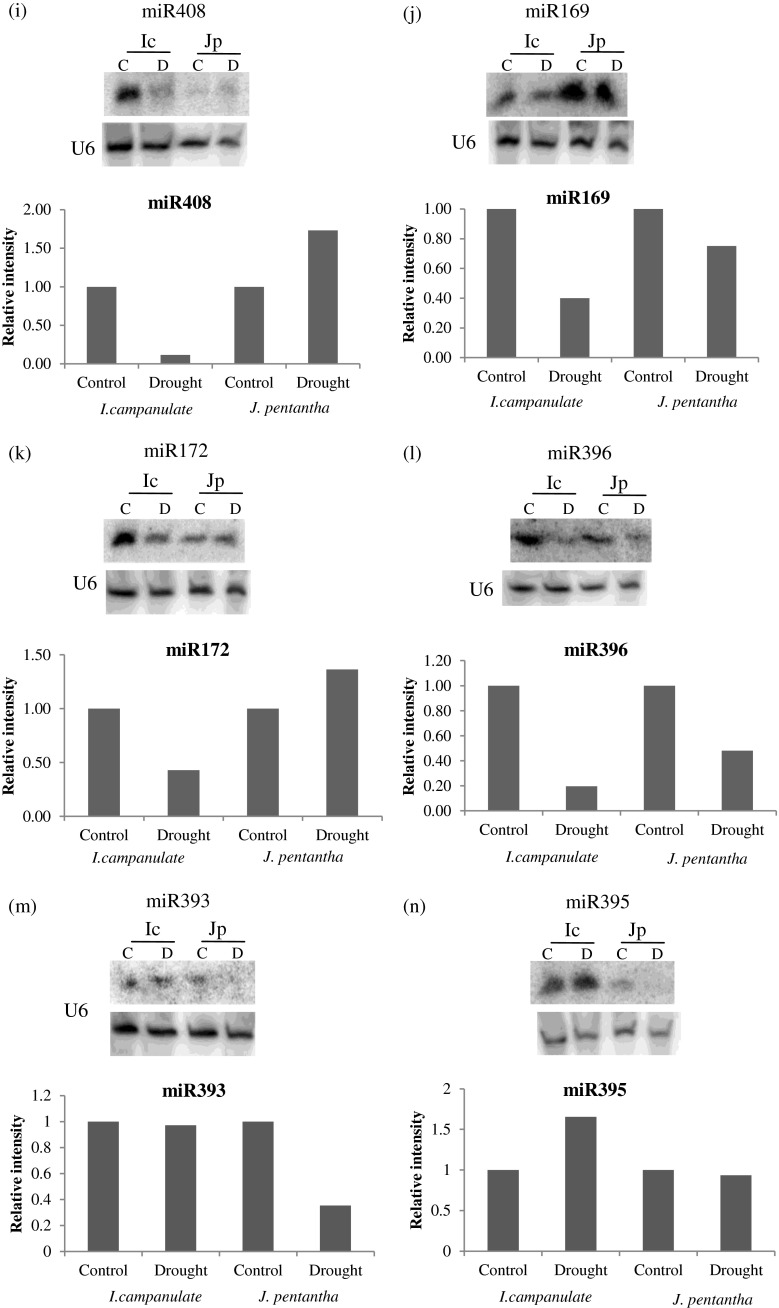
In response to water deficit, miR398, miR319, miR395, miR172 and miR408 showed opposite pattern of expression in I. campanulata and J. pentantha, revealing differences in the expression levels of these conserved miRNAs (Table 3) (Fig. 3g,h,k,n and i). Expression of miR319, miR398, miR172, and miR408 were downregulated by approximately 1.25, 2, 2.5 and 10 fold respectively in I. campanulata exposed to water deficit. While in J. pentantha expression of miR319, miR398 miR172 and miR408 were upregulated by approximately 1.2, 2.3, 1.4 and 1.8 fold respectively in response to water deficit. In contrast, miR395 expression was observed to be upregulated (~1.6 fold) in I. campanulata while downregulated (~1fold) in J. pentantha. Other miRNAs (such as miR156, miR160, miR397, miR168, miR171, miR169, miR396 and miR393) showed similar pattern of expression in both the species in response to water deficit (Table 3) (Fig. 3a,c,d,e,f,j,l,and m). However, the degree of variation was different in both the species. miR156, miR160, miR168, miR171 and miR393 showed prominent downregulation (~1–5 fold lower) in J. pentantha than I. campanulata; while others (miR169, miR396 and miR397) showed prominent downregulation (~1–2 fold lower) in I. campanulata than J. pentantha. Only miR159 showed almost similar level of reduction in its expression in both the species (Fig. 3b).
