Abstract
Low temperature during germination and early seedling growth is one of the most significant limiting factors in the productivity of plants. Tomato seedling germination is sensitive to chilling stress. Gamma-aminobutyric acid (GABA), as a non-protein amino acid, involved in various stress tolerances in plants. In this study, 5-day old tomato seedlings were exposed to chilling stress (2 ± 0.05 °C for 48 h) and then the effects of 0, 100, 250, 500 and 750 μmolL−1 concentrations of GABA on electrolyte leakage, proline and malondialdehyde (MDA) content were investigated. The resultS showed that the antioxidant enzyme activity, electrolyte leakage, MDA and proline content were significantly reduced by GABA treatments. However under chilling stress seedlings treated with GABA exhibited significantly higher sugar and proline contents as compared to un-treated seedlings. These results suggest that GABA treatment protects tomato seedlings from chilling stress by enhancing some antioxidant enzymes activity and reducing MDA content which results in maintaining membrane integrity.
Keywords: Tomato seedling, Gamma-aminobutyric acid, Chilling stress, Antioxidant enzyme
Introduction
The temperature on the Earth’s surface is very different, changing during the seasons as well as during the day and night. In plants chilling temperature usually ranges from 0 to 15 °C (Guan et al. 2009; Malekzadeh et al. 2012). Low temperature during germination and early seedling growth is one of the most significant limiting factors in the productivity of plants (Aghdam et al. 2012) and induces considerable changes in biochemistry and physiology of plants such as damage to membranes, generation of reactive oxygen species (ROS), protein denaturation and accumulation of toxic compounds at various organizational levels of the cells (Nayyar et al. 2005). Antioxidant capacity increases during cold acclimation in several plants as an adaptive mechanism to low temperature (Foyer and Noctor 2005; Aroca et al. 2001). Malondialdehyde (MDA) is produced when polyunsaturated fatty acids in the membrane undergo peroxidation (Foyer and Noctor 2005). Thus, MDA is usually considered to be an indicator of plant oxidative stress (Hodges et al. 1999) and the structural integrity of the membranes in plants subjected to low temperatures (Posmyk et al. 2005).
Tomato (Lycopersicon esculentum) is a warm season crop that grows best in warm days of 25 to 28 °C, and stops growing when the temperature is lower than 5 to 13 °C (Aghdam et al. 2012). Chilling injury in tomato is a common problem in Iran, especially in Azerbaijan state. Exposure to temperatures below 13 °C may inhibit fruit-set (Atherton and Rudich 1986), while extended exposure to temperatures below 6 °C can kill tomato plants.
Gamma-aminobutyric acid (GABA) is a non-protein amino acid whose intracellular levels are typically low in plants, for instance, tomato plants do not naturally accumulate GABA (Malekzadeh et al. 2012). However, GABA can be greatly and rapidly accumulated and involved in responses to drought, salt and low temperature stresses (Mazzucotelli et al. 2006; Xing et al. 2007; Yang et al. 2011). Moreover, GABA synthesis system functions as a pH-stat, regulating H+ in cytosol (Sawaki et al. 2009). Furthermore, GABA can be applied exogenously to plants as a solution. Exogenous GABA could alleviate oxidative damage caused by aluminum and proton stresses on barley seedlings (Song et al. 2010). Also, Shang et al. (2011) showed that exogenous application of GABA can reduce chilling stress in peach fruit. Results of a new research showed that exogenous GABA can mitigate chilling stress in wheat seedlings (Malekzadeh et al. 2012). However, little information is available about the role of exogenous GABA as a plant growth regulator; also, the mechanism by which GABA increases stress tolerance is poorly understood.
Our study includes effects of different concentrations of exogenous GABA in chilling stressed tomato seedlings and comparing its effect with normal seedlings which were not under chilling stress and also did not receive GABA. In fact, we compared normal seedlings (control) as an indicator with under stressed seedling which did not receive GABA and other seedlings that receive different concentration of exogenous GABA.
Thus, this study was undertaken to evaluate (a) the effects of different concentrations of exogenous GABA treatment on chilling injury and biochemical metabolism in tomato seedling under chilling stress and (b) to elucidate the underlying mechanism by which GABA alleviated the damage caused by chilling stress.
