Abstract
Patients with primary immunodeficiency (PID) provide rare opportunities to study the impact of specific gene mutations on the regulation of human B cell tolerance. Alterations in B cell receptor and Toll-like receptor signaling pathways result in a defective central checkpoint and a failure to counterselect developing autoreactive B cells in the bone marrow. In contrast, CD40L- and MHC class II–deficient patients only displayed peripheral B cell tolerance defects, suggesting that decreased numbers of regulatory T cells and increased concentration of B cell activating factor (BAFF) may interfere with the peripheral removal of autoreactive B cells. The pathways regulating B cell tolerance identified in PID patients are likely to be affected in patients with rheumatoid arthritis, systemic lupus erythematosus, and type 1 diabetes who display defective central and peripheral B cell tolerance checkpoints. Indeed, risk alleles encoding variants altering BCR signaling, such as PTPN22 alleles associated with the development of these diseases, interfere with the removal of developing autoreactive B cells. Hence, insights into B cell selection from PID patients are highly relevant to the understanding of the etiology of autoimmune conditions.
Keywords: B cell tolerance, B cell receptor, Toll-like receptors, receptor editing
Introduction
Autoimmune diseases affect about 5% of the population and are often characterized by the production of autoantibodies directed against self-antigens.1 An important role for B cells in autoimmune diseases is demonstrated by the successful treatment of patients with rheumatoid arthritis (RA), type 1 diabetes (T1D), multiple sclerosis (MS), and other autoimmune syndromes with anti-CD20 monoclonal antibodies that eliminate B cells.2–4 However, the underlying mechanisms that account for autoreactive B cells and autoantibody production in autoimmune diseases remain elusive. We developed a method that allows us to analyze the frequency of autoreactive clones in diverse B cell subpopulations by amplifying and cloning immunoglobulin heavy and light chain genes from single B cells. The reactivities of recombinant antibodies are then tested by two ELISA assays detecting polyreactivity and HEp-2 reactivity as well as in indirect immunofluorescence assays on slides coated with HEp-2 cells to detect antinuclear antibodies (ANAs).5–7
We review herein data demonstrating that central B cell tolerance is mostly controlled by intrinsic B cell factors regulating B cell receptor (BCR) and Toll-like receptor (TLR) signaling, whereas peripheral B cell tolerance seems to involve extrinsic B cell factors such as regulatory T (Treg) cells and serum B cell activating factor (BAFF) concentrations.
Central B cell tolerance requires proper BCR signaling
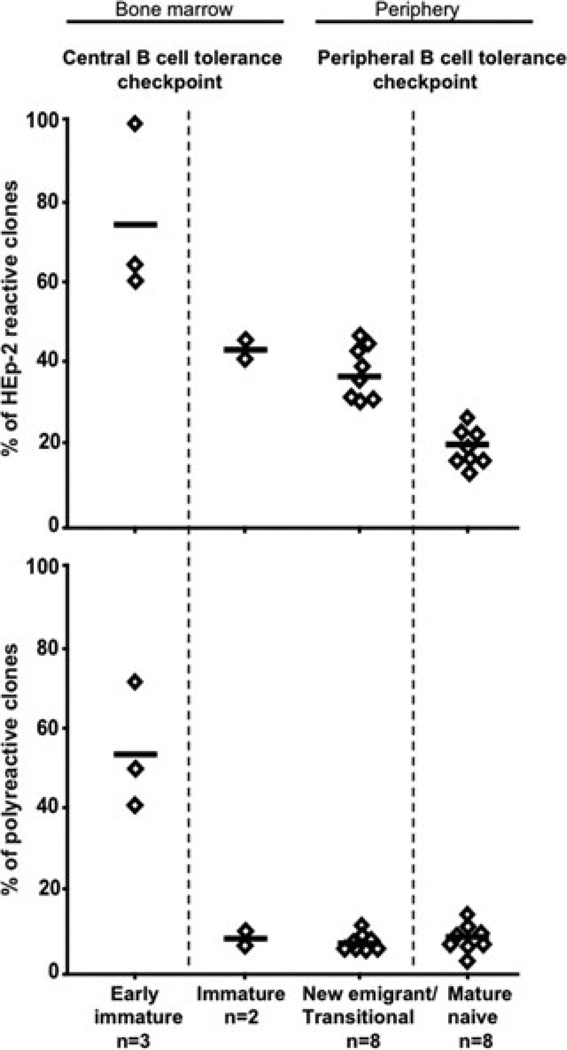
Using a single cell PCR method, we found that random V(D)J joining in healthy donors generated a large number of autoreactive B cells that were removed at two discrete checkpoints.5,8 First, a central checkpoint in the bone marrow between early immature and immature B cells removed most B cells expressing polyreactive and ANAs5 (Fig. 1). Interestingly, we can assess the functionality of the central B cell tolerance checkpoint in a subject without a bone marrow sample simply by following the frequency of polyreactive and antinuclear clones in the new emigrant/transitional B cell compartment in peripheral blood because bone marrow immature B cells and peripheral new emigrant/transitional B cells express similar antibody repertoire and reactivity uninfluenced by proliferation steps.5,9 In addition, the frequencies of polyreactive and antinuclear clones are remarkably similar among the eight analyzed healthy donors who did not carry the PTPN22 risk allele associated to the development of autoimmunity (Fig. 1).10 Indeed, polyreactive clones ranged from 5.0% to 11.1% in new emigrant/transitional B cells from healthy donors, whereas the frequencies of antinuclear new emigrant B cells averaged 1.6% (0%–5.6%), reflecting the proper removal of polyreactive and antinuclear clones in the bone marrow (Figs. 1 and 2).5,10 Hence, increased frequencies of polyreactive and/or antinuclear new emigrant/transitional B cells reflect an abnormal failure to remove autoreactive clones in the bone marrow, thereby revealing a defective central B cell tolerance checkpoint.10 A second checkpoint at which additional autoreactive B cells were removed from the population was detected in the periphery of healthy donors at the transition between new emigrant and mature naive B cells (Fig. 1).5,8 Indeed, anti-HEp-2 frequencies ranged from 30.0% to 46.2% in new emigrant/transitional B cells, which decreased to 16.7–26.3% in the mature naive B cell compartment, potentially reflecting counters-election of some autoreactive immature B cells that encounter peripheral autoantigens not expressed in the bone marrow environment (Fig. 1).5,8
Figure 1.