Table 3.
Conserved stress responsive miRNA expression in I. campanulata, J. pentantha and Arabidopsis where ‘↑’- upregulated, ‘↓’-downregulated and ‘-’ -expression not known
| miRNAs | Targets | Water deficit | ||
|---|---|---|---|---|
| I. campanulata | J. pentantha | Arabidopsis | ||
| miR156 | SBP-LIKE | ↓ | ↓ | ↑ |
| miR159 | TCP/MYB | ↓ | ↓ | ↑ |
| miR160 | ARF | ↓ | ↓ | – |
| miR397 | Laccases | ↓ | ↓ | ↑ |
| miR168 | AGO1 | ↓ | ↓ | ↑ |
| miR171 | SCL | ↓ | ↓ | ↑ |
| miR319 | TCP/MYB | ↓ | ↓ | ↑ |
| miR398 | CSD1-2,Cox 5b | ↓ | ↑ | ↓ |
| miR408 | Plantacyanin | ↓ | ↑ | ↑ |
| miR169 | NFY | ↓ | ↓ | ↓ |
| miR172 | AP2-LIKE | ↓ | ↑ | ↑ |
| miR396 | GRF | ↓ | ↓ | ↑ |
| miR393 | TIR1/AFB | ↓ | ↓ | ↑ |
| miR395 | SULTR1-2 | ↑ | ↓ | – |
Fig. 3.
Expression level of conserved miRNAs in I. campanulata (Ic) and J.pentantha (Jp) leaves growing under Control (c) and Water deficit (d) conditions analysed through Northern blotting. U6 (small nuclear RNA) was used as loading control and relative accumulation of all miRNAs (to that of Control) was quantified by normalizing their intensity values in accordance to that of U6
Discussion
Water is a major limiting factor for the growth of plants. In the current study, I. campanulata and J. pentantha responded to water deficit by showing reduction in measured leaf traits such as leaf area and SLA. Changes were more prominent in J. pentantha than I. campanulata. Decrease associated with these parameters has a negative impact on plant growth. It was reported earlier (Xu and Zhou 2008; Pereira and Chaves 1993) that reduction in leaf area directly affects photosynthesis, thereby affecting plant growth rate and biomass production. Liu and Stutzel 2004 reported that decrease in SLA leads to reduction in photosynthesis capacity. Higher reduction in leaf area and SLA in J. pentantha than I. campanulata indicated its higher sensitivity to water stress.
