Abstract
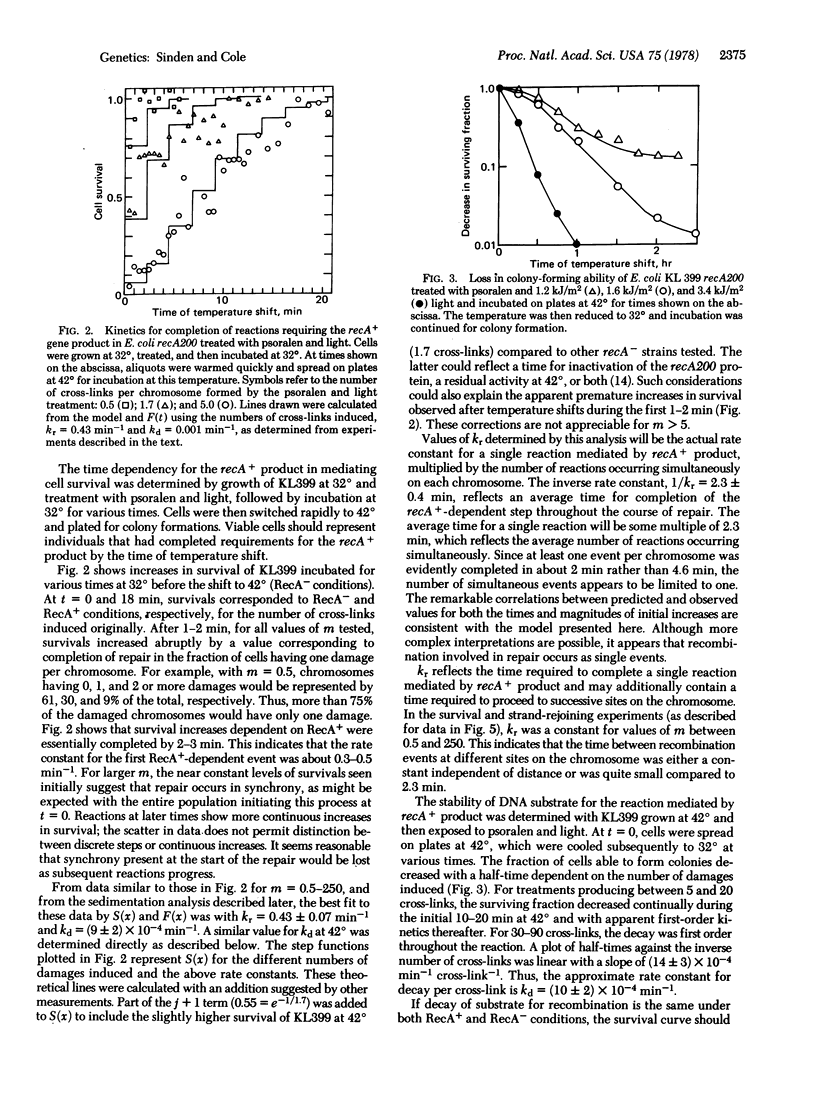
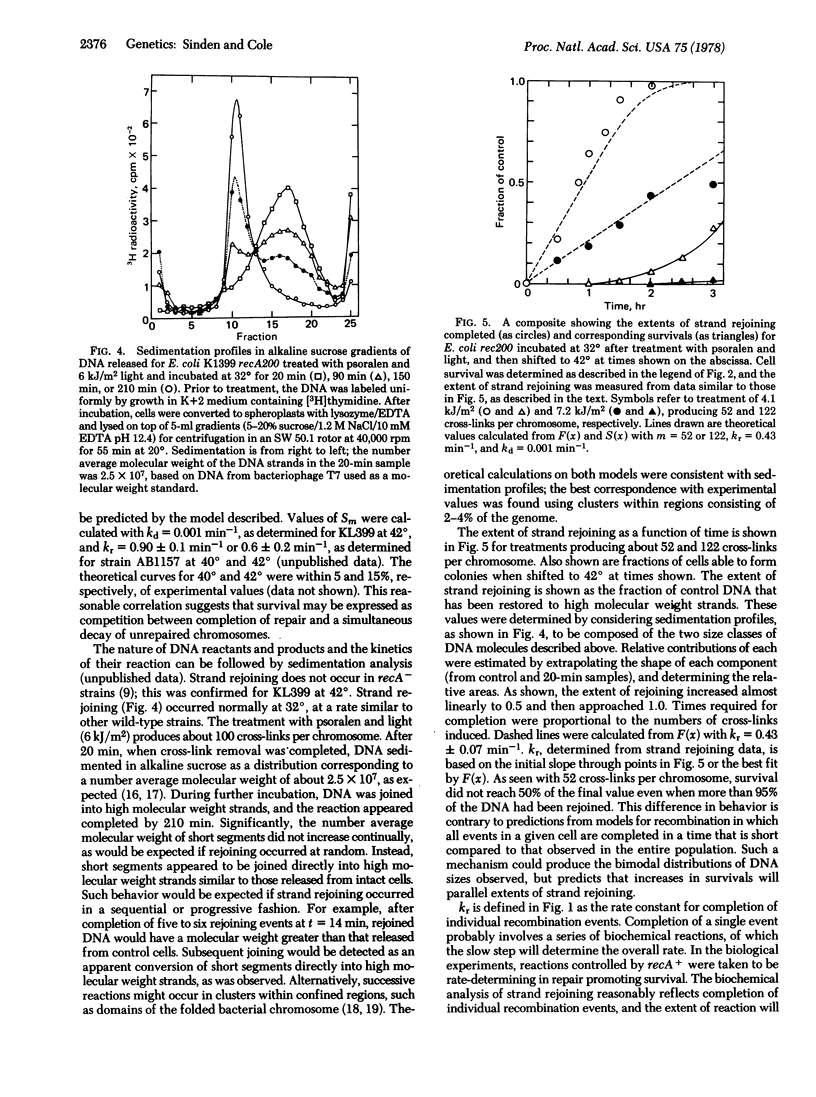
Genetic exchanges appear to be involved in repair of cross-linked DNA. Kinetics for completion of repair and strand rejoining controlled by the recA+ gene were examined in Escherichia coli treated with psoralen and light. The results suggest the following model for genetic recombination. After cross-linking treatment, cells in a population initiate repair in near synchrony. Removal of DNA cross-links, preparation of substrate for recombination, and initiation of the first recA-dependent event are completed in less than 1 min. Recombination events occur singly in each cell or chromosome, and require 2.3 ± 0.4 min at 32° for the recA+-dependent step. After completion of the first event, subsequent recombination events occur in a sequential or progressive fashion around the chromosome or in clusters which may consist of one or more domains of the folded chromosome. The time required to proceed to successive sites is either a constant, independent of the distance on the chromosome, or is quite small compared to 2.3 min. DNA substrate for recombination decays with approximate first-order kinetics and the rate is dependent on the number of unrepaired sites. Cell survival can be expressed as a competition between completion of all repair events and the simultaneous decay of chromosomes to forms not reparable by recombination.
Equations relating kinetics for completion of repair, the size distribution of DNA molecules, and cell survival are derived for the above model, using as parameters only rate constants for recombination and decay of substrate, and number of events per chromosome. An excellent correlation is found between experimentally determined and theoretical values.
