Abstract
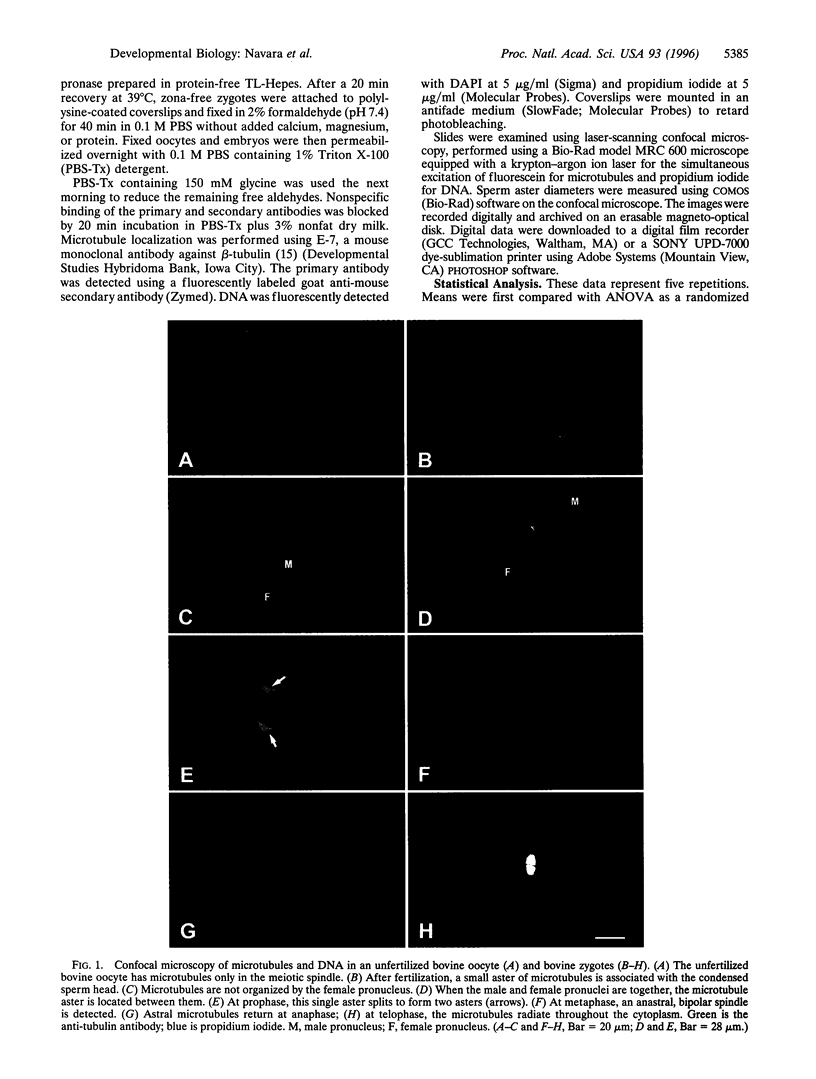
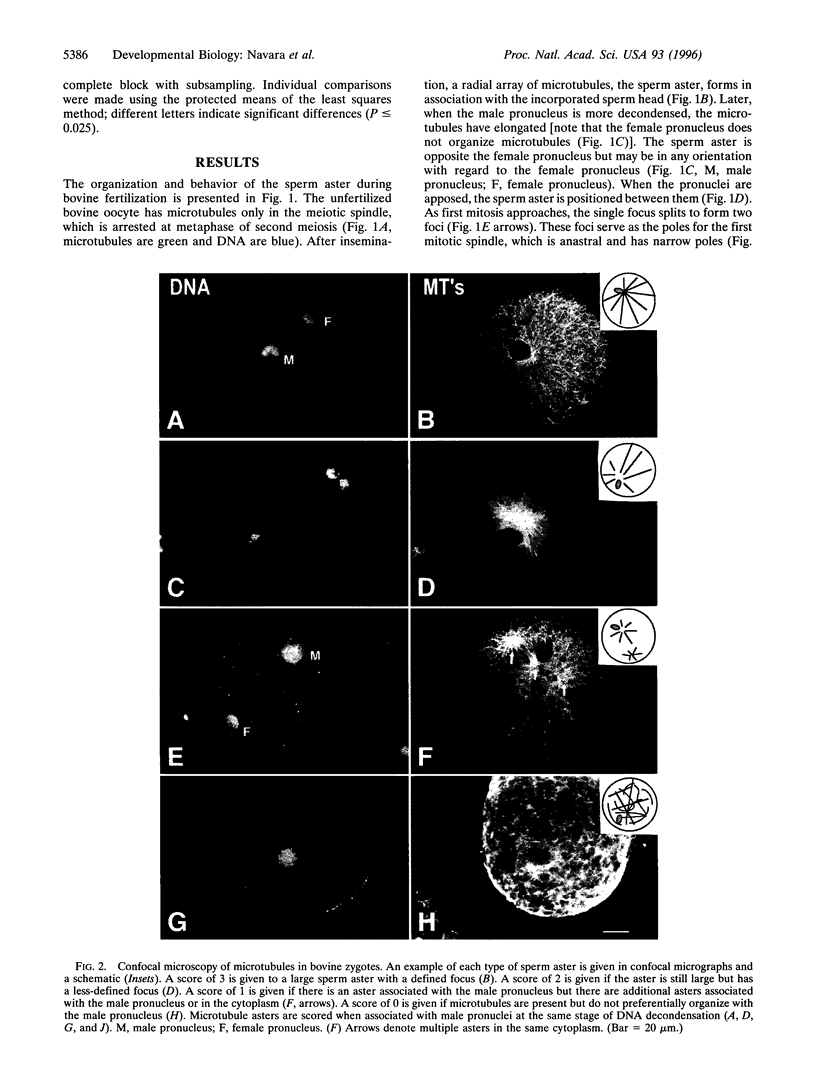
This study describes a paternal effect on sperm aster size and microtubule organization during bovine fertilization. Immunocytochemistry using tubulin antibodies quantitated with confocal microscopy was used to measure the diameter of the sperm aster and assign a score (0-3) based on the degree of radial organization (0, least organized; 3, most organized). Three bulls (A-C) were chosen based on varying fertility (A, lowest fertility; C, highest fertility) as assessed by nonreturn to estrus after artificial insemination and in vitro embryonic development to the blastocyst stage. The results indicate a statistically significant bull-dependent difference in diameter of the sperm aster and in the organization of the sperm astral microtubules. Insemination from bull A resulted in an average sperm aster diameter of 101.4 microm (76.3% of oocyte diameter). This significantly differs (P < or = 0.0001) from the average sperm aster diameters produced after inseminations from bull B (78.2 microm; 60.8%) or bull C (77.9 microm; 57.8%), which themselves displayed no significant differences. The degree of radial organization of the sperm aster was also bull-dependent. Sperm asters organized by bull A-derived sperm had an average quality score of 1.8, which was higher than that of bull B (1.4; P < or = 0.0005) or bull C (1.2; P < or = 0.0001). Results with bulls B and C were also significantly different (P < or = 0.025). These results indicate that the paternally derived portion of the centrosome varies among males and that this variation affects male fertility, the outcome of early development, and, therefore, reproductive success.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bavister B. D., Leibfried M. L., Lieberman G. Development of preimplantation embryos of the golden hamster in a defined culture medium. Biol Reprod. 1983 Feb;28(1):235–247. doi: 10.1095/biolreprod28.1.235. [DOI] [PubMed] [Google Scholar]
- Brinkley B. R. Microtubule organizing centers. Annu Rev Cell Biol. 1985;1:145–172. doi: 10.1146/annurev.cb.01.110185.001045. [DOI] [PubMed] [Google Scholar]
- Chu D. T., Klymkowsky M. W. The appearance of acetylated alpha-tubulin during early development and cellular differentiation in Xenopus. Dev Biol. 1989 Nov;136(1):104–117. doi: 10.1016/0012-1606(89)90134-6. [DOI] [PubMed] [Google Scholar]
- Fukui Y., Glew A. M., Gandolfi F., Moor R. M. Ram-specific effects on in-vitro fertilization and cleavage of sheep oocytes matured in vitro. J Reprod Fertil. 1988 Jan;82(1):337–340. doi: 10.1530/jrf.0.0820337. [DOI] [PubMed] [Google Scholar]
