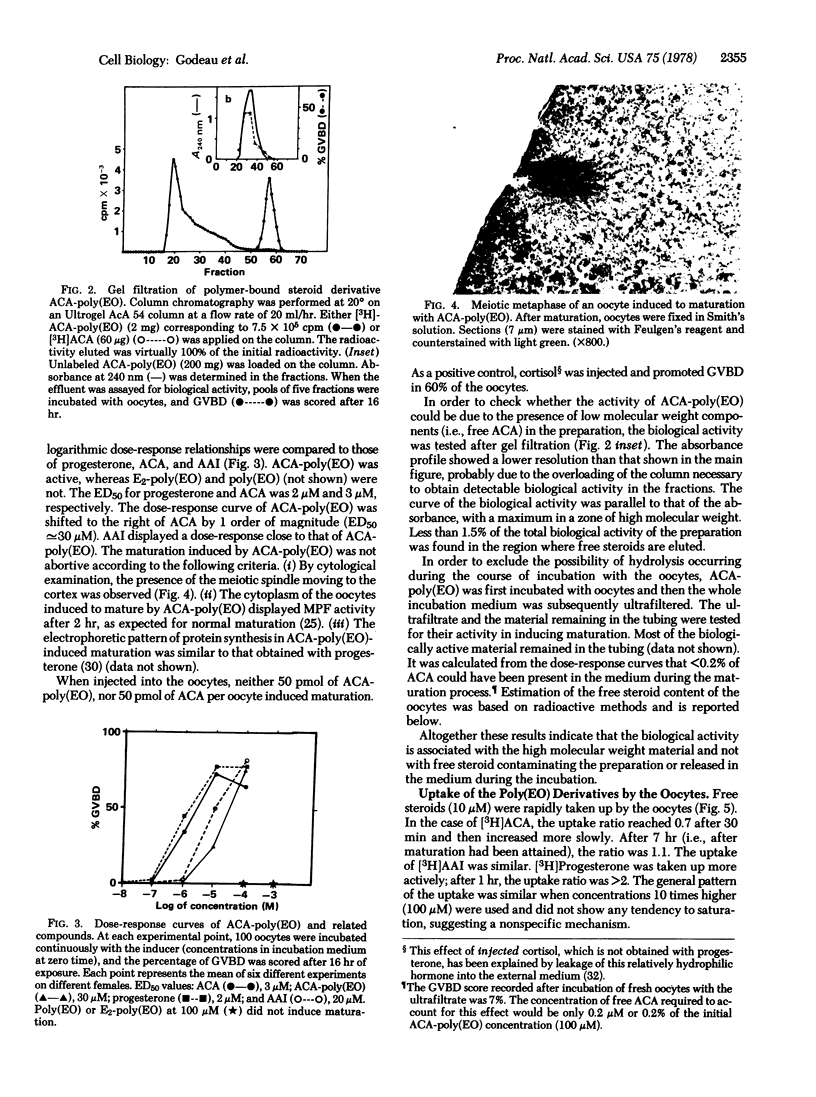
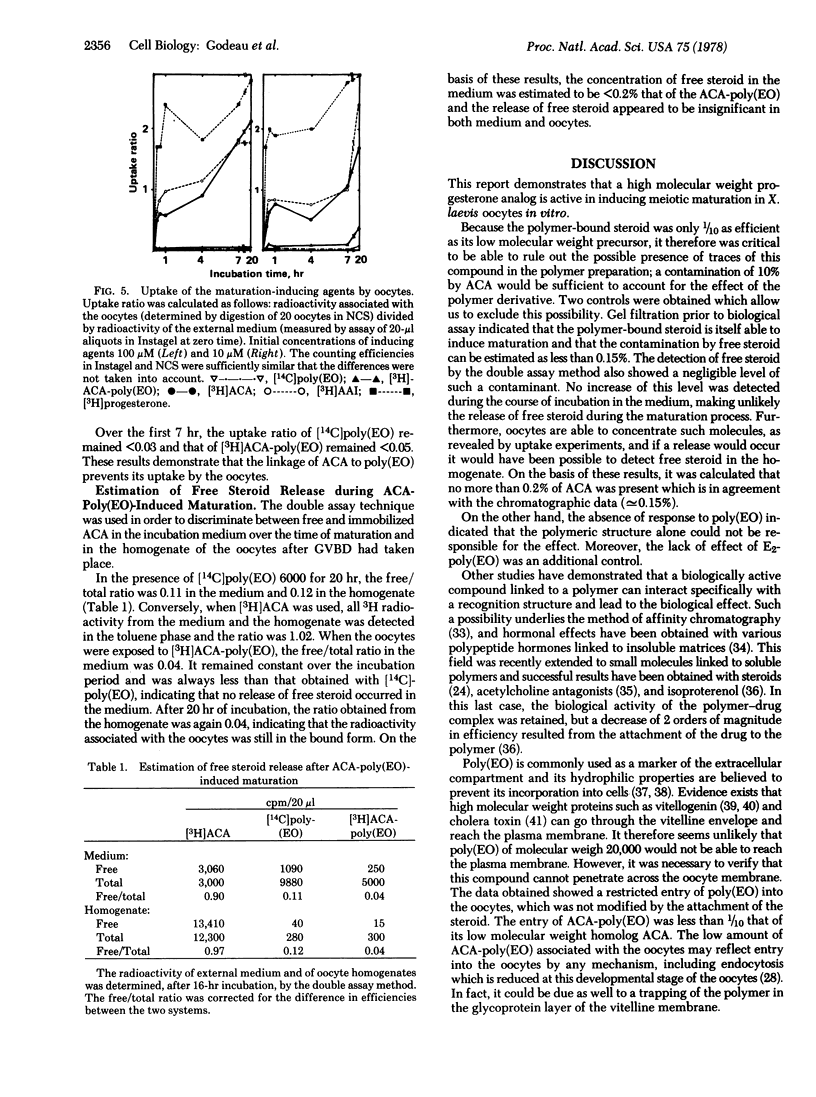
Abstract
A progesterone analog has been covalently linked via an amide bond to polyethylene oxide (molecular weight, 20,000). This macromolecular steroid molecule displays the biological activity of progesterone in inducing meiotic maturation when incubated with Xenopus laevis oocytes (stage VI) in vitro. Its efficiency (half-maximum effective concentration, 30 μM) is approximately 10 times lower than that of its low molecular weight homolog (3 μM). Control experiments with polyethylene oxide and an estradiol derivative (up to 1 mM) assessed the specificity of the progesterone macromolecular analog. Uptake experiments using radioactive derivatives revealed a small (if not negligible) intake of the macromolecular progesterone analog by the oocytes compared to that of free steroids, and no parallelism was found between radioactivity incorporation and effect. The possibility of cleavage of the macromolecular derivative during the incubation was ruled out. Furthermore, injection of the polymer-linked progesterone into the oocytes did not induce maturation. These observations suggest that the macromolecular progesterone analog itself is responsible for the biological effect and that the presence of this compound inside the cell is neither necessary nor sufficient for triggering reinitiation of meiosis. These conclusions are in agreement with the proposal that interaction with the plasma membrane of the oocyte is necessary for progesterone action in this particular system, in contrast to the case of somatic cells which have intracellular steroid receptors.
Keywords: mechanism of progesterone action, meiosis, plasma membrane, immobilized hormones, polyethylene oxide
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baulieu E. E., Atger M., Best-Belpomme M., Corvol P., Courvalin J. C., Mester J., Milgrom E., Robel P., Rochefort H., De Catalogne D. Steroid hormone receptors. Vitam Horm. 1975;33:649–736. doi: 10.1016/s0083-6729(08)60974-7. [DOI] [PubMed] [Google Scholar]
- Bellé R., Schorderet-Slatkine S., Drury K. C., Ozon R. In vitro progesterone binding to Xenopus laevis oocytes. Gen Comp Endocrinol. 1975 Mar;25(3):339–345. doi: 10.1016/0016-6480(75)90124-0. [DOI] [PubMed] [Google Scholar]
- Brachet J., Hanocq F., Van Gansen P. A cytochemical and ultrastructural analysis of in vitro maturation in amphibian oocytes. Dev Biol. 1970 Feb;21(1):157–195. doi: 10.1016/0012-1606(70)90067-9. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Affinity chromatography. Annu Rev Biochem. 1971;40:259–278. doi: 10.1146/annurev.bi.40.070171.001355. [DOI] [PubMed] [Google Scholar]