Drought stress is identified to severely decrease chlorophyll levels whereas it increases the accumulation of anthocyanins, MDA and SODs (Taulavuori et al. 2010; Gould 2004; Cruz de Carvalho 2008; Kliebenstein et al. 1998). Decreased chlorophyll is recognized to directly affect photosynthesis which plays a vital role in plant growth and development (Chaves et al. 2009; Flexas et al. 2004; Lawlor and Tezara 2009). Larger fall in the chlorophyll content of J. pentantha may depict its susceptibility to water deficit. Anthocyanin levels are an indicator of sensitivity to diverse stresses like drought, UV-B and heavy metals (Gould 2004). Anthocyanin production is a metabolically expensive process and competes with chlorophyll for light harvesting (Chalker-Scott 1999). In the present study, J. pentantha showed higher (~3 fold) accumulation of anthocyanins compared to unstressed controls suggesting that it is experiencing a high degree of stress as compared to I. campanulata under approximately similar level of water deficit. Similarly lipid peroxidation was recorded higher in J. pentantha indicating larger damage. Relatively lower values were seen in I. campanulata showing its tolerance to water stress. Levels of lipid peroxidation were observed to be lower in drought tolerant Phaseolous acutifolius than the drought sensitive species Phaseolous vulgaris(Turkan et al. 2005). Similarly, drought tolerant invasive plant species Alternanthera philoxeroides depicted lower levels of lipid peroxidation than the sensitive crop plant Oryza sativa (Gao et al. 2008). Observations of our study go together with these findings. SODs are produced as a preliminary line of defense for oxidative stress (Foyer and Noctor 2005). SODs are classified into FeSODs (Iron SODs), MnSODs (manganese SODs) and CuZnSODs (copper zinc SODs) based on the use of metal cofactor, with a critical role of CuZnSODs under oxidative stress (Mittler 2002; Sunkar et al. 2006). The SOD isoenzymes identified in these two plant species were based on their differential sensitivity to KCN and H2O2, which were comparable to the ones observed in Glycyrrhiza uralensis (Pan et al. 2006). In the current study both the species showed rise in the activity of all SOD isoforms in response to water deficit, however it was comparatively higher in I. campanulata. A. thaliana exposed to oxidative stress showed rise in the activity of CuZnSODs, however MnSOD isoforms showed no change (Kliebenstein et al. 1998). In Glycyrrhiza uralensis, drought stress induced no change in MnSOD and FeSOD activities; however CuZnSOD showed most abundant activity (Pan et al. 2006). Increase in the activity of CuZnSODs was also observed in rice and pea plants exposed to drought (Ke et al. 2009; Moran et al. 1994). Although MnSOD activity was not detected in above described species, transgenic plant species (alfalfa and rice) overexpressing MnSOD were reported as more drought tolerant compared to non-transgenic plants (McKersie et al. 1996; Wang et al. 2005). Similarly, transgenic Maize overproducing FeSOD was shown to be more oxidative stress-tolerant (Van Breusegem et al. 1999). Better adaptability of I. campanulata to water deficit can be attributed to the greater rise in activity of MnSOD and FeSOD isoforms. Transgenic sweet potato (Ipomoea batatus) overexpressing CuZnSOD and APX displayed not only better drought tolerance but could also recover from drought (Lu et al. 2010). Furthermore, relative drought and other abiotic stress tolerance of A. thaliana ecotype Cvi has been attributed to elevated level of CuZnSOD (CSD2) expression (Abarca et al. 2001). Therefore, the observed higher activity of all SOD isoforms (specifically CuZnSOD) in I. campanulata could potentially contribute for its drought tolerance. On the contrary, transient/less induction of SODs (specifically CuZnSOD) observed in J. pentantha may demonstrate its sensitivity to water deficit.
miRNAs are critical regulators of gene expression as they respond spontaneously to stress by regulating the existing pool of mRNAs (Leung and Sharp 2007; Sunkar et al. 2012). Drought tolerant and sensitive soybean cultivars showed opposite pattern of miRNA expression (Kulcheski et al. 2011). Similar to this report, the expression of five miRNA families (miR398, miR408, miR395, miR319 and miR172) differed between I. campanulata and J. pentantha. miR398, miR408 and miR395 largely regulate the expression of genes coding for CuZnSODs (CSD), plantacyanin, and sulfate transport and assimilation (APS; ATP sulfurylases and SULTR; sulfate transporter) respectively, which are also associated with stress responses in plants (Sunkar et al 2006; Abdel-Ghany and Pilon 2008; Jones-Rhoades et al. 2006). miR319 targets TCP transcription factor that controls leaf morphogenesis (Palatnik et al. 2003). Differences seen in both the species with respect to regulating CuZnSODs and measured leaf traits could partly be attributed to the variations in the expression of miR398, miR408, miR395, and miR319. miR398 levels were downregulated in drought-stressed Arabidopsis but upregulated in drought-stressed M. truncatula and T. dicoccoides (Sunkar and Zhu 2004; Trindade et al. 2010; Kantar et al. 2011). Protection against oxidative stress in Arabidopsis could be achieved by downregulation of miR398, which consequently induces CuZnSODs (Sunkar et al. 2006). Decreased miR398 levels coupled with the rise in levels of CuZnSODs (as evident from in-gel activity staining and immunoblotting) in drought-stressed I. campanulata supports a role for miR398 in drought tolerance. However expression of miR398 did not correlate with CuZnSOD accumulation in J. pentantha (unlike in I. campanulata) suggesting the existence of yet unknown regulatory mechanisms in this sensitive species.