Materials and methods
Plant material and growth conditions
Seeds of tomato (Lycopersicon esculentum CV. Moneymaker) were disinfected in 1 % sodium hypochlorite solution for 10 min to eliminate possible seed-borne microorganisms, rinsed for 1 min under running water and then were dried for 30 min at room temperature. For germination, seeds were soaked in distilled water for 2 h and then placed in a petri dish with moist filter paper and kept in the dark for 24 h at 22–24 °C. Germinated seeds were transferred onto a mesh tray floating in a continuously aerated Hoagland nutrient solution (Hoagland and Arnon 1950). Seedlings were kept in dark at 22–24 °C for 24 h and then transferred to a growth chamber at 24 ± 2 °C with a 12/12 h light/dark photoperiod. Hoagland nutrient solution applied to the seedlings replaced daily. Five-day old seedlings were exposed to different concentrations of GABA (0, 100, 250, 500 and 750 μmol L−1) (Table 1). Each treatment contained three replicates of 15 seedlings and the entire experiment repeated twice. All plants were subjected to chilling stress at 2 ± 0.5 °C for 48 h under same light regime as mentioned above 3 days after spray application of GABA. All plants were watered 2 h prior to and after the chilling stress to determine the extent of chilling injury.
Table 1.
Treatments applied to 5-day-old tomato seedlings
| No | Chilling stress | GABA (μmol L−1) |
|---|---|---|
| Control | – | 0 (control) |
| 0 | + | 0 |
| 1 | + | 100 |
| 2 | + | 250 |
| 3 | + | 500 |
| 4 | + | 750 |
(−): not chilling treated seedlings, (+): chilling treated seedlings
Determination of electrolyte leakage, proline, soluble sugar and malondealdehyde content
Electrolyte leakage (EL) was measured by method of Jiang et al. (2001). Proline was quantified by using ninhydrin reagent and measured according to (Bates et al. 1973). Lipid peroxidation was measured using 2-thiobarbituric acid (TBA) reaction (Heath and Packer 1968). The concentration of soluble sugar in seedlings was measured by anthrone colorimetric method (Li et al. 2000).
Determination of antioxidant enzyme activity and H2O2 content
The level of H2O2 was determined according to method of Chen et al. (2010). Superoxide dismutase (SOD) activity was assayed by measuring its ability to inhibit the photochemical reduction of nitro blue tetrazolium chloride (NBT) using the method of Posmyk et al. (2005). Catalase (CAT) activity was assayed by measuring the rate of disappearance of hydrogen peroxide (Hernandez et al. 2001). Ascorbate peroxidase (APX) activity was determined according to the method of Chen et al. (2010).
Determination of endogenous GABA content
GABA concentration was determined by method described by Guijin and Bown (1997). The absorbance at 340 nm was monitored before and after adding α-ketoglutarate for 10 min at room temperature using a Genesys 5 spectrophotometer (Spectronic Instruments, Waltham, MA).
Statistical analysis
Experiments were performed using a completely randomized design. Each treatment contained three replicates of 15 seedlings and the entire experiment was repeated twice. All statistical analyses were performed with SPSS (version 16). Data were analyzed by one-way analysis of variance (ANOVA). Mean separations were performed by Duncan’s multiple range tests. Differences at P < 0.05 were considered as significant.
Results and discussion
Effects of exogenous GABA on electrolyte leakage, soluble sugar, MDA and proline contents
Chilling injury involves in membrane damage, and can be measured indirectly by electrolyte leakage. Electrolyte leakage (EL) reflects the damage of stresses to the plasma lemma. Zhao et al. (2009) has found that the coefficient between chilling injury and electrolyte leakage was higher irrespective of differences in chilling susceptibility between tomato cultivars. In this study, electrolyte leakage was significantly reduced in GABA treated seedlings (Table 2; p < 0.05). Under chilling stress the EL amount in tomato seedlings increased with treatment time. Although the EL increased in GABA treated seedlings under chilling stress, the magnitude of this increase was less than seedlings not treated with GABA (Table 2). Results indicated that tomato seedlings treated by 500 and 750 μmolL−1 GABA underwent less chilling damage in comparison with 0 and 100 μmolL-1 of GABA. Based on the above results, it may be concluded that exogenous GABA can help to reduce chilling stress effects in tomato seedlings by keeping the stability of membrane. As shown in Table 2, the MDA content increased significantly (p < 0.05) both in GABA treated and seedlings not treated with GABA under chilling stress compared with control plants. The amount of MDA in chilling-stressed seedlings was significantly (P < 0.05) increased to 9.6867 in seedlings not treated with GABA and to 6.550 in 750 μmol L−1 concentration of GABA compared to control plants. Although the MDA concentration increased in GABA treated seedlings, the magnitude of this increase was less than seedlings not treated with GABA (Table 2). It seems that GABA decreased the accumulation of lipid peroxidation product, MDA, which is regarded as an indicator of the loss of structural integrity in membranes subjected to chilling stress (Posmyk et al. 2005).