- Drury K., Schorderet-Slatkin S., Courrier M. R. Les effets du cortisol et de la progestérone directement injectés à l'intérieur de l'ovocyte de Xenopus Laevis. C R Acad Sci Hebd Seances Acad Sci D. 1975 Mar 10;280(10):1273–1275. [PubMed] [Google Scholar]
- Dumont J. N. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J Morphol. 1972 Feb;136(2):153–179. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Edelman I. S. Mechanism of action of steroid hormones. J Steroid Biochem. 1975 Mar-Apr;6(3-4):147–159. doi: 10.1016/0022-4731(75)90125-9. [DOI] [PubMed] [Google Scholar]
- Eppig J. J., Dumont J. N. Defined nutrient medium for the in vitro maintenance of Xenopus laevis oocytes. In Vitro. 1976 Jun;12(6):418–427. doi: 10.1007/BF02806021. [DOI] [PubMed] [Google Scholar]
- Flanagan S. D., Taylor P., Barondes S. H. Affinity partitioning of acetylcholine receptor enriched membranes and their purification. Nature. 1975 Apr 3;254(5499):441–443. doi: 10.1038/254441a0. [DOI] [PubMed] [Google Scholar]
- Godeau F., Boquet P., Schorderet M., Schorderet-Slatkine S., Baulieu E. E. Inhibition par l'entéro-toxine de Vibrio cholerae de la réinitiation méiotique de l'ovocyte de Xenopus laevis induite in vitro par la progestérone. C R Acad Sci Hebd Seances Acad Sci D. 1978 Mar 6;286(9):685–688. [PubMed] [Google Scholar]
- Guerrier P., Doree M. Hormonal control of reinitiation of meiosis in starfish. The requirement of 1-methyladenine during nuclear maturation. Dev Biol. 1975 Dec;47(2):341–348. doi: 10.1016/0012-1606(75)90288-2. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B. Changes in somatic cell nuclei inserted into growing and maturing amphibian oocytes. J Embryol Exp Morphol. 1968 Nov;20(3):401–414. [PubMed] [Google Scholar]
- Hubert P., Dellacherie E., Neel J., Baulieu E. E. Affinity partitioning of steroid-binding proteins. The use of polyethylene oxide-bound estradiol for purifying delta 5 goes to 4 3-oxosteroid isomerase. FEBS Lett. 1976 Jun 1;65(2):169–174. doi: 10.1016/0014-5793(76)80472-3. [DOI] [PubMed] [Google Scholar]
- Jacobelli S., Hanocq J., Baltus E., Brachet J. Hormone-induced maturation of Xenopus laevis oocytes: effects of different steroids and study of the properties of a progesterone receptor. Differentiation. 1974 Jun;2(3):129–135. doi: 10.1111/j.1432-0436.1974.tb00346.x. [DOI] [PubMed] [Google Scholar]
- Jensen E. V., DeSombre E. R. Mechanism of action of the female sex hormones. Annu Rev Biochem. 1972;41:203–230. doi: 10.1146/annurev.bi.41.070172.001223. [DOI] [PubMed] [Google Scholar]