- Félix M. A., Antony C., Wright M., Maro B. Centrosome assembly in vitro: role of gamma-tubulin recruitment in Xenopus sperm aster formation. J Cell Biol. 1994 Jan;124(1-2):19–31. doi: 10.1083/jcb.124.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard D. L. Gamma-tubulin is asymmetrically distributed in the cortex of Xenopus oocytes. Dev Biol. 1994 Jan;161(1):131–140. doi: 10.1006/dbio.1994.1015. [DOI] [PubMed] [Google Scholar]
- Joshi H. C., Palacios M. J., McNamara L., Cleveland D. W. Gamma-tubulin is a centrosomal protein required for cell cycle-dependent microtubule nucleation. Nature. 1992 Mar 5;356(6364):80–83. doi: 10.1038/356080a0. [DOI] [PubMed] [Google Scholar]
- Kirschner M., Mitchison T. Beyond self-assembly: from microtubules to morphogenesis. Cell. 1986 May 9;45(3):329–342. doi: 10.1016/0092-8674(86)90318-1. [DOI] [PubMed] [Google Scholar]
- Kuriyama R., Borisy G. G., Masui Y. Microtubule cycles in oocytes of the surf clam, Spisula solidissima: an immunofluorescence study. Dev Biol. 1986 Mar;114(1):151–160. doi: 10.1016/0012-1606(86)90391-x. [DOI] [PubMed] [Google Scholar]
- Long C. R., Pinto-Correia C., Duby R. T., Ponce de Leon F. A., Boland M. P., Roche J. F., Robl J. M. Chromatin and microtubule morphology during the first cell cycle in bovine zygotes. Mol Reprod Dev. 1993 Sep;36(1):23–32. doi: 10.1002/mrd.1080360105. [DOI] [PubMed] [Google Scholar]
- Mazia D. Centrosomes and mitotic poles. Exp Cell Res. 1984 Jul;153(1):1–15. doi: 10.1016/0014-4827(84)90442-7. [DOI] [PubMed] [Google Scholar]
- Navara C. S., First N. L., Schatten G. Microtubule organization in the cow during fertilization, polyspermy, parthenogenesis, and nuclear transfer: the role of the sperm aster. Dev Biol. 1994 Mar;162(1):29–40. doi: 10.1006/dbio.1994.1064. [DOI] [PubMed] [Google Scholar]
- Parrish J. J., Susko-Parrish J. L., First N. L. Effect of heparin and chondroitin sulfate on the acrosome reaction and fertility of bovine sperm in vitro. Theriogenology. 1985 Nov;24(5):537–549. doi: 10.1016/0093-691x(85)90060-3. [DOI] [PubMed] [Google Scholar]
- Parrish J. J., Susko-Parrish J. L., Leibfried-Rutledge M. L., Critser E. S., Eyestone W. H., First N. L. Bovine in vitro fertilization with frozen-thawed semen. Theriogenology. 1986 Apr;25(4):591–600. doi: 10.1016/0093-691x(86)90143-3. [DOI] [PubMed] [Google Scholar]