Table 2.
Effect of exogenous GABA on MDA, proline, soluble sugar and electrolyte leakage in tomato seedlings under chilling stress
| Chilling stress | GABA concentration (μmolL−1) | MDA content (μmolg−1FW) | Proline content (μmolg−1FW) | Soluble sugar (mg g−1FW) | Electrolyte leakage (%) |
|---|---|---|---|---|---|
| – | Control | 5.3267 ± 1.2 a | 17.51 ± 3.5 a | 8.69 ± 0.46 a | 30.66 ± 3.51 a |
| + | 0 | 9.6867 ± 1.3 d | 86.15 ± 10.7 d | 22.15 ± 2.03 d | 73.66 ± 8.02 c |
| + | 100 | 8.6833 ± 0.92 cd | 53.66 ± 11c | 18.83 ± 1.30 c | 59.33 ± 1.52 b |
| + | 250 | 8.9000 ± 0.30 cd | 45.40 ± 4 c | 15.76 ± 1.16 b | 54.66 ± 3.78 b |
| + | 500 | 7.3867 ± 0.93 bc | 22.06 ± 3 ab | 10.60 ± 2.16 a | 35.66 ± 2.08 a |
| + | 750 | 6.5500 ± 0.47 ab | 32.00 ± 4 b | 11.23 ± 1.40 a | 33.00 ± 2.64 a |
| Significance | * | * | * | * | |
Mean values ± Standard Error (SE), Values in the column followed by the different letters (a, b,c and d) in the same column indicate significant difference (P < 0.05) according to Duncan’s Multiple Range Test analysis. Combined letters (ab, cd and bc) in the same column indicate that rows with similar letters are not significant (P < 0.05)
(*): Significance at 0.05 levels
(−): not chilling treated seedlings, (+): chilling treated seedlings
It was observed that proline amount increased in tomato seedlings after chilling stress (86.15 μmolg-1FW compared to control 17.1 μmolg-1FW). Although GABA treatments caused a slight reduction in the proline accumulation it was maintained at levels higher to that observed in control plants growing at normal temperatures (Table 2). Guan et al. (2009) found that under chilling stress there was an increase in proline content in maize seedlings. In addition, the primary response of chilling stressed tomato plants was osmotic adjustment through proline accumulation, which is well established in many plant species (Malekzadeh et al. 2012). It seems that exogenous GABA treatment partly reduced sensitivity of tomato seedling to chilling stress by increasing proline content. Soluble sugar increased during chilling stress compared with control seedlings, but soluble sugar content significantly reduced in presence of exogenous GABA (Table 2; p < 0. 05). The same trend was observed by Yadegari et al. (2007) who found that under chilling stress, the soluble sugar content in soybean seedlings would increase. Accumulation of soluble sugar increases the resistance to chilling stress in plants (Malekzadeh et al. 2012). Our results may suggest that applying exogenous GABA can protect tomato seedlings against chilling stress; therefore, less soluble sugar might produce in GABA treated seedlings.