I. campanulata and J. pentantha or both demonstrated opposite expression pattern of regulation of several miRNAs (miR156, miR168, miR171, miR172, miR393, miR319, miR396, miR397 and miR408) to that of drought-stressed Arabidopsis (Liu et al. 2008). Most importantly, decreased miR156, miR168 and miR171 levels under water deficit in both the species was quite different from the observations made in Arabidopsis and other cultivated species, indicating the distinct response of I. campanulata and J. pentantha (Sunkar and Zhu 2004; Liu et al. 2008; Arenas-Huertero et al. 2009; Hwang et al. 2011).
Better tolerance of I. campanulata to water deficit can be attributed to specific morpho-physiological and biochemical traits (like less oxidative stress as revealed by lower degree of lipid peroxidation and higher accumulation of CuZnSODs) and differential miRNA expression. miRNA expression seen in I. campanulata is different from J. pentantha and also from other model plant species such as Arabidopsis. Further studies are required to understand the adaptive response of wild I. campanulata to water deficit compared to cultivated J. pentantha.
Acknowledgments
VG, NSRK, KP and SI are thankful to UGC-DRS program for financial assistance, RS is thankful to Oklahoma Agricultural Experiment Station and an NSF-EPSCoR award EPS0814361.
References
- Abarca D, Roldan M, Martin M, Sabater B. Arabidopsis thaliana ecotype Cvi shows an increased tolerance to photo-oxidative stress and contains a new chloroplastic copper/zinc superoxide dismutase isoenzymes. J Exp Bot. 2001;52(360):1417–1425. doi: 10.1093/jexbot/52.360.1417. [DOI] [PubMed] [Google Scholar]
- Abdel-Ghany SE, Pilon M. MicroRNA-mediated systemic down-regulation of copper protein expression in response to low copper availability in Arabidopsis. J Biol Chem. 2008;283(23):15932–15945. doi: 10.1074/jbc.M801406200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alley R, Berntsen T, Bindoff NL, Chen Z, Chidthaisong A, Friedlingstein P, Gregory J, Hegerl G, Heimann M, Hewitson B, Hoskins B, Joos F, Jouzel J, Kattsov V, Lohmann U, Manning M, Matsuno T, Molina M, Nicholls N, Overpeck J, Qin D, Raga G, Ramaswamy V, Ren J, Rusticucci M, Solomon S, Somerville R, Stocker TF, Stott P, Stouffer RJ, Whetton P, Wood RA, Wratt D (2007) Climate change 2007: The physical science basis. summary for policymakers. Intergovernmental panel on climate change: Geneva, CH pp 5–11
- Arenas-Huertero C, Perez B, Rabanal F, Blanco-Melo D, De la Rosa C, Estrada-Navarrete G, Sanchez F, Covarrubias A, Reyes J. Conserved and novel miRNAs in the legume Phaseolus vulgaris in response to stress. Plant Mol Biol. 2009;70:385–401. doi: 10.1007/s11103-009-9480-3. [DOI] [PubMed] [Google Scholar]
- Axtell MJ, Bartel DP. Antiquity of microRNAs and their targets in land plants. Plant Cell Online. 2005;17(6):1658–1673. doi: 10.1105/tpc.105.032185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels D, Sunkar R. Drought and salt tolerance in plants. Crit Rev Plant Sci. 2005;24:23–58. doi: 10.1080/07352680590910410. [DOI] [Google Scholar]
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Carroll AB, Pallardy SG, Galen C. Drought stress plant water status and floral trait expression in fireweed Epilobium angustifolium (Onagraceae) Am J Bot. 2001;88(3):438–446. doi: 10.2307/2657108. [DOI] [PubMed] [Google Scholar]
