Abstract
Toxoplasma gondii is obligate coccidian zoonotic parasite. Felidae family is definitive and wide ranges of warm-blooded vertebrates are intermediate hosts for the parasite. Rodents are measured as an important source of T. gondii infection for the definitive host. Thus, this study aimed to investigate Toxoplasm infection in rodents of Ahvaz district, southwest of Iran. A total of 100 rodents (73 Rattus norvegicus, 21 Rattus rattus, and 6 Mus musculus) were collected and studied by GRA6PCR and mouse bioassay. The finding indicated that 6 out of 100 (6%) and 2 out of 100 (2%) samples were positive by PCR and mouse bioassay, respectively. The results show notable chronic infection in the rodent and potential transmission of the infection among animal and men in the region. Accordingly, this study recommended investigating of the T. gondii infection in definitive and other intermediate hosts in other points of Khuzestan province, Southwest, Iran.
1. Introduction
Toxoplasma gondii is an intracellular and obligate coccidian zoonotic parasite. The definitive hosts of the parasite are members of the Felidae family (mainly domestic cats) and the intermediate hosts are warm-blooded vertebrates including humans, rodents, birds, livestock, and marine mammals [1].
Felids with excreting of the environmentally resistant oocysts are the most important hosts in the life cycle of T. gondii. Cats become infected with T. gondii by eating infected tissues from intermediate hosts. While foraging on the ground, rodents can get infected by Toxoplasma gondii. Toxoplasmosis is one of the opportunistic infections which in people with immunocompetent is largely asymptomatic, but in immunocompromised individuals, the parasite can become widely disseminated, causing severe toxoplasmosis [2–4]. In Iran, toxoplasmosis continues to be a public health problem, and a seroprevalence of 41.4% has been reported in central part of the country [5].
Assessment of Toxoplasma infection in the rodents as a main prey for cats with a key role in the ecological food chain and also in the transmission of parasites to other animals is very important. Studies in Iran revealed existence of different species of rodent including the brown rat (Rattus. norvegicus), the black rat (R. rattus), the Himalayan rat (R. pectoris), and house mice, Mus musculus [6, 7]. In Ahvaz, Southwest Iran, mainly due to unsupervised housing constructions and lack of health sewage system, large numbers of wild and domestic rodents are found on residential streets. These populations are potentially an important host reservoir for the transmission of zoonotic parasites such as T. gondii [8]. Although several studies were conducted on Toxoplasma infection in rodents in Iran [6, 7, 9], there is no report of molecular and change for mouse bioassay studies of Toxoplasma isolates from southwest of Iran.
The polymerase chain reaction (PCR) is very sensitive, highly specific, and rapid molecular technique to detect the parasite animal and human tissues [10]. Mouse bioassay is the principal method used to detected cysts in tissues [11]. The objective of this study was to determine Toxoplasma infection in domestic and wild rodents in Ahvaz, southwest of Iran by bioassay in mice, polymerase chain reaction (PCR), and sequencing.
2. Materials and Methods
2.1. Geographical Information on Study Area
Ahvaz is the capital of Khuzestan province, southwest of Iran. At the 2006 census, its population was 1,425,891, in 212,097 families. The city is built on the banks of the Karun River and has an average elevation of 20 meters above sea level. Ahvaz has a desert climate with long, extremely hot summers and mild, short winters. Maximum temperature in summer routinely exceeds 50 degrees while in winters the minimum temperature could fall around −5 degrees Celsius. The average annual rainfall is around 230 mm. In Ahvaz there is high density of rodents and rat-man proximity is considerable [7].
2.2. Rodent Collection
In the year 2011, trappings were performed at different parts of the city using live traps. In each locality, traps were baited with favorite foods of rodents at different seasons. On consecutive days, traps were collected and transported to medicine school, Parasitology department, where rodents were bled. Species identification and sex and age determination were done as described by Roberts [12].
2.3. Restraining of the Rodents and Tissue Collection
The trapped rodents were anaesthetized by putting the live traps in a thick transparent polythene bag and then a cotton swab soaked in ether as described by Singla et al. [13].
A total of 100 rodents (73 Rattus norvegicus, 21 Rattus rattus, and 6 Mus musculus) were bled and following microscopic examination for the presence of tissue cysts, all the tissues were artificially digested (0.5% pepsin, 1% HCl) [14–16].
2.4. Detection of T. gondii in Tissue by the Polymerase Chain Reaction and Sequencing
DNA was extracted from digested-tissue samples of brain, skeletal muscle, and liver using Bioneer Co., Seoul, South Korea, according to the manufacturer's instructions. Purified DNA concentration was determined by UV absorption [17].