Effects of exogenous GABA on antioxidant enzymes and H2O2 content
The content of H2O2 was higher in tomato seedlings under chilling stress in comparison with control (Table 3; P < 0.05). The level of H2O2 content in tomato seedlings treated with GABA was lower (P < 0.05) than seedlings not treated with GABA. Results of this study indicated that chilling stress had different effects on the activity of antioxidant enzymes. Chilling had no effect on APX activity in tomato seedling, while it increased activity of CAT and SOD (Table 3). CAT activity was increased both in GABA treated and tomato seedlings not treated with GABA under chilling stress; though, CAT activity was lower in GABA treated seedlings than seedlings not treated with GABA (Table 3). Also similar changes in SOD activity were found in tomato seedlings under chilling stress both in GABA treated and seedlings not treated with GABA. However, the results did not show any significant difference between APX activity both in GABA treated and not-treated tomato seedlings (Table 3). Based on Table 3, chilling stress increased the levels of H2O2, while exogenous GABA supplementation reduced increase in H2O2 amount. This suggests that exogenous GABA supplementation decreases the accumulation of H2O2 and thereby reduces lipid peroxidation in chilling-stressed seedlings. In this study, the high levels of SOD and CAT observed in GABA-treated tomato seedling compared with seedlings not treated with GABA under chilling stress suggest that GABA treatment induces the activity of antioxidant enzymes in tomato seedling. Similar results were reported in pea leaves under chilling stress (Hernandez et al. 2001).
Table 3.
Effect of exogenous GABA on H2O2 content, CAT, SOD and APX activities in tomato seedlings under chilling stress
| Chilling stress | GABA concentration (μmolL−1) | H2O2 content (μmol·g−1 F) | CAT activity (U·g−1FW) | SOD activity (U·g−1FW) | APX activity (U·g−1FW) |
|---|---|---|---|---|---|
| – | Control | 3.50 ± 028 a | 2.93 ± 0.28 a | 24.54 ± 6.8 a | 2.89 ± 1.05 |
| + | 0 | 9.97 ± 1.08 c | 5.56 ± 0.6 b | 41.01 ± 7.6 c | 4.40 ± 0.91 |
| + | 100 | 7.76 ± 0.49 c | 7.44 ± 0.81 c | 35.64 ± 3.3 bc | 3.93 ± 0.90 |
| + | 250 | 6.09 ± 0.46 b | 7.87 ± 0.69 c | 31.90 ± 3.2 abc | 4.09 ± 0.62 |
| + | 500 | 4.26 ± 0.45 a | 14.59 ± 0.43 e | 31.40 ± 3.1 ab | 3.34 ± 0.71 |
| + | 750 | 4.13 ± 1.00 a | 12.21 ± 1.1 d | 26.73 ± 2.9 ab | 3.66 ± 0.58 |
| Significance | * | * | * | ns | |
Mean values ± Standard Error (SE), Values in the column followed by the different letters (a, b,c and d) in the same column indicate significant difference (P < 0.05) according to Duncan’s Multiple Range Test analysis. Combined letters (ab, cd and bc) in the same column indicate that rows with similar letters are not significant (P < 0.05)
(ns): non-significance at P < 0.05, (*): Significance at 0.05 levels
(−): not chilling treated seedlings, (+): chilling treated seedlings
Effects of exogenous GABA treatment on endogenous GABA content
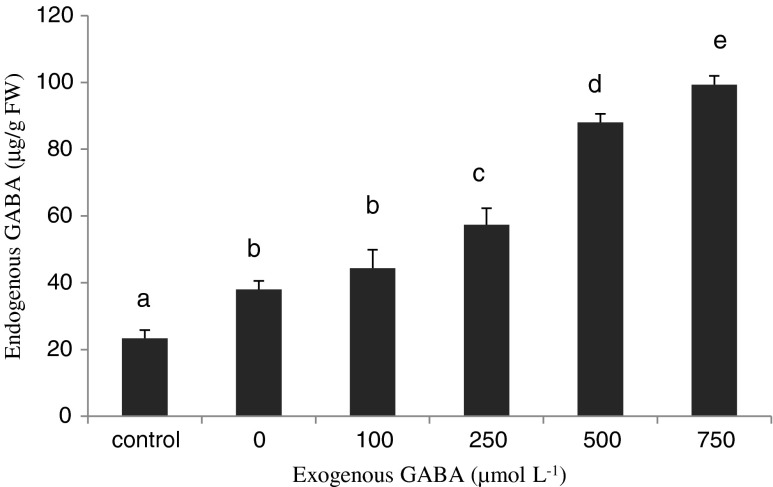
Many studies have demonstrated the GABA accumulation in plants in response to environmental stresses (Kinnersley and Turano 2000; Song et al. 2010). In the present study, the accumulation of GABA was also observed in tomato seedling under chilling stress. Exogenous GABA treatment induced a larger amount of endogenous GABA in GABA-treated tomato seedlings than the control plants (Fig. 1). As per Fig. 1, the amount of endogenous GABA in control seedlings is 23.33 μg/g FW and in seedlings not treated with GABA chilling stress induced endogenous GABA to 38 μg/g FW, while exogenous application of GABA increased endogenous GABA content to 99.33 μg/g FW in 750 μmol L−1 in tomato seedlings. It seems that the higher amount of endogenous GABA is due to uptake of exogenous GABA. Thus, it may be concluded that the higher level of endogenous GABA is one of the major factors that trigger the chilling resistance in tomato seedlings when treated with exogenous GABA.