- Chalker-Scott L. Environmental significance of anthocyanins in plant stress responses. Photochem Photobiol. 1999;70(1):1–9. doi: 10.1111/j.1751-1097.1999.tb01944.x. [DOI] [Google Scholar]
- Chaves MM, Flexas J, Pinheiro C. Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot. 2009;103(4):551–560. doi: 10.1093/aob/mcn125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz de Carvalho MH. Drought stress and reactive oxygen species: production scavenging and signaling. Plant Signal Behav. 2008;3(3):156. doi: 10.4161/psb.3.3.5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazeli F, Ghorbanli M, Niknam V. Effect of drought on biomass protein content lipid peroxidation and antioxidant enzymes in two sesame cultivars. Biol Plant. 2007;51(1):98–103. doi: 10.1007/s10535-007-0020-1. [DOI] [Google Scholar]
- Flexas J, Bota J, Cifre J, Mariano-Escalona J, Galmes J, Gulias J, Lefi E, Martinez-Canellas S, Moreno M, Ribas-Carbo M, Riera D, Sampol B, Medrano H. Understanding down-regulation of photosynthesis under water stress: future prospects and searching for physiological tools for irrigation management. Ann Appl Biol. 2004;144(3):273–283. doi: 10.1111/j.1744-7348.2004.tb00343.x. [DOI] [Google Scholar]
- Foyer CH, Noctor G. Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell Online. 2005;17(7):1866–1875. doi: 10.1105/tpc.105.033589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Xiao Q, Ding L, Chen M, Yin L, Li J, Zhou S, He G. Differential responses of lipid peroxidation and antioxidants in Alternanthera philoxeroides and Oryza sativa subjected to drought stress. Plant Growth Regul. 2008;56(1):89–95. doi: 10.1007/s10725-008-9291-6. [DOI] [Google Scholar]
- Gould KS. Nature’s Swiss army knife: the diverse protective roles of anthocyanins in leaves. BioMed Res Int. 2004;5:314–320. doi: 10.1155/S1110724304406147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granier C, Aguirrezabal L, Chenu K, Cookson SJ, Dauzat M, Hamard P, Yioux J, Rolland G, Combaud S, Lebaudy A, Muller B, Simonneau T, Tardieu F. PHENOPSIS an automated platform for reproducible phenotyping of plant responses to soil water deficit in Arabidopsis thaliana permitted the identification of an accession with low sensitivity to soil water deficit. New Phytol. 2006;169(3):623–635. doi: 10.1111/j.1469-8137.2005.01609.x. [DOI] [PubMed] [Google Scholar]
- Heath RL, Packer L. Photoperoxidation in isolated chloroplasts: I Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125(1):189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Hwang EW, Shin SJ, Yu BK, Byun MO, Kwon HB. miR171 family members are involved in drought response in Solanum tuberosum. J Plant Biol. 2011;54(1):43–48. doi: 10.1007/s12374-010-9141-8. [DOI] [Google Scholar]
- Isaacson T, Damasceno CM, Saravanan RS, He Y, Catala C, Saladie M, Rose JK. Sample extraction techniques for enhanced proteomic analysis of plant tissues. Nat Protoc. 2006;1(2):769–774. doi: 10.1038/nprot.2006.102. [DOI] [PubMed] [Google Scholar]
- Jaleel CA, Manivannan P, Wahid A, Farooq M, Al-Juburi HJ, Somasundaram R, Panneerselvam R. Drought stress in plants: a review on morphological characteristics and pigments composition. Int J Agric Biol. 2009;11(1):100–105. [Google Scholar]
- Jones-Rhoades MW, Bartel DP, Bartel B. MicroRNAs and their regulatory roles in plants. Annu Rev Plant Biol. 2006;57:19–53. doi: 10.1146/annurev.arplant.57.032905.105218. [DOI] [PubMed] [Google Scholar]