For positive control, confirmed genomic DNA (Genbank accession number AB733004) was used in PCR amplification.
Detection of T. gondii DNA was carried out using a single PCR assay targeting the GRA6 region. For T. gondii-specific amplification, two forward primer, 5′- GTAGCGTGCTTGTTGGCGAC-3′, and reverse primer, 5′-ACAAGACATAGAGTGCCCC-3′, primers were used as described by Fazaeli et al. [18].
The PCR reaction was performed in a 25 μL reaction mixture containing 10 pmol of each primer and 10 μL of extracted DNA, 75 mM Tris-HCl (PH 8.5), 20 mM (NH4)2SO4, 1.5 mM MgCl2, 0.1% Tween 20, 0.2 mM dNTPs, 0.025 unite/μL Amplicon Taq DNA polymerase, inert red dye, and a stabilizer. The PCR conditions were 5 min at 95°C followed by 35 cycles of 30 s at 94°C, 1 min at 60°C, 2 min at 72°C, and a final elongation of 72°C for 7 min [18].
The PCR products were separated on a 1.5% agarose gel with 1x TAE buffer and visualized by staining with ethidium bromide.
The PCR positive samples were purified using an Accuprep Gel Purification kit (Bioneer, Deajeon, Korea) then sequenced (MWG-Biotech, Ebersberg, Germany) by the primers employed in the PCR. Sequence alignments were constructed using the program CLUSTAL W version 1.83 (http://www.ddbj.nig.ac.jp/search/clustalwe.html).
2.5. Bioassay in Mice
White laboratory mice weighing 20–25 g with 6-week-old specific-pathogen free were used in the bioassay. Tissue samples of liver, muscle, and brain from trapped rodent were submitted to artificial digestion and 1 mL of each tissue homogenate was intraperitoneally inoculated into 2 mice per each tissue sample. Mice were daily inspected for signs that might indicate acute toxoplasmosis. Mice were killed 50 days after infection and the brain of all inoculated mice was studied by direct microscopy for presence of Toxoplasma gondii cysts.
3. Results and Discussion
Brain, muscle, and liver tissues of 100 wild and domestic rodents were analyzed by GRA6PCR method. PCR showed 6/100 (6%) positive samples (4 from Rattus norvegicus and 2 from Rattus rattus), being all from brain tissues. No positive result was detected from Mus musculus species. Of the 4 infected Rattus norvegicus isolates, 3 were collected from urban points and one was from rural region, whereas both of the infected Rattus rattus ones were collected from urban sit. This finding indicated that Rattus norvegicus and Rattus rattus could be a significant source of T. gondii infection for stray cats in the urban region.
In addition, No PCR-positive value was found for muscle and liver tissues. Tissue cysts of T. gondii were identified in 2 brain tissues of inoculated mice studied by direct microscopy examination (Table 1).
Table 1.
The results of Toxoplasma gondii detection in rodent of Ahvaz, southwest Iran, using PCR, mouse bioassay, and direct microscopy examination methods.
| Species | N | PCR | Mouse bioassay | Direct microscopy examination | Total positive samples |
|---|---|---|---|---|---|
| Rattus rattus | 21 | 2 (9.5%) | 0 | 1 | 2 |
| Rattus norvegicus | 73 | 4 (5.4%) | 2 (2.7%) | 0 | 4 |
| Mus musculus | 6 | 0 | 0 | 0 | 0 |
|
| |||||
| Total | 100 | 6% | 2% | 1% | 6 |
The size of the PCR products was about 800 bp [18] (Figure 1). The finding of GRA6 PCR technique indicated highest rate of T. gondii infection in Rattus norvegicus with 4/73 (5.4%).
Figure 1.
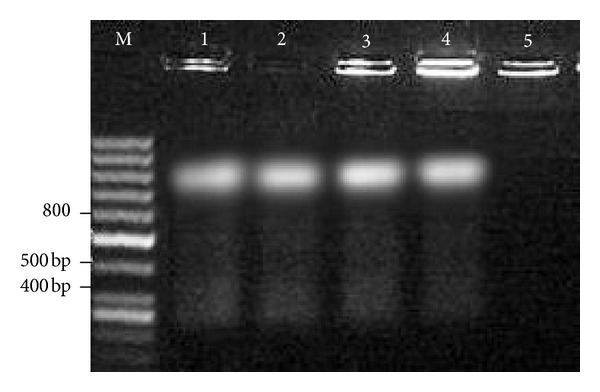
PCR amplification products of Toxoplasma gondii GRA6 in Rodent tissue samples. Lanes: M: molecular weight marker; 1: positive control (Toxoplasma tachyzoites); 2, 3, 4: positive samples; 5: negative control (H2O dest instead of DNA).