Early B cell tolerance checkpoints in healthy donors. Single CD34−CD19+CD10+IgM− early immature B cells and CD34−CD19+CD10+IgM+ immature B cells from bone marrow and CD19+CD10+IgM++CD27− new emigrant/transitional and CD19+CD10−IgM+CD27− mature naive B cells from peripheral blood of healthy controls were isolated by flow cytometry based on the indicated surface markers. IgH and IgL chain genes from single purified B cells were cloned, and the monoclonal antibodies were expressed in vitro.7 The frequency of HEp-2 reactive antibodies (top panel) was determined by HEp-2 cell ELISA and indirect immunofluorescence on HEp-2 cells. The frequency of polyreactive antibodies (bottom panel) was determined by ELISA with ssDNA, dsDNA, insulin, and lipopolysaccharide as antigens. Polyreactive antibodies recognized at least two structurally diverse antigens and often all four. Each diamond represents an individual; the average is shown with a bar. The central and peripheral B cell tolerance checkpoints are indicated.
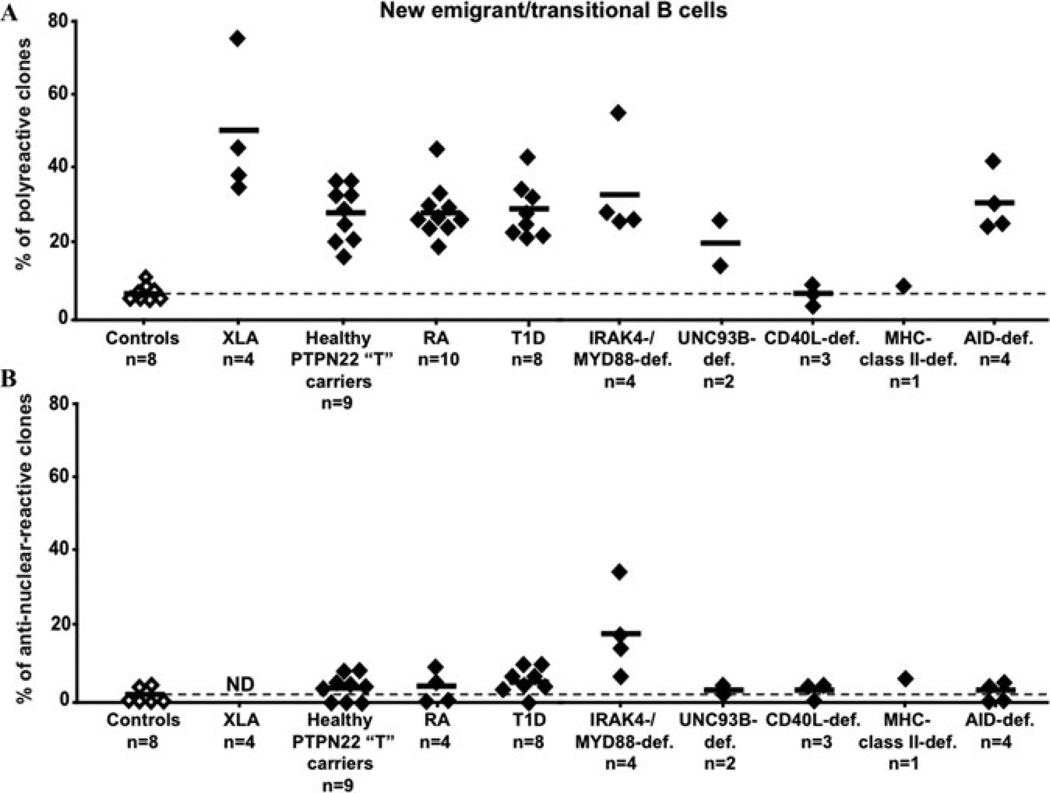
Figure 2.
Central B cell tolerance requires proper BCR and TLR signaling. The frequencies of polyreactive (A) and antinuclear (B) new emigrant/transitional B cells are compared between controls (open diamonds), subjects with the PTPN22 “T” risk allele, patients with diverse PID, rheumatoid arthritis (RA), and type 1 diabetes (T1D) (black diamonds). Alteration in either BCR or TLR signaling results in a failure to counterselect developing autoreactive B cells in the bone marrow and results in increased frequencies of polyreactive new emigrant/transitional B cells. IRAK4- and MYD88-deficient new emigrant/transitional B cells were especially enriched in antinuclear clones, as shown in B.
We conclude that autoreactive B cells generated by random V(D)J recombination are eliminated at two distinct early B cell tolerance checkpoints in healthy donors, first in the bone marrow and then in the periphery.
Central B cell tolerance relies on proper BCR signaling
We analyzed the molecules and pathways that regulate the establishment of human B cell tolerance by studying PID patients. Many mouse models suggest that B cell tolerance is regulated by BCR signaling.11 While it has been postulated that increased BCR signaling led to autoimmunity, new data in mice and humans suggest instead that decreased BCR signaling interfere with autoreactive B cell counterselection at immature B cell stages by failing to induce proper tolerance mechanisms.10,12,13
Indeed, we reported that patients with X-linked agammaglobulinemia (XLA)12 who carry mutations in the BTK gene that encodes an essential BCR signaling component14,15 display a high frequency of autoreactive new emigrant/transitional B cells including antinuclear B cells, demonstrating that BTK and therefore BCR signaling were essential in regulating the central B cell tolerance checkpoint (Fig. 2).12 A major role for BCR signaling in the establishment of human B cell tolerance was further demonstrated by the analysis of healthy individuals carrying the PTPN22 allele encoding an R620W variant associated with the development of many autoimmune diseases including RA, systemic lupus erythematosus (SLE), and T1D.16–19 Current data indicate that the R620W polymorphism in PTPN22/Lyp leads to decreased BCR signaling, which in turn regulates the establishment of human B cell tolerance.10,20,21 Indeed, we recently reported that new emigrant/transitional and mature naive B cells from PTPN22 risk allele carriers contained high frequencies of autoreactive clones compared to noncarrier donors, revealing defective central and peripheral B cell tolerance checkpoints (Figs. 2 and 3).10 Hence, a single PTPN22 risk allele has a dominant effect on altering autoreactive B cell counterselection before any onset of autoimmunity. In addition, similar central and peripheral B cell tolerance defects were also identified in active RA, SLE, and T1D patients, suggesting that these early B cell tolerance defects common to RA, SLE, and T1D may result from specific polymorphisms and precede the onset of these autoimmune diseases (Figs. 2 and 3).10 These data also further suggest that naive autoreactive B cells produced in RA, SLE, and T1D patients before disease onset may promote the development of autoimmunity, potentially by recognizing and presenting self-antigens to T cells.