Fig. 1.
Effect of exogenous GABA on endogenous GABA in tomato seedlings under chilling stress. Different letters in the same column indicate statistically significant differences (p < 0.05)
In conclusion, the present study has proven that the beneficial effects of GABA on reducing chilling stress are valid also in tomato seedling under low temperature. Our results suggest that the growth of tomato seedlings under chilling stress includes an increase in antioxidant enzyme activity and increase of osmolyte such as proline and soluble sugar content and loss of membrane integrity. Consequently, GABA can be applied in order to reduce chilling stress effect in tomato seedlings during low temperature. Due to useful effects of GABA, future studies are required to clarify its effect on fruit-set and post-harvest in different fruits.
Contributor Information
Parviz Malekzadeh, Phone: +98-914-8022362, FAX: +98-426-2232457, Email: p.malekzadeh@urmia.ac.ir, Email: par_malek@yahoo.com.
Jalil Khara, Email: jkhara@yahoo.com.
Reza Heydari, Email: r.heydari@urmia.ac.ir.
References
- Aghdam MS, Asghari M, Farmani B, Mohayeji M, Moradbeygi H. Impact of postharvest brassinosteroids treatment on PAL activity in tomato fruit in response to chilling stress. Sci Hortic. 2012;144:116–120. doi: 10.1016/j.scienta.2012.07.008. [DOI] [Google Scholar]
- Aroca R, Tognoni F, Irigoyen JJ, Sánchez-Díaz M, Pardossi A. Different root low temperature response of two maize genotypes differing in chilling sensitivity. Plant Physiol Biochem. 2001;39(12):1067–1073. doi: 10.1016/S0981-9428(01)01335-3. [DOI] [Google Scholar]
- Atherton JG, Rudich J. The tomato crop: a scientific basis for improvement. England: Chapman and Hall London; 1986. [Google Scholar]
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39(1):205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- Chen YP, Jia JF, Yue M. Effect of CO2 laser radiation on physiological tolerance of wheat seedlings exposed to chilling stress. Photochem Photobiol. 2010;86(3):600–605. doi: 10.1111/j.1751-1097.2010.00723.x. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell. 2005;17(7):1866–1875. doi: 10.1105/tpc.105.033589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan YJ, Hu J, Wang XJ, Shao CX. Seed priming with chitosan improves maize germination and seedling growth in relation to physiological changes under low temperature stress. J Zhejiang Univ Sci B. 2009;10(6):427–433. doi: 10.1631/jzus.B0820373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guijin Z, Bown AW. The rapid determination of γ-aminobutyric acid. Phytochemistry. 1997;44(6):1007–1009. doi: 10.1016/S0031-9422(96)00626-7. [DOI] [Google Scholar]
- Heath RL, Packer L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125(1):189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Hernandez JA, Ferrer MA, Jimenez A, Barcelo AR, Sevilla F. Antioxidant systems and O2.-/H2O2 production in the apoplast of pea leaves. Its relation with salt-induced necrotic lesions in minor veins. Plant Physiol. 2001;127(3):817–831. doi: 10.1104/pp.010188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoagland D, Arnon D. The water-culture method for growing plants without soil. Circular 347. Berkeley: University of California; 1950. [Google Scholar]