The amplified GRA6 genes of the 6 isolates were sequenced. The results were submitted to DDBJ/Genbank at accession nos: AB743592–AB743597. The nucleotide sequences of GRA6 gene from isolates were aligned with nucleotide sequences of T. gondii (GenBank accession nos. AB703303, AB703299, AB703305, and AB733005) (Figure 2). Figure 2 showed different nucleotide substitutions. These sequences were previously detected in domestic and wild birds in the region [19]. The obtained GRA6 sequences indicated atypical type of T. gondii in the region. This result suggested that there are probably different T. gondii genotypes circulating in this region.
Figure 2.

Multiple alignments of nucleotide sequences of GRA6 gene from 6 isolates and 4 nucleotide sequences of T. gondii (GenBank accession nos. AB703303, AB703299, AB703305, and AB733005). Asterisks indicates identical nucleotides.
Two positive brain samples of inoculated lab mice, detected by direct microscopic observation, were also studied by PCR technique. The obtained sequences were recorded as AB743592.1 and AB743593.1 accession numbers. Comparisons of these sequences with sequences that have been previously obtained from brain samples of hunted rodents (inoculated in the lab mice) showed 100% homology.
In this study, we used a PCR and sequencing at GRA6 geneand bioassay in mice for detection of T. gondii infection in rodent populations in Ahvaz, southwest Iran. Finding of this investigation indicated 6% of T. gondii infection in Ahvaz rodent which is in accordance with previous reports in Tehran and Zanjan, Iran, which showed 5.5% (8/145) of T. gondii infection in mouse brain tissues by PCR-RFLP. Seroprevalence study of Toxoplasma gondii among wild Rats (Rattus rattus) in Ahvaz district, Southwestern Iran, in 2011 showed thirty-one of the 127 serum samples (24.41%) had antibodies against T. gondii [9]. It could be due to the kind of test which seroprevalence results indicating exposure to T. gondii and not still associated with forming cysts in tissues.
In the present study, in bioassay method, T. gondii was isolated from the brain of two lab mice inoculated brain suspensions of trapped mice. This result is corresponding with Sreekumar et al. [20] in bioassay of chicken tissues in mice who reported that T. gondii could not be isolated from any of the positive samples by bioassay in mice, whereas they could isolate T. gondii from the feces of five cats fed tissues pooled from 89 chickens with titers of 1 : 5 or less. The lower prevalence rate in this assay could be due to various reasons. It is possible that the samples that were analyzed herein originated from younger mice. It is likely that storage of samples under unrefrigerated conditions during preparation affected the viability of T. gondii. PCR is a sensitive and specific for detecting T. gondii even when the tissues available for testing are in state of decomposition; bioassays, in contrast, can only detect viable parasites [10, 21, 22]. The present results are similar to Yai et al. [10] who detect lower levels of identification via bioassays (four out of eight pigs) than with nested-PCR (seven out of eight pigs). However, many studies worldwide reported higher sensitivity for murine bioassays than PCR with respect to the isolation of T. gondii [23–25]. The present results showed that the brain was the most frequently infected compared with the other tissues. These results concur with those of Esteban-Redondo and Innes [26].
In this study, the most abundant species was Rattus norvegicus (73/100) and highest prevalence of T. gondii infection (5.4%, 4/73) was detected in this species. This finding is in agreement with previous study in Ahvaz that indicated Rattus norvegicus in 80% of the trapped rodent [7]. The highest rate of T. gondii infection detected in Rattus norvegicus may be due to the fact that repeat congenital transmission happens in this species [27]. Nonetheless, recent studies have used PCR in The Netherlands and the United Kingdom demonstrating a very high prevalence in Mus musculus (51.9%, 120/231) and lower in R. norvegicus (10.3%, 4/39) [28, 29].
4. Conclusions
This is the first molecular and bioassay survey of T. gondii infection among wild and domestic rodent in Ahvaz district, southwestern Iran, and the results indicate notable chronic infection in the rodent and potential transmission of the infection among animal and men. We suggest the study of the T. gondii infection in definitive and other intermediate hosts in other points of Khuzestan province, Southwest, Iran.