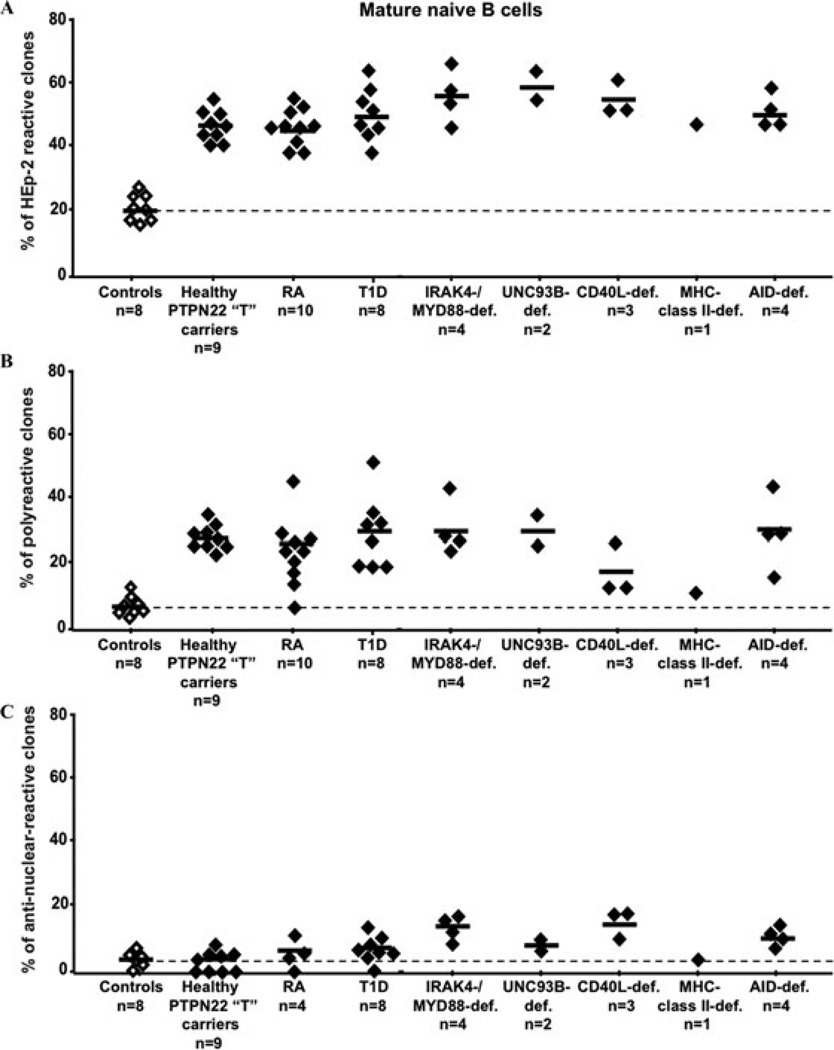
Figure 3.
Specific defective peripheral B cell tolerance checkpoint in CD40L- and MHC class II-deficient patients. The frequencies of HEp-2 reactive (A), polyreactive (B), and antinuclear (C) mature naive B cells are compared between controls (open diamonds), subjects with the PTPN22 “T” risk allele, patients with diverse PID, rheumatoid arthritis (RA), and type 1 diabetes (T1D) (black diamonds). Defects in CD40L expression or antigen presentation through MHC class II molecules specifically either interfere with the removal or fail to prevent the accumulation of autoreactive B cells in the periphery. All other subjects who presented central B cell tolerance defects also display large numbers of autoreactive B cells in their mature naive B cell compartment.
Central B cell tolerance is IRAK4/MYD88 dependent
In addition to their BCRs, B cells also express germline-encoded transmembrane receptors called Toll-like receptors (TLRs) that were originally described to bind microbial components but that are also able to recognize self-antigens.22 All TLRs except TLR3 as well as transmembrane activator and calcium-modulating cyclophilin ligand interactor (TACI) use IRAK4 and MYD88 to signal.23–25 In addition, the UNC-93B protein interacts with intracellular TLRs including TLR3, TLR7, TLR8, and TLR9 and seems essential to mediate their functions.26–30 Additional clues on the regulation of central B cell tolerance came from the analysis of IRAK4-, MYD88-, and UNC-93B–deficient patients. IRAK4-, MYD88-, and UNC-93B–deficient patients showed increased frequencies of polyreactive new emigrant/transitional B cells indicative of a defective central B cell tolerance checkpoint, revealing the importance of the IRAK4/MYD88 pathway in the establishment of central tolerance (Fig. 2A).30
In addition, we discovered that ANA clones including those reacting with chromatin were enriched in the new emigrant/transitional B cell compartment when the MYD88/IRAK4 signaling pathway was defective (Fig. 2B).30 It is tempting to propose that TLR7 and TLR9, which bind nucleic acid containing antigens also recognized by ANAs, are responsible for the MYD88/IRAK4-dependent removal of ANA-expressing clones. However, the proper silencing of ANA-expressing B cells in UNC-93B–deficient patients argues against an involvement of TLR7 and TLR9 in this process, since UNC-93B has been reported to be required for these TLRs to function (Fig. 2B).27,28,30 Nevertheless, we can hypothesize that TLR7 and TLR9 may mediate the elimination of ANA clones if nucleic acid containing autoantigens bound to antinuclear BCRs may reach these TLRs in the absence of UNC-93B.28,29,31 Other IRAK4/MYD88-dependent receptors such as TACI25 may also contribute to the counterselection of B cells expressing ANAs. Altogether, these data suggest that the TLR/BCR coengagement paradigm for B cell activation and proliferation may also apply to the selection of developing B cells in the bone marrow.