- Kantar M, Lucas S, Budak H. miRNA expression patterns of Triticum dicoccoides in response to shock drought stress. Planta. 2011;233:471–484. doi: 10.1007/s00425-010-1309-4. [DOI] [PubMed] [Google Scholar]
- Karl TR, Trenberth KE. Modern global climate change. Science. 2003;302:1719–1723. doi: 10.1126/science.1090228. [DOI] [PubMed] [Google Scholar]
- Ke Y, Han G, He H, Li J. Differential regulation of proteins and phosphoproteins in rice under drought stress. Biochem Biophys Res Commun. 2009;379(1):133–138. doi: 10.1016/j.bbrc.2008.12.067. [DOI] [PubMed] [Google Scholar]
- Kliebenstein DJ, Monde RA, Last RL. Superoxide dismutase in Arabidopsis : an eclectic enzyme family with disparate regulation and protein localization. Plant Physiol. 1998;118(2):637–650. doi: 10.1104/pp.118.2.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulcheski FR, Oliveira LFV, Molina LG, Almerao MP, Rodrigues FA, Marcolino J, Barbosa JF, Stolf-Moreira R, Nepomuceno AL, Marcelino-Guimaraes FC, Abdelnooe RV, Nascimento LC, Carazzolle MF, Pereira GA, Margis R. Identification of novel soybean microRNAs involved in abiotic and biotic stress. BMC Genomics. 2011;12:e307–e414. doi: 10.1186/1471-2164-12-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;277:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawlor DW, Tezara W. Causes of decreased photosynthetic rate and metabolic capacity in water-deficient leaf cells: a critical evaluation of mechanisms and integration of processes. Ann Bot. 2009;103(4):561–579. doi: 10.1093/aob/mcn244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung AK, Sharp PA. microRNAs: a safeguard against turmoil? Cell. 2007;130:581–585. doi: 10.1016/j.cell.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Li RH, Guo PG, Michael B, Stefania G, Salvatore C. Evaluation of chlorophyll content and fluorescence parameters as indicators of drought tolerance in barley. Agric Sci China. 2006;5(10):751–757. doi: 10.1016/S1671-2927(06)60120-X. [DOI] [Google Scholar]
- Li W-X, Oono Y, Zhu J, He X-J, Wu J-M, Iida K, Lu X-Y, Cui X, Jin H, Zhu J-K. The Arabidopsis NFYA5 transcription factor is regulated transcriptionally and posttranscriptionally to promote drought resistance. Plant Cell. 2008;20:2238–2251. doi: 10.1105/tpc.108.059444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Stutzel H (2004) Biomass partitioning, specific leaf area, and water use efficiency of vegetable amaranth (Amaranthus spp.) in response to drought stress. Scie Hort 102(1):15–27
- Liu HH, Tian X, Li YJ, Wu CA, Zheng CC. Microarray-based analysis of stress-regulated microRNAs in Arabidopsis thaliana. RNA. 2008;14:836–843. doi: 10.1261/rna.895308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S, Sun YH, Chiang VL (2008) Stress-responsive microRNAs in Populus. Plant J 55:131–151 [DOI] [PubMed]
- Lu YY, Deng XP, Kwak SS. Over expression of CuZn superoxide dismutase (CuZn SOD) and ascorbate peroxidase (APX) in transgenic sweet potato enhances tolerance and recovery from drought stress. Afr J Biotechnol. 2010;9(49):8378–8391. [Google Scholar]
- Maroco JP, Pereira JS, Manuela Chaves M. Growth photosynthesis and water-use efficiency of two C4 Sahelian grasses subjected to water deficits. J Arid Environ. 2000;45(2):119–137. doi: 10.1006/jare.2000.0638. [DOI] [Google Scholar]
- Mayrose M, Kane NC, Mayrose I, Dlugosch KM, Rieseberg LH. Increased growth in sunflower correlates with reduced defences and altered gene expression in response to biotic and abiotic stress. Mol Ecol. 2011;20(22):4683–4694. doi: 10.1111/j.1365-294X.2011.05301.x. [DOI] [PubMed] [Google Scholar]