Acknowledgments
This study is conducted and financially supported by grants from Ahvaz Jundishapur Medical sciences. The authors would like to appreciate personnel of animal house of the University for their kind cooperation and Mr. Nezam Salehi for his collaboration in sample collection.
Conflict of Interests
The authors wish to declare that there is no known conflict of interests associated with this publication and there has been no competing financial interests.
Funding
Research Deputy, Ahvaz Jundishapur University of Medical Sciences.
References
- 1.Dubey JP. Toxoplasma gondii infections in chickens (Gallus domesticus): prevalence, clinical disease, diagnosis and public health significance. Zoonoses and Public Health. 2010;57(1):60–73. doi: 10.1111/j.1863-2378.2009.01274.x. [DOI] [PubMed] [Google Scholar]
- 2.Velimirovic B. Toxoplasmosis in immunosuppresion and AIDS. Infection. 1984;12(5):315–317. doi: 10.1007/BF01651143. [DOI] [PubMed] [Google Scholar]
- 3.Gallino A, Maggiorini M, Kiowski W, et al. Toxoplasmosis in heart transplant recipients. European Journal of Clinical Microbiology and Infectious Diseases. 1996;15(5):389–393. doi: 10.1007/BF01690095. [DOI] [PubMed] [Google Scholar]
- 4.Wanke C, Tuazon CU, Kovacs A. Toxoplasma encephalitis in patients with acquired immune deficiency syndrome: diagnosis and response to therapy. The American Journal of Tropical Medicine and Hygiene. 1987;36(3):509–516. doi: 10.4269/ajtmh.1987.36.509. [DOI] [PubMed] [Google Scholar]
- 5.Mostafavi SN, Ataei B, Nokhodian Z, Yaran M, Babak A. Seroepidemiology of Toxoplasma gondii infection in Isfahan province, central Iran: a population based study. Journal of Research in Medical Sciences. 2011;16(4):496–501. [PMC free article] [PubMed] [Google Scholar]
- 6.Akbary Rad S, Jalal R, Darvish J, Matin MM. Identification of three Iranian species of the genus Rattus (Rodentia, Muridae) using a PCR-RFLP technique on mitochondrial DNA. Hystrix. 2009;20(1):69–77. [Google Scholar]
- 7.Kia EB, Homayouni MM, Farahnak A, Mohebali M, Shojai S. Study of endoparasites of rodents and their zoonotic importance in Ahvaz, south west Iran. Iranian Journal of Public Health. 2001;30(1-2):49–52. [Google Scholar]
- 8.Dubey JP, Frenkel JK. Toxoplasmosis of rats: a review, with considerations of their value as an animal model and their possible role in epidemiology. Veterinary Parasitology. 1998;77(1):1–32. doi: 10.1016/s0304-4017(97)00227-6. [DOI] [PubMed] [Google Scholar]
- 9.Mosallanejad B, Avizeh R, Jalali MHR, Hamidinejat H. Seroprevalence of Toxoplasma gondii among wild rats (Rattus rattus) in Ahvaz district, southwestern Iran. Jundishapur Journal of Microbiology. 2012;5(1):332–335. [Google Scholar]
- 10.Yai LEO, Vianna MCB, Soares RM, et al. Evaluation of experimental Toxoplasma gondii (Nicolle and Manceaux, 1909) infection in pigs by bioassay in mice and polymerase chain reaction. Brazilian Journal of Veterinary Research and Animal Science. 2003;40(3):227–234. [Google Scholar]
- 11.Tsutsui VS, Freire RL, Garcia JL, et al. Detection of Toxoplasma gondii by PCR and mouse bioassay in commercial cuts of pork from experimentally infected pigs. Arquivo Brasileiro de Medicina Veterinária e Zootecnia. 2007;59(1):30–34. [Google Scholar]
- 12.Roberts TJ. The Mammals of Pakistan. 2nd edition. Oxford, UK: Oxford University Press; 1997. [Google Scholar]
- 13.Singla LD, Singla N, Parshad VR, Juyal PD, Sood NK. Rodents as reservoirs of parasites in India. Integrative Zoology. 2008;3(1):21–26. doi: 10.1111/j.1749-4877.2008.00071.x. [DOI] [PubMed] [Google Scholar]
- 14.Stojcevic D, Mihaljevic Z, Marinculic A. Parasitological survey of rats in rural regions of Croatia. Veterinary Medicine Czech. 2004;49(3):70–74. [Google Scholar]
- 15.Conlogue G, Foreyt W, Adess M, Levine H. Capillaria hepatica (Bancroft) in select rat populations of Hartford, Connecticut, with possible public health implications. The Journal of Parasitology. 1979;65(1):105–108. [PubMed] [Google Scholar]