Activation-induced cytidine deaminase is essential for central B cell tolerance
Hyper IgM (HIGM) syndromes are PIDs characterized by defects in class switch recombination (CSR) resulting in severely decreased numbers of circulating isotype-switched memory B cells.32 The genetic basis of HIGM is diverse and is caused by defects in either the CD40L/CD40 pathway essential for B cell activation, germinal center (GC) formation and CSR induction, or the enzymes such as activation-induced cytidine deaminase (AID) required for CSR and somatic hypermutation (SHM).33–35 Aside from the susceptibility to bacterial infections, HIGM patients are prone to develop autoimmune diseases, suggesting that B cell tolerance is not properly established and/or maintained in the absence of CD40L or AID.36,37 Antibody characteristics and specificity from CD40L-deficient new emigrant/transitional B cells were similar to those from healthy donors suggesting that CD40L, which is not expressed in developing B cells, does not play an important role in the establishment of central B cell tolerance (Fig. 2). In contrast, new emigrant/transitional B cells from autosomal recessive AID-deficient patients express an abnormal immunoglobulin repertoire and a high frequency of polyreactive antibodies, demonstrating that AID is required for the establishment of central B cell tolerance (Fig. 2A).38 How AID affects central B cell tolerance is currently unknown but because the mechanisms that ensure human central B cell tolerance seem to be mostly controlled by intrinsic B cell factors, AID expression in immature B cells might be relevant to tolerance induction.8,11,39 Although AID expression was previously believed to be restricted to activated B cells and GCs, we and others have now detected AID transcripts in human and mouse immature B cells, further supporting an earlier role for AID during bone marrow B cell development.38,40–44 In addition, AID expression is upregulated in mature and immature B cells by BCR, TLR7, TLR9, and TACI triggering.25,38 Interestingly, all of these receptors have been demonstrated, or are suspected, to be involved in the establishment of early B cell tolerance, potentially further arguing for a relevant intrinsic role for AID in central B cell tolerance (Ref. 38 and data not shown).
Several scenarios could explain how AID might regulate early B cell tolerance. AID might induce DNA lesions that eventually lead to cell death and the elimination of autoreactive clones; AID-deficient B cells may therefore be less sensitive to apoptosis, a mechanism involved in central B cell tolerance, as reported in mice.44,45 AID deamination of methylated cytidines might also induce DNA demethylation potentially required for the epigenetic regulation of gene expression (and perhaps V(D)J recombination and receptor editing, the most important mechanism for central B cell tolerance46). In line with this hypothesis, changes in DNA methylation in individuals carrying a mutated DNA methyltransferase 3B (DNMT3B) gene have been reported to interfere with the central counterselection of B cell clones.47 However, because AID gene transcription in human immature B cells is 20–25 times lower than in GC B cells,38 these low AID transcript levels may not be relevant to immature B cell physiology and the removal of developing autoreactive B cells. In this case, early B cell tolerance alteration observed in AID-deficient patients may not result from intrinsic B cell defects but perhaps from a failure to control intestinal microflora.48 The expansion of autoreactive IgM+ B cells in AID-deficient patients may then represent a compensatory mechanism to counterbalance the loss of protection by B cells with high affinity in the absence of functional AID. Nonetheless, a major and previously unsuspected role for AID in the removal of developing autoreactive B cells in humans has been reported.38 Interestingly, a requirement for AID expression in central B cell tolerance was also reported in mice,44 demonstrating a conserved role for AID on tolerance through evolution.
Central B cell tolerance defects correlate with altered receptor editing regulation
Receptor editing is a major central B cell tolerance mechanism by which developing autoreactive B cells can be silenced, especially those that express ANAs.49,50 Secondary recombination events, first on the kappa locus and then on the lambda locus, provide attempts to edit autoreactive antibodies by substituting light chains until BCR autoreactivity is either abolished or diminished to levels that allow B cell development to proceed.11,39,50 As a result, upstream variable (V) gene usage combined with downstream joining (J) segments is a signature for secondary recombination events. A first correlation between abnormal regulation of secondary recombination mediating receptor editing and impaired central B cell tolerance in humans was found in XLA patients.12 The immunoglobulin kappa (Igκ) and lambda chain (Igλ) gene repertoires of new emigrant/transitional B cells from XLA patients were consistent with extensive secondary recombination activity, revealing that BCR signaling play an important role in downregulating such recombination events likely through the termination of RAG gene expression.12,51 Similarly, extensive secondary recombination on both Igκ and Igλ loci was also observed in human Igμ-deficient pro-B cells, further suggesting that IgL gene secondary recombination is a default mechanism in the absence of IgM signaling.52 Hence, as expected, receptor editing at the pre-B/immature B cell stage likely requires appropriate BCR signaling to be downregulated in order to properly counterselect autoreactive developing B cells in the bone marrow.12
Decreased secondary recombination potentially corresponding to a failure to induce receptor editing may also lead to abnormal central B cell tolerance. Indeed, a subset of common variable immunodeficiency disease (CVID) patients with expanded autoreactive CD21−/lo B cell populations (CVID group Ia53) suffer from a defective central B cell tolerance checkpoint associated with an Igκ repertoire characterized by a dearth of secondary recombination events.54,55 Interestingly, new emigrant B cells from untreated active RA patients who also suffer from defective central B cell tolerance10,56,57 express distinct patterns of Igκ light chain antibody repertoires, some of which altered by defective regulation of secondary recombination, a feature also reported in SLE patient’s B cells.58–60 An alteration in the regulation of secondary recombination events was also reported in IRAK4- and MYD88-deficient patients with defective central B cell tolerance; most ANA-expressing new emigrant/transitional B cells that escaped central tolerance in these patients used kappa chains, demonstrating that editing using lambda chains, which are more efficient in humans at silencing autoreactive antibodies than kappa chains,61 was not induced in these B cells.30 In addition, lambda chain usage in IRAK4- and MYD88-deficient B cells did not diminish antibody autoreactivity compared to kappa chain expressing clones, which further suggest defects in receptor editing in these patients. Additional analysis of the Igλ gene repertoire of new emigrant/transitional from IRAK4- and MYD88-deficient patients revealed increased downstream Vλ gene usage combined with upstream Jλ1 usage, the most upstream of all Jλ gene segments, attesting a dearth of secondary recombination on this locus when functional IRAK4/MYD88 complexes could not be expressed.
We conclude that kappa and lambda light chain receptor editing is not efficiently regulated to silence autoreactive and ANA expressing developing B cells in many PID patients who suffer from impaired central B cell tolerance.