- McKersie BD, Bowley SR, Harjanto E, Leprince O. Water-deficit tolerance and field performance of transgenic alfalfa overexpressing superoxide dismutase. Plant Physiol. 1996;111(4):1177–1181. doi: 10.1104/pp.111.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R. Oxidative stress antioxidants and stress tolerance. Trends Plant Sci. 2002;7(9):405–410. doi: 10.1016/S1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- Moran JF, Becana M, Iturbe-Ormaetxe I, Frechilla S, Klucas RV, Aparicio-Tejo P. Drought induces oxidative stress in pea plants. Planta. 1994;194(3):346–352. doi: 10.1007/BF00197534. [DOI] [Google Scholar]
- Palatnik JF, Allen E, Wu X, Schommer C, Schwab R, Carrington JC, Weigel D. Control of leaf morphogenesis by microRNAs. Nature. 2003;425:257–263. doi: 10.1038/nature01958. [DOI] [PubMed] [Google Scholar]
- Pan Y, Wu LJ, Yu ZL. Effect of salt and drought stress on antioxidant enzymes activities and SOD isoenzymes of liquorice (Glycyrrhiza uralensis Fisch) Plant Growth Regul. 2006;49:157–165. doi: 10.1007/s10725-006-9101-y. [DOI] [Google Scholar]
- Pereira JS, Chaves MM. Plant water deficits in Mediterranean ecosystems. In: Smith JAC Griffiths H, editor. Plant responses to water deficits-from cell to community. Oxford: BIOS Scientific; 1993. pp. 237–251. [Google Scholar]
- Rabino I, Mancinelli AL. Light temperature and anthocyanin production. Plant Physiol. 1986;81(3):922–924. doi: 10.1104/pp.81.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampino P, Pataleo S, Gerardi C, Mita G, Perrotta C. Drought stress response in wheat: physiological and molecular analysis of resistant and sensitive genotypes. Plant Cell Environ. 2006;29(12):2143–2152. doi: 10.1111/j.1365-3040.2006.01588.x. [DOI] [PubMed] [Google Scholar]
- Reddy AR, Chaitanya KV, Vivekanandan M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J Plant Physiol. 2004;161(11):1189–1202. doi: 10.1016/j.jplph.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Sakuma Y, Maruyama K, Osakabe Y, Qin F, Seki M, Shinozaki K, Yamaguchi-Shinozaki K. Functional analysis of an Arabidopsis transcription factor DREB2A involved in drought-responsive gene expression. Plant Cell Online. 2006;18(5):1292–1309. doi: 10.1105/tpc.105.035881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo PJ, Xiang F, Qiao M, Park JY, Lee YN, Kim SG, Park CM. The MYB96 transcription factor mediates abscisic acid signaling during drought stress response in Arabidopsis. Plant Physiol. 2009;151(1):275–289. doi: 10.1104/pp.109.144220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (eds) (2007) Climate change 2007-the physical science basis: Working group I contribution to the fourth assessment report of the IPCC, vol 4. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA pp 105–108
- Sunkar R, Chinnusamy V, Zhu J, Zhu J K (2007) Small RNAs as big players in plant abiotic stress responses and nutrient deprivation. Trends Plant Sci 12(7): 301–309 [DOI] [PubMed]
- Sunkar R, Jagadeeswaran G. In silico identification of conserved microRNAs in large number of diverse plant species. BMC Plant Biol. 2008;8(1):37. doi: 10.1186/1471-2229-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunkar R, Zhu J-K. Novel and stress-regulated microRNAs and other small RNAs from Arabidopsis. Plant Cell. 2004;16:2001–2019. doi: 10.1105/tpc.104.022830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunkar R, Zhu J-K. Micro RNAs and short-interfering RNAs in plants. J Integr Plant Biol. 2007;49:817–826. doi: 10.1111/j.1744-7909.2007.00499.x. [DOI] [Google Scholar]