- Kanatani H., Hiramoto Y. Site of action of 1-methyladenine in inducing oocyte maturation in starfish. Exp Cell Res. 1970 Aug;61(2):280–284. doi: 10.1016/0014-4827(70)90448-9. [DOI] [PubMed] [Google Scholar]
- Krag E., Krag B., Lenz K. A comparison of stable and 3H- labelled polyethylene glycol 4000 as non-absorbable water phase markers in the human ileum and faeces. Scand J Gastroenterol. 1975;10(1):105–108. [PubMed] [Google Scholar]
- Le Gaillard F., Racadot A., Racadot-Leroy N., Dautrevaux M. Isolement de la transcortine humaine par chromatographie d'affinité. Biochimie. 1974;56(1):99–108. doi: 10.1016/s0300-9084(74)80359-7. [DOI] [PubMed] [Google Scholar]
- Moreau M., Doree M., Guerrier P. Electrophoretic introduction of calcium ions into the cortex of Xenopus laevis oocytes triggers meiosis neinitiation. J Exp Zool. 1976 Sep;197(3):443–449. doi: 10.1002/jez.1401970318. [DOI] [PubMed] [Google Scholar]
- O'Connor C. M., Smith L. D. Inhibition of oocyte maturation by theophylline: possible mechanism of action. Dev Biol. 1976 Sep;52(2):318–322. doi: 10.1016/0012-1606(76)90249-9. [DOI] [PubMed] [Google Scholar]
- Pennequin P., Schorderet-Slatkine S., Drury K. C., Baulieu E. E. Decreased uptake of (3H)leucine during progesterone induced maturation of Xenopus laevis oocytes. FEBS Lett. 1975 Mar 1;51(1):156–160. doi: 10.1016/0014-5793(75)80875-1. [DOI] [PubMed] [Google Scholar]
- Reynhout J. K., Smith L. D. Studies on the appearance and nature of a maturation-inducing factor in the cytoplasm of amphibian oocytes exposed to progesterone. Dev Biol. 1974 Jun;38(2):394–400. doi: 10.1016/0012-1606(74)90016-5. [DOI] [PubMed] [Google Scholar]
- Roth J., Kahn C. R., Lesniak M. A., Gorden P., De Meyts P., Megyesi K., Neville D. M., Jr, Gavin J. R., 3rd, Soll A. H., Freychet P. Receptors for insulin, NSILA-s, and growth hormone: applications to disease states in man. Recent Prog Horm Res. 1975;31:95–139. doi: 10.1016/b978-0-12-571131-9.50007-4. [DOI] [PubMed] [Google Scholar]
- Schorderet-Slatkine S. Action of progesterone and related steroids on oocyte maturation in Xenopus laevis. An in vitro study. Cell Differ. 1972 Aug;1(3):179–189. doi: 10.1016/0045-6039(72)90027-9. [DOI] [PubMed] [Google Scholar]
- Schorderet-Slatkine S. Discussion paper: induction by progesterone and a "maturation-promoting factor" of soluble proteins in Xenopus laevis oocytes in vitro. Ann N Y Acad Sci. 1977 Mar 11;286:421–433. doi: 10.1111/j.1749-6632.1977.tb29434.x. [DOI] [PubMed] [Google Scholar]
- Schorderet-Slatkine S., Drury K. C. Progesterone induced maturation in oocytes of Xenopus laevis. Appearance of a 'maturation promoting factor' in enucleated oocytes. Cell Differ. 1973 Oct;2(4):247–254. doi: 10.1016/0045-6039(73)90013-4. [DOI] [PubMed] [Google Scholar]