Defects in peripheral B cell tolerance
Transgenic mouse models have suggested that CD4+ T cells may play an important role in the elimination of peripheral autoreactive B cells through MHC class II/T cell receptor; CD40/CD40L and Fas/FasL interactions.62 To investigate the impact of CD40/CD40L interactions and MHC class II expression on human B cell tolerance, we tested the reactivity of recombinant antibodies isolated from single B cells from CD40L-deficient and MHC class II–deficient patients.63 We found that although developing autoreactive B cells were properly counterselected in the bone marrow of these patients, mature naive B cells from CD40L- and MHC class II–deficient patients expressed a high proportion of autoreactive antibodies, including ANAs (Figs. 2 and 3).63 Thus, CD40/CD40L interactions and antigen presentation are essential for peripheral B cell tolerance. In addition, all patients who suffered from a defective central B cell tolerance checkpoint (IRAK4-, MYD88-, UNC-93B–, and AID-deficiencies) also displayed additional selection defects in their periphery, resulting in the accumulation of autoreactive mature naive B cells.30,38
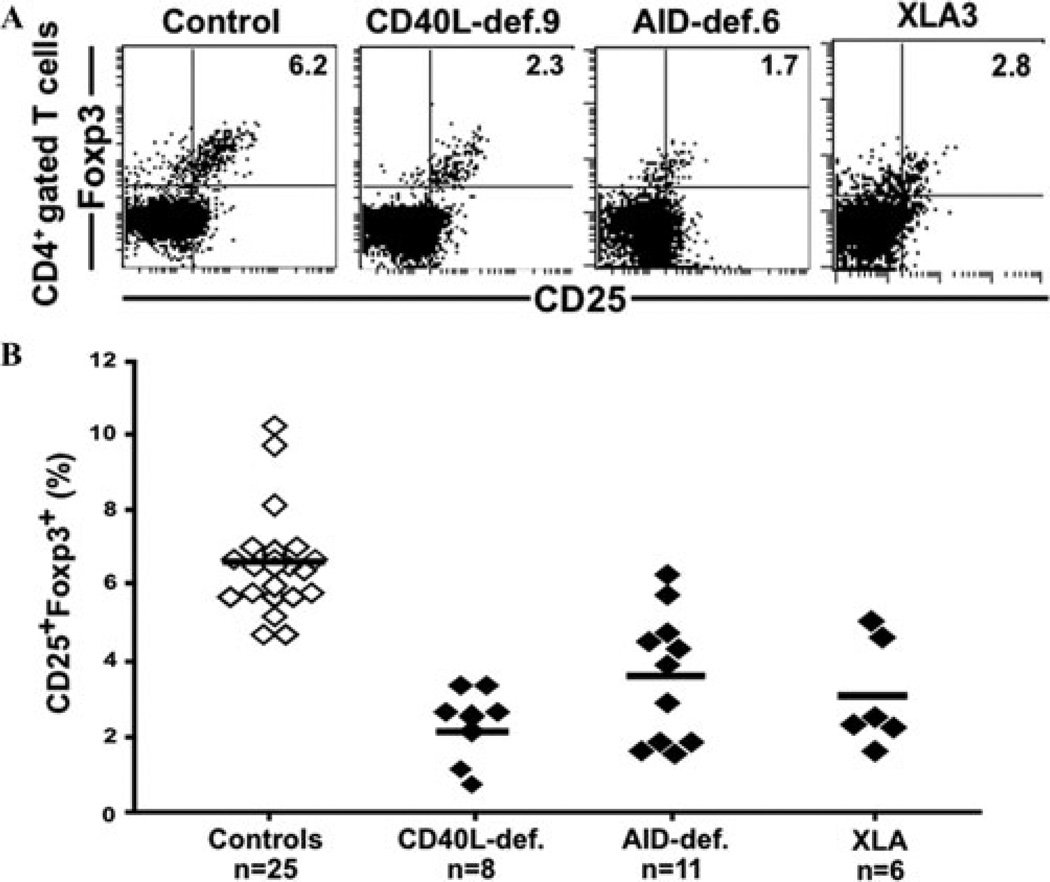
The specific defects at the peripheral B cell tolerance checkpoint in CD40L- and MHC class II–deficient patients suggested that a T cell population is involved in the removal of autoreactive B cells in the periphery.63 We found that CD40L- and MHC class II–deficient patients displayed decreased Treg cell numbers (Fig. 4).63 The importance of Treg cells in the establishment and/or the maintenance of peripheral tolerance is demonstrated in mice and humans deficient in Foxp3, who suffer from a severe autoimmune syndrome.64–66 Similar to CD40L- and MHC class II–deficient patients, individuals with BTK- and AID-deficiency, who suffer from a defective peripheral B cell tolerance checkpoint, also lack isotype-switched memory B cells and display decreased Treg cell frequencies, suggesting a potential involvement for B cells in either the generation or the maintenance of some Treg cells in humans (Fig. 4).38
Figure 4.
Decreased Treg cell frequency in CD40L-, AID-deficient, and XLA patients. Treg cell frequencies among peripheral CD4+ T cells were assessed by analyzing the proportion of CD25+Foxp3+ cells. Dot plots representative for a healthy control and CD40L-, AID-deficient, and XLA patients are displayed in (A). (B) Treg cell frequencies from all patients were significantly lower than those in healthy controls (P < 0.0001 for CD40L-, AID-deficient, and XLA patients).