- Schatten G. The centrosome and its mode of inheritance: the reduction of the centrosome during gametogenesis and its restoration during fertilization. Dev Biol. 1994 Oct;165(2):299–335. doi: 10.1006/dbio.1994.1256. [DOI] [PubMed] [Google Scholar]
- Schatten H., Schatten G., Mazia D., Balczon R., Simerly C. Behavior of centrosomes during fertilization and cell division in mouse oocytes and in sea urchin eggs. Proc Natl Acad Sci U S A. 1986 Jan;83(1):105–109. doi: 10.1073/pnas.83.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu H. B., Joshi H. C. Gamma-tubulin can both nucleate microtubule assembly and self-assemble into novel tubular structures in mammalian cells. J Cell Biol. 1995 Sep;130(5):1137–1147. doi: 10.1083/jcb.130.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly C., Wu G. J., Zoran S., Ord T., Rawlins R., Jones J., Navara C., Gerrity M., Rinehart J., Binor Z. The paternal inheritance of the centrosome, the cell's microtubule-organizing center, in humans, and the implications for infertility. Nat Med. 1995 Jan;1(1):47–52. doi: 10.1038/nm0195-47. [DOI] [PubMed] [Google Scholar]
- Sirard M. A., Parrish J. J., Ware C. B., Leibfried-Rutledge M. L., First N. L. The culture of bovine oocytes to obtain developmentally competent embryos. Biol Reprod. 1988 Oct;39(3):546–552. doi: 10.1095/biolreprod39.3.546. [DOI] [PubMed] [Google Scholar]
- Sluder G., Miller F. J., Lewis K., Davison E. D., Rieder C. L. Centrosome inheritance in starfish zygotes: selective loss of the maternal centrosome after fertilization. Dev Biol. 1989 Feb;131(2):567–579. doi: 10.1016/s0012-1606(89)80027-2. [DOI] [PubMed] [Google Scholar]
- Stearns T., Evans L., Kirschner M. Gamma-tubulin is a highly conserved component of the centrosome. Cell. 1991 May 31;65(5):825–836. doi: 10.1016/0092-8674(91)90390-k. [DOI] [PubMed] [Google Scholar]
- Stearns T., Kirschner M. In vitro reconstitution of centrosome assembly and function: the central role of gamma-tubulin. Cell. 1994 Feb 25;76(4):623–637. doi: 10.1016/0092-8674(94)90503-7. [DOI] [PubMed] [Google Scholar]
- Tucker M. J., Chan S. Y. Origins and effects of variations in spermatozoal quality. Int J Fertil Menopausal Stud. 1993 Jul-Aug;38(4):197–209. [PubMed] [Google Scholar]
- Vale R. D. Severing of stable microtubules by a mitotically activated protein in Xenopus egg extracts. Cell. 1991 Feb 22;64(4):827–839. doi: 10.1016/0092-8674(91)90511-v. [DOI] [PubMed] [Google Scholar]
- Wyrobek A. J. Methods and concepts in detecting abnormal reproductive outcomes of paternal origin. Reprod Toxicol. 1993;7 (Suppl 1):3–16. doi: 10.1016/0890-6238(93)90064-e. [DOI] [PubMed] [Google Scholar]