- Hodges DM, DeLong JM, Forney CF, Prange RK. Improving the thiobarbituric acid-reactivesubstances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta. 1999;207(4):604–611. doi: 10.1007/s004250050524. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Shiina T, Nakamura N, Nakahara A. Electrical conductivity evaluation of postharvest strawberry damage. J Food Sci. 2001;66(9):1392–1395. doi: 10.1111/j.1365-2621.2001.tb15220.x. [DOI] [Google Scholar]
- Kinnersley AM, Turano FJ. Gamma aminobutyric acid (GABA) and plant responses to stress. Crit Rev Plant Sci. 2000;19(6):479–509. doi: 10.1016/S0735-2689(01)80006-X. [DOI] [Google Scholar]
- Li H, Sun Q, Zhao S. Principles and techniques of plant physiological biochemical experiment. Beijing: Higher Education; 2000. pp. 186–191. [Google Scholar]
- Malekzadeh P, Khara J, Heidari R (2012) Effect of exogenous Gama-aminobutyric acid on physiological tolerance of wheat seedlings exposed to chilling stress. Iran J Plant Physiol 3 (1)
- Mazzucotelli E, Tartari A, Cattivelli L, Forlani G. Metabolism of γ-aminobutyric acid during cold acclimation and freezing and its relationship to frost tolerance in barley and wheat. J Exp Bot. 2006;57(14):3755–3766. doi: 10.1093/jxb/erl141. [DOI] [PubMed] [Google Scholar]
- Nayyar H, Chander K, Kumar S, Bains T. Glycine betaine mitigates cold stress damage in Chickpea. Agron Sustain Dev. 2005;25(3):381–388. doi: 10.1051/agro:2005033. [DOI] [Google Scholar]
- Posmyk MM, Bailly C, Szafranska K, Janas KM, Corbineau F. Antioxidant enzymes and isoflavonoids in chilled soybean (Glycine max (L.) Merr.) seedlings. J Plant Physiol. 2005;162(4):403–412. doi: 10.1016/j.jplph.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Sawaki Y, Iuchi S, Kobayashi Y, Ikka T, Sakurai N, Fujita M, Shinozaki K, Shibata D, Kobayashi M, Koyama H. STOP1 regulates multiple genes that protect arabidopsis from proton and aluminum toxicities. Plant Physiol. 2009;150(1):281–294. doi: 10.1104/pp.108.134700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang H, Cao S, Yang Z, Cai Y, Zheng Y. Effect of exogenous gamma-aminobutyric acid treatment on proline accumulation and chilling injury in peach fruit after long-term cold storage. J Agric Food Chem. 2011;59(4):1264–1268. doi: 10.1021/jf104424z. [DOI] [PubMed] [Google Scholar]
- Song H, Xu X, Wang H, Wang H, Tao Y. Exogenous γ–aminobutyric acid alleviates oxidative damage caused by aluminium and proton stresses on barley seedlings. J Sci Food Agric. 2010;90(9):1410–1416. doi: 10.1002/jsfa.3951. [DOI] [PubMed] [Google Scholar]
- Xing SG, Jun YB, Hau ZW, Liang LY. Higher accumulation of γ-aminobutyric acid induced by salt stress through stimulating the activity of diamine oxidases in Glycine max (L.) Merr. roots. Plant Physiol Biochem. 2007;45(8):560–566. doi: 10.1016/j.plaphy.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Yadegari LZ, Heidari R, Carapetian J. The influence of cold acclimation on proline, malondialdehyde (MDA), total protein and pigments contents in soybean (Glycine max) seedlings. J Biol Sci. 2007;7(8):1436–1141. doi: 10.3923/jbs.2007.1436.1441. [DOI] [Google Scholar]
- Yang A, Cao S, Yang Z, Cai Y, Zheng Y. γ-Aminobutyric acid treatment reduces chilling injury and activates the defence response of peach fruit. Food Chem. 2011;129(4):1619–1622. doi: 10.1016/j.foodchem.2011.06.018. [DOI] [Google Scholar]
- Zhao D, Shen L, Fan B, Liu K, Yu M, Zheng Y, Ding Y, Sheng J. Physiological and genetic properties of tomato fruits from 2 cultivars differing in chilling tolerance at cold storage. J Food Sci. 2009;74(5):C348–C352. doi: 10.1111/j.1750-3841.2009.01156.x. [DOI] [PubMed] [Google Scholar]