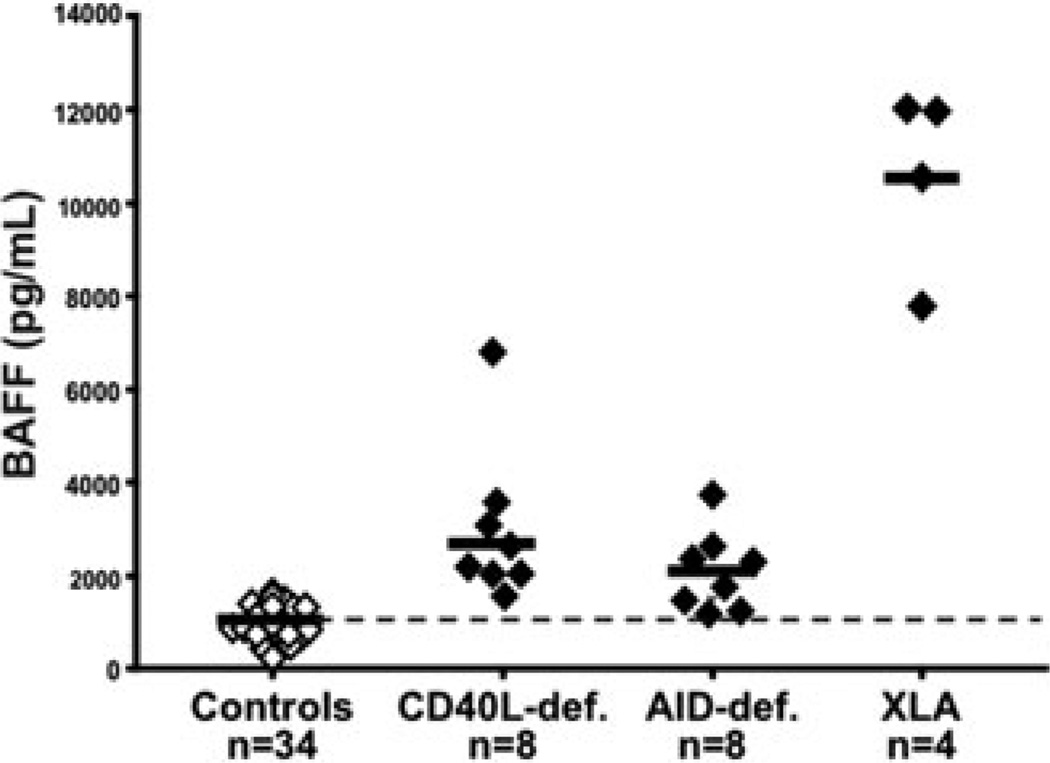
BAFF is a serum cytokine that promotes transitional and mature naive B cell survival. BAFF-deficient mice display profoundly decreased numbers of peripheral B cells.67 In contrast, mice overexpressing BAFF develop autoimmune disorders similar to SLE and Sjorgren syndrome characterized by the production of autoreactive antibodies, including rheumatoid factor, anti-DNA, and other ANAs.68 Elevated BAFF concentration inhibits the counterselection of autoreactive new emigrant/transitional B cells that failed to be removed from the B cell population.69,70 BAFF may also favor the proliferation of some clones by promoting the entry into the cell cycle.71 Hence, the elevated serum BAFF concentration in CD40L-, MHC class II-, and AID-deficient patients is therefore likely to contribute to the accumulation of autoreactive mature naive B cells in the blood of these patients (Fig. 5).38,63We conclude that Treg cells and serum BAFF concentrations may be involved in the regulation of peripheral human B cell tolerance.38,63
Figure 5.
Elevated serum BAFF concentrations in CD40L-, AID-deficient, and XLA patients. BAFF concentrations (pg/mL) in the serum of healthy donor controls (open diamonds), CD40L-, and AID-deficient patients as well as XLA patients (black diamonds) were measured by ELISA. Each diamond represents an individual, and the average is shown with a bar.
Perspectives
We have reviewed herein the current data on the establishment of central and peripheral B cell tolerance selecting the naive B cell repertoire. It is obvious that the analysis of additional PID will further increase and refine our understanding on the steps controlling the selection of B cell clones in the mature naive B cell compartment. Several important aspects of early B cell tolerance remain to be investigated: Are TLR7 and TLR9 the receptors responsible for the central removal of antinuclear clones? How does AID deficiency affect central B cell tolerance? and Are Treg cells essential for the removal of autoreactive B cells in the periphery? PID patients deficient for TLR7 or TLR9 have not yet been identified to assess the first question. The mechanisms by which AID deficiency impairs central tolerance may be explored by studying early B cell tolerance checkpoints in PID patients deficient in molecules acting downstream of AID such as Uracil N-glycosylase (UNG), MSH6, and PMS2, the latter two belonging to the mismatch repair system (MMR) complexes. Finally, the investigation of immune deficiency, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) patients who carry a mutation in the FOXP3 gene and suffer from defective Treg cell functions, should determine if Treg cells are involved in the establishment of peripheral B cell tolerance in humans.
Acknowledgments
This work was supported by Grants AI061093, AI071087, AI082713 from NIH-NIAID.
Footnotes
Conflicts of interest
The author declares no conflicts of interest.
References
- 1.Leslie D, Lipsky P, Notkins AL. Autoantibodies as predictors of disease. J. Clin. Invest. 2001;108:1417–1422. doi: 10.1172/JCI14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edwards JCW, et al. Efficacy of B cell-targeted therapy with Rituximab in patients with rheumatoid arthritis. N. Engl. J. Med. 2004;350:2572–2581. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 3.Hauser SL, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N. Engl. J. Med. 2008;358:676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 4.Pescovitz MD, et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N. Engl. J. Med. 2009;361:2143–2152. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wardemann H, et al. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 6.Meffre E, et al. Surrogate light chain expressing human peripheral B cells produce self-reactive antibodies. J. Exp. Med. 2004;199:145–150. doi: 10.1084/jem.20031550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tiller T, et al. Efficient generation of monoclonal antibodies from single human B cells by single cell RT-PCR and expression vector cloning. J. Immunol. Methods. 2008;329:112–124. doi: 10.1016/j.jim.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meffre E, Wardemann H. B-cell tolerance checkpoints in health and autoimmunity. Curr. Opin. Immunol. 2008;20:632–638. doi: 10.1016/j.coi.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 9.van Zelm MC, Szczepanski T, van der Burg M, van Dongen JJ. Replication history of B lymphocytes reveals homeostatic proliferation and extensive antigen-induced B cell expansion. J. Exp. Med. 2007;204:645–655. doi: 10.1084/jem.20060964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menard L, et al. The PTPN22 allele encoding an R620W variant interferes with the removal of developing autoreactive B cells in humans. J. Clin. Invest. 2011;121:3635–3644. doi: 10.1172/JCI45790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodnow CC. Balancing immunity and tolerance: deleting and tuning lymphocyte repertoires. Proc. Natl. Acad. Sci. USA. 1996;93:2264–2271. doi: 10.1073/pnas.93.6.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ng Y-S, Wardemann H, Chelnis J, et al. Bruton’s tyrosine kinase (Btk) is essential for human B cell tolerance. J. Exp. Med. 2004;200:927–934. doi: 10.1084/jem.20040920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grimaldi CM, Hicks R, Diamond B. B cell selection and susceptibility to autoimmunity. J. Immunol. 2005;174:1775–1781. doi: 10.4049/jimmunol.174.4.1775. [DOI] [PubMed] [Google Scholar]
- 14.deWeers M, et al. B-cell antigen receptor stimulation activates the human Bruton’s tyrosine kinase, which is deficient in X-linked agammaglobulinemia. J. Biol. Chem. 1994;269:23857–23860. [PubMed] [Google Scholar]
- 15.Kurosaki T, Tsukada S. BLNK: connecting Syk and Btk to calcium signals. Immunity. 2000;12:1–5. doi: 10.1016/s1074-7613(00)80153-3. [DOI] [PubMed] [Google Scholar]