- Schorderet-Slatkine S., Schorderet M., Baulieu E. E. Inhibition of the progesterone-dependent induction of meiosis by gammexane in Xenopus laevis oocytes. Biochem Biophys Res Commun. 1977 Jul 25;77(2):496–502. doi: 10.1016/s0006-291x(77)80007-7. [DOI] [PubMed] [Google Scholar]
- Schorderet-Slatkine S., Schorderet M., Baulieu E. E. Progesterone-induced meiotic reinitation in vitro in Xenopus laevis oocytes: a role for the displacement of membrane-bound calcium. Differentiation. 1977 Oct 20;9(1-2):67–76. doi: 10.1111/j.1432-0436.1977.tb01520.x. [DOI] [PubMed] [Google Scholar]
- Schorderet-Slatkine S., Schorderet M., Baulieu E. Initiation of meiotic maturation in Xenopus laevis oocytes by lanthanum. Nature. 1976 Jul 22;262(5566):289–290. doi: 10.1038/262289a0. [DOI] [PubMed] [Google Scholar]
- Schoredert-Slatkine S., Schorderet M. O. Maturation in vitro de l'ovocyte de Xenopus laevis sous l'effet d'agents pharmacologiques du type propranolol. C R Acad Sci Hebd Seances Acad Sci D. 1976 May 17;282(19):1733–1736. [PubMed] [Google Scholar]
- Schuetz A. W. Action of hormones on germinal vesicle breakdown in frog (Rana pipiens) oocytes. J Exp Zool. 1967 Dec;166(3):347–354. doi: 10.1002/jez.1401660307. [DOI] [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- Smith L. D., Ecker R. E. Regulatory processes in the maturation and early cleavage of amphibian eggs. Curr Top Dev Biol. 1970;5:1–38. [PubMed] [Google Scholar]
- Smith L. D., Ecker R. E. The interaction of steroids with Rana pipiens Oocytes in the induction of maturation. Dev Biol. 1971 Jun;25(2):232–247. doi: 10.1016/0012-1606(71)90029-7. [DOI] [PubMed] [Google Scholar]
- Verlander M. S., Venter J. C., Goodman M., Kaplan N. O., Saks B. Biological activity of catecholamines covalently linked to synthetic polymers: proof of immobilized drug theory. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1009–1013. doi: 10.1073/pnas.73.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. A., Ho T. Protein incorporation by isolated amphibian oocytes. II. A survey of inhibitors. J Exp Zool. 1972 Sep;181(3):303–317. doi: 10.1002/jez.1401810303. [DOI] [PubMed] [Google Scholar]
- Wasserman W. J., Masui Y. Initiation of meiotic maturation in Xenopus laevis oocytes by the combination of divalent cations and ionophore A23187. J Exp Zool. 1975 Sep;193(3):369–375. doi: 10.1002/jez.1401930313. [DOI] [PubMed] [Google Scholar]
- Williams W. M., Chen T. S., Huang K. C. Renal handling of aromatic amino acids, sugar, and standard glomerular markers in winter flounder. Am J Physiol. 1974 Dec;227(6):1380–1384. doi: 10.1152/ajplegacy.1974.227.6.1380. [DOI] [PubMed] [Google Scholar]