- 16.Ceruti R, Sonzogni O, Origgi F, et al. Capillaria hepatica infection in wild brown rats (Rattus norvegicus) from the urban area of Milan, Italy. Journal of Veterinary Medicine, Series B. 2001;48(3):235–240. doi: 10.1046/j.1439-0450.2001.00436.x. [DOI] [PubMed] [Google Scholar]
- 17.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY, USA: Cold Spring Harbor Laboratory; 1997. [Google Scholar]
- 18.Fazaeli A, Carter PE, Darde ML, Pennington TH. Molecular typing of Toxoplasma gondii strains by GRA6 gene sequence analysis. International Journal for Parasitology. 2000;30(5):637–642. doi: 10.1016/s0020-7519(00)00036-9. [DOI] [PubMed] [Google Scholar]
- 19.Khademvatan S, Saki J, Yousefi E, Abdizadeh R. Detection and genotyping of Toxoplasma gondii strains isolated from birds in the southwest of Iran. British Poultry Science. 2013;54(1):76–80. doi: 10.1080/00071668.2013.763899. [DOI] [PubMed] [Google Scholar]
- 20.Sreekumar C, Graham DH, Dahl E, et al. Genotyping of Toxoplasma gondii isolates from chickens from India. Veterinary Parasitology. 2003;118(3-4):187–194. doi: 10.1016/j.vetpar.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 21.Burg JL, Grober CM, Pouletty P, Boothroyd JC. Direct and sensitive detection of a pathogenic protozoan, Toxoplasma gondii, by polymerase chain reaction. Journal of Clinical Microbiology. 1989;27(8):1787–1792. doi: 10.1128/jcm.27.8.1787-1792.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hurtado A, Aduriz G, Moreno B, Barandika J, Garcia-Pérez AL. Single tube nested PCR for the detection of Toxoplasma gondii in fetal tissues from naturally aborted ewes. Veterinary Parasitology. 2001;102(1-2):17–27. doi: 10.1016/s0304-4017(01)00526-x. [DOI] [PubMed] [Google Scholar]
- 23.Wastling JM, Nicoll S, Buxton D. Comparison of two gene amplification methods for the detection of Toxoplasma gondii in experimentally infected sheep. Journal of Medical Microbiology. 1993;38(5):360–365. doi: 10.1099/00222615-38-5-360. [DOI] [PubMed] [Google Scholar]
- 24.da Silva AV, Langoni H. The detection of Toxoplasma gondii by comparing cytology, histopathology, bioassay in mice, and the polymerase chain reaction (PCR) Veterinary Parasitology. 2001;97(3):191–198. doi: 10.1016/s0304-4017(01)00404-6. [DOI] [PubMed] [Google Scholar]
- 25.García JL, Gennari SM, Machado RZ, Navarro IT. Toxoplasma gondii: detection by mouse bioassay, histopathology, and polymerase chain reaction in tissues from experimentally infected pigs. Experimental Parasitology. 2006;113(4):267–271. doi: 10.1016/j.exppara.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Esteban-Redondo I, Innes EA. Detection of Toxoplasma gondii in tissues of sheep orally challenged with different doses of oocysts. International Journal for Parasitology. 1998;28(9):1459–1466. doi: 10.1016/s0020-7519(98)00116-7. [DOI] [PubMed] [Google Scholar]
- 27.Dubey JP, Shen SK, Kwok OC, Thulliez P. Toxoplasmosis in rats (Rattus norvegicus): congenital transmission to first and second generation offspring and isolation of Toxoplasma gondii from seronegative rats. Parasitology. 1997;115(1):9–14. doi: 10.1017/s0031182097008950. [DOI] [PubMed] [Google Scholar]
- 28.Kijlstra A, Meerburg B, Cornelissen J, de Craeye S, Vereijken P, Jongert E. The role of rodents and shrews in the transmission of Toxoplasma gondii to pigs. Veterinary Parasitology. 2008;156(3-4):183–190. doi: 10.1016/j.vetpar.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 29.Murphy RG, Williams RH, Hughes JM, Hide G, Ford NJ, Oldbury DJ. The urban house mouse (Mus domesticus) as a reservoir of infection for the human parasite Toxoplasma gondii an unrecognised public health issue? International Journal of Environmental Health Research. 2008;18(3):177–185. doi: 10.1080/09603120701540856. [DOI] [PubMed] [Google Scholar]