- 16.Begovich AB, et al. A missense single-nucleotide polymorphism in a gene encoding a protein tyrosine phosphatase (PTPN22) is associated with rheumatoid arthritis. Am. J. Hum. Genet. 2004;75:330–337. doi: 10.1086/422827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kyogoku C, et al. Genetic association of the R620W polymorphism of protein tyrosine phosphatase PTPN22 with human SLE. Am. J. Hum. Genet. 2004;75:504–507. doi: 10.1086/423790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bottini N, et al. A functional variant of lymphoid tyrosine phosphatase is associated with type 1 diabetes. Nat. Genet. 2004;36:337–338. doi: 10.1038/ng1323. [DOI] [PubMed] [Google Scholar]
- 19.Michou L, et al. Linkage proof for PTPN22, a rheumatoid arthritis susceptibility gene and a human autoimmunity gene. Proc. Natl. Acad. Sci. USA. 2007;104:1649–1654. doi: 10.1073/pnas.0610250104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vang T, et al. Autoimmune-associated lymphoid tyrosine phosphatase is a gain-of-function variant. Nat. Genet. 2005;37:1317–1319. doi: 10.1038/ng1673. [DOI] [PubMed] [Google Scholar]
- 21.Arechiga AF, et al. Cutting edge: the PTPN22 allelic variant associated with autoimmunity impairs B cell signaling. J. Immunol. 2009;182:3343–3347. doi: 10.4049/jimmunol.0713370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat. Rev. Immunol. 2006;6:823–835. doi: 10.1038/nri1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akira S, Takeda K. Toll-like receptor signaling. Nat. Rev. Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 24.Beutler B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature. 2004;430:257–263. doi: 10.1038/nature02761. [DOI] [PubMed] [Google Scholar]
- 25.He B, et al. The transmembrane activator TACI triggers immunoglobulin class switching by activating B cells through the adaptor MyD88. Nat. Immunol. 2010;11:836–845. doi: 10.1038/ni.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brinkmann MM, et al. The interaction between the ER membrane protein UNC93B and TLR3, 7, and 9 is crucial for TLR signaling. J. Cell. Biol. 2007;177:265–275. doi: 10.1083/jcb.200612056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Casrouge A, et al. Herpes simplex virus encephalitis in human UNC-93B deficiency. Science. 2006;314:308–312. doi: 10.1126/science.1128346. [DOI] [PubMed] [Google Scholar]
- 28.Kim YM, Brinkmann MM, Paquet ME, Ploegh HL. UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature. 2008;452:234–238. doi: 10.1038/nature06726. [DOI] [PubMed] [Google Scholar]
- 29.Tabeta K, et al. The Unc93b1 mutation 3d disrupts exogenous antigen presentation and signaling via Toll-like receptors 3, 7 and 9. Nat. Immunol. 2006;7:156–164. doi: 10.1038/ni1297. [DOI] [PubMed] [Google Scholar]
- 30.Isnardi I, et al. IRAK-4- and MyD88-dependent pathways are essential for the removal of developing autoreactive B cells in humans. Immunity. 2008;29:746–757. doi: 10.1016/j.immuni.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim YM, Brinkmann MM, Ploegh HL. TLRs bent into shape. Nat. Immunol. 2007;8:675–677. doi: 10.1038/ni0707-675. [DOI] [PubMed] [Google Scholar]
- 32.Gulino AV, Notarangelo LD. Hyper IgM syndromes. Curr. Opin. Rheumatol. 2003;15:422–429. doi: 10.1097/00002281-200307000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Lee WI, et al. Molecular analysis of a large cohort of patients with the hyper immunoglobulin M (IgM) syndrome. Blood. 2005;105:1881–1890. doi: 10.1182/blood-2003-12-4420. [DOI] [PubMed] [Google Scholar]
- 34.Muramatsu M, et al. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [see comments]. [DOI] [PubMed] [Google Scholar]
- 35.Revy P, et al. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the Hyper-IgM syndrome (HIGM2) Cell. 2000;102:565–575. doi: 10.1016/s0092-8674(00)00079-9. [see comments]. [DOI] [PubMed] [Google Scholar]
- 36.Quartier P, et al. Clinical, immunologic and genetic analysis of 29 patients with autosomal recessive hyper-IgM syndrome due to activation-induced cytidine deaminase deficiency. Clin. Immunol. 2004;110:22–29. doi: 10.1016/j.clim.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 37.Durandy A, Peron S, Fischer A. Hyper-IgM syndromes. Curr. Opin. Rheumatol. 2006;18:369–376. doi: 10.1097/01.bor.0000231905.12172.b5. [DOI] [PubMed] [Google Scholar]
- 38.Meyers G, et al. Activation-induced cytidine deaminase (AID) is required for B-cell tolerance in humans. Proc. Natl. Acad. Sci. USA. 2011;108:11554–11559. doi: 10.1073/pnas.1102600108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nemazee D, et al. B-cell-receptor-dependent positive and negative selection in immature B cells. Curr. Top.Microbiol. Immunol. 2000;245:57–71. doi: 10.1007/978-3-642-59641-4_3. [DOI] [PubMed] [Google Scholar]
- 40.Mao C, et al. T cell-independent somatic hypermutation in murine B cells with an immature phenotype. Immunity. 2004;20:133–144. doi: 10.1016/s1074-7613(04)00019-6. [DOI] [PubMed] [Google Scholar]
- 41.Han JH, et al. Class switch recombination and somatic hypermutation in early mouse B cells are mediated by B cell and Toll-like receptors. Immunity. 2007;27:64–75. doi: 10.1016/j.immuni.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ueda Y, Liao D, Yang K, et al. T-independent activation-induced cytidine deaminase expression, classs-witch recombination, and antibody production by immature/transitional 1 B cells. J. Immunol. 2007;178:3593–3601. doi: 10.4049/jimmunol.178.6.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuraoka M, et al. Activation-induced cytidine deaminase expression and activity in the absence of germinal centers: insights into hyper-IgM syndrome. J. Immunol. 2009;183:3237–3248. doi: 10.4049/jimmunol.0901548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuraoka M, et al. Activation-induced cytidine deaminase mediates central tolerance in B cells. Proc. Natl. Acad. Sci. USA. 2011;108:11560–11565. doi: 10.1073/pnas.1102571108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zaheen A, et al. AID constrains germinal center size by rendering B cells susceptible to apoptosis. Blood. 2009;114:547–554. doi: 10.1182/blood-2009-03-211763. [DOI] [PubMed] [Google Scholar]