- Sunkar R, Kapoor A, Zhu J-K. Posttranscriptional induction of two Cu/Zn superoxide dismutase genes in Arabidopsis is mediated by downregulation of miR398 and important for oxidative stress tolerance. Plant Cell. 2006;18:2051–2065. doi: 10.1105/tpc.106.041673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunkar R, Li YF, Jagadeeswaran G. Functions of microRNAs in plant stress responses. Trends Plant Sci. 2012;17:196–203. doi: 10.1016/j.tplants.2012.01.010. [DOI] [PubMed] [Google Scholar]
- Taulavuori E, Tahkokorpi M, Laine K, Taulavuori K. Drought tolerance of juvenile and mature leaves of a deciduous dwarf shrub Vaccinium myrtillus L in a boreal environment. Protoplasma. 2010;241:19–27. doi: 10.1007/s00709-009-0096-x. [DOI] [PubMed] [Google Scholar]
- Trindade I, Capitao C, Dalmay T, Fevereiro M, Santos D. miR398 and miR408 are up-regulated in response to water deficit in Medicago truncatula. Planta. 2010;231(3):705–716. doi: 10.1007/s00425-009-1078-0. [DOI] [PubMed] [Google Scholar]
- Turkan I, Bor M, Ozdemir F, Koca H. Differential responses of lipid peroxidation and antioxidants in the leaves of drought-tolerant P. acutifolius Gray and drought-sensitive P. vulgaris L subjected to polyethylene glycol mediated water stress. Plant Sci. 2005;168(1):223–231. doi: 10.1016/j.plantsci.2004.07.032. [DOI] [Google Scholar]
- Turner IM. Sclerophylly: primarily protective. Funct Ecol. 1994;8(6):669–675. doi: 10.2307/2390225. [DOI] [Google Scholar]
- Urano K, Kurihara Y, Seki M, Shinozaki K. ‘Omics’ analyses of regulatory networks in plant abiotic stress responses. Curr Opin Plant Boil. 2010;13(2):132–138. doi: 10.1016/j.pbi.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Van Breusegem F, Slooten L, Stassart JM, Moens T, Botterman J, Van Montagu M, Inze D. Overproduction of Arabidopsis thaliana FeSOD confers oxidative stress tolerance to transgenic maize. Plant Cell Physiol. 1999;40(5):515–523. doi: 10.1093/oxfordjournals.pcp.a029572. [DOI] [PubMed] [Google Scholar]
- Wang FZ, Wang QB, Kwon SY, Kwak SS, Su WA. Enhanced drought tolerance of transgenic rice plants expressing a pea manganese superoxide dismutase. J Plant Physiol. 2005;162(4):465–472. doi: 10.1016/j.jplph.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Wendel JF, Weeden NF (1989) Visualization and interpretation of plant isozymes. In: Soltis DE, Soltis PE (eds) Isoenzymes in plant biology. dioscorides, Oregon, pp 5–45
- Xu Z, Zhou G. Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. J Exp Bot. 2008;59(12):3317–3325. doi: 10.1093/jxb/ern185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K, Shinozaki K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu Rev Plant Biol. 2006;57:781–803. doi: 10.1146/annurev.arplant.57.032905.105444. [DOI] [PubMed] [Google Scholar]
- Zhao B, Liang R, Ge L, Li W, Xiao H, Lin H, Ruan K, Jin Y. Identification of drought-induced microRNAs in rice. Biochem Biophys Res Commun. 2007;354:585–590. doi: 10.1016/j.bbrc.2007.01.022. [DOI] [PubMed] [Google Scholar]
- Zhou L, Liu Y, Liu Z, Kong D, Duan M, Luo L. Genome-wide identification and analysis of drought-responsive microRNAs in Oryza sativa. J Exp Bot. 2010;61(15):4157–4168. doi: 10.1093/jxb/erq237. [DOI] [PubMed] [Google Scholar]