- 46.Goodhardt M, et al. Methylation status of immunoglobulin kappa gene segments correlates with their recombination potential. Eur. J. Immunol. 1993;23:1789–1795. doi: 10.1002/eji.1830230809. [DOI] [PubMed] [Google Scholar]
- 47.Blanco-Betancourt CE, et al. Defective B-cell-negative selection and terminal differentiation in the ICF syndrome. Blood. 2004;103:2683–2690. doi: 10.1182/blood-2003-08-2632. [DOI] [PubMed] [Google Scholar]
- 48.Fagarasan S, et al. Critical roles of activation-induced cytidine deaminase in the homeostasis of gut flora. Science. 2002;298:1424–1427. doi: 10.1126/science.1077336. [DOI] [PubMed] [Google Scholar]
- 49.Halverson R, Torres RM, Pelanda R. Receptor editing is the main mechanism of B cell tolerance toward membrane antigens. Nat. Immunol. 2004;6:645–650. doi: 10.1038/ni1076. [DOI] [PubMed] [Google Scholar]
- 50.Radic MZ, Weigert M. Origins of anti-DNA antibodies and their implications for B-cell tolerance. Ann. N.Y. Acad. Sci. 1995;764:384–396. doi: 10.1111/j.1749-6632.1995.tb55853.x. [DOI] [PubMed] [Google Scholar]
- 51.Verkoczy L, et al. A role for nuclear factor kappa B/rel transcription factors in the regulation of the recombinase activator genes. Immunity. 2005;22:519–531. doi: 10.1016/j.immuni.2005.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meffre E, et al. Immunoglobulin heavy chain expression shapes the B cell receptor repertoire in human B cell development. J. Clin. Invest. 2001;108:879–886. doi: 10.1172/JCI13051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Warnatz K, et al. Severe deficiency of switched memory B cells (CD27(+)IgM(−)IgD(−)) in subgroups of patients with common variable immunodeficiency: a new approach to classify a heterogeneous disease. Blood. 2002;99:1544–1551. doi: 10.1182/blood.v99.5.1544. [DOI] [PubMed] [Google Scholar]
- 54.Isnardi I, et al. Complement receptor 2/CD21- human naive B cells contain mostly autoreactive unresponsive clones. Blood. 2010;115:5026–5036. doi: 10.1182/blood-2009-09-243071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Romberg N, Ng YS, Cunningham-Rundles C, Meffre E. Common variable immunodeficiency patients with increased CD21−/lo B cells suffer from altered receptor editing and defective central B cell tolerance. 2011 In press. [Google Scholar]
- 56.Samuels J, Ng Y-S, Coupillaud C, et al. Impaired early B cell tolerance in patients with rheumatoid arthritis. J. Exp. Med. 2005;201:1659–1667. doi: 10.1084/jem.20042321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Menard L, Samuels J, Ng YS, Meffre E. Inflammation-independent defective early B cell tolerance checkpoints in rheumatoid arthritis. Arthritis Rheum. 2011;63:1237–1245. doi: 10.1002/art.30164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bensimon C, Chastagner P, Zouali M. Human lupus anti-DNA autoantibodies undergo essentially primary V kappa gene rearrangements. EMBO J. 1994;13:2951–2962. doi: 10.1002/j.1460-2075.1994.tb06593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suzuki N, Harada T, Mihara S, Sakane T. Characterization of a germline Vk gene encoding cationic anti-DNA antibody and role of receptor editing for development of the autoantibody in patients with systemic lupus erythematosus. J. Clin. Invest. 1996;98:1843–1850. doi: 10.1172/JCI118985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dorner T, Foster SJ, Farner NL, Lipsky PE. Immunoglobulin kappa chain receptor editing in systemic lupus erythematosus. J. Clin. Invest. 1998;102:688–694. doi: 10.1172/JCI3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wardemann H, Hammersen J, Nussenzweig MC. Human autoantibody silencing by immunoglobulin light chains. J. Exp. Med. 2004;200:191–199. doi: 10.1084/jem.20040818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rathmell JC, et al. CD95 (Fas)-dependent elimination of self-reactive B cells upon interaction with CD4+ T cells. Nature. 1995;376:181–184. doi: 10.1038/376181a0. [DOI] [PubMed] [Google Scholar]
- 63.Hervé M, et al. CD40 ligand and MHC class II expression are essential for human peripheral B cell tolerance. J. Exp. Med. 2007;204:1583–1593. doi: 10.1084/jem.20062287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brunkow ME, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat. Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 65.Wildin RS, et al. X-linked neonatal diabetes mellitus, enteropathy, and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat. Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 66.Bennett CL, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 67.Schiemann B, et al. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293:2111–2114. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- 68.Mackay F, et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J. Exp. Med. 1999;190:1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lesley R, et al. Reduced competitiveness of autoantigen-engaged B cells due to increased dependence on BAFF. Immunity. 2004;20:441–453. doi: 10.1016/s1074-7613(04)00079-2. [DOI] [PubMed] [Google Scholar]
- 70.Thien M, et al. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. 2004;20:785–798. doi: 10.1016/j.immuni.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 71.Huang X, et al. Homeostatic cell-cycle control of Blys: induction of cell-cycle entry but not G1/S transition in opposition to p18INK4c and p27KIP1. Proc. Natl. Acad. Sci. USA. 2004;101:17789–17794. doi: 10.1073/pnas.0406111101. [DOI] [PMC free article] [PubMed] [Google Scholar]