Keywords: DNA cross-links, recA, DNA repair
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cassuto E., Gross N., Bardwell E., Howard-Flanders P. Genetic effects of photoadducts and photocross-links in the DNA of phage lambda exposed to 360 nm light and tri-methylpsoralen or khellin. Biochim Biophys Acta. 1977 Apr 19;475(4):589–600. doi: 10.1016/0005-2787(77)90319-7. [DOI] [PubMed] [Google Scholar]
- Clark A. J. Recombination deficient mutants of E. coli and other bacteria. Annu Rev Genet. 1973;7:67–86. doi: 10.1146/annurev.ge.07.120173.000435. [DOI] [PubMed] [Google Scholar]
- Cole R. S. Inactivation of Escherichia coli, F' episomes at transfer, and bacteriophage lambda by psoralen plus 360-nm light: significance of deoxyribonucleic acid cross-links. J Bacteriol. 1971 Sep;107(3):846–852. doi: 10.1128/jb.107.3.846-852.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R. S., Levitan D., Sinden R. R. Removal of psoralen interstrand cross-links from DNA of Escherichia coli: mechanism and genetic control. J Mol Biol. 1976 May 5;103(1):39–59. doi: 10.1016/0022-2836(76)90051-6. [DOI] [PubMed] [Google Scholar]
- Cole R. S. Properties of F' factor deoxyribonucleic acid transferred from ultraviolet-irradiated donors: photoreactivation in the recipient and the influence of recA, recB, recC, and uvr genes. J Bacteriol. 1971 Apr;106(1):143–149. doi: 10.1128/jb.106.1.143-149.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R. S. Repair of DNA containing interstrand crosslinks in Escherichia coli: sequential excision and recombination. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1064–1068. doi: 10.1073/pnas.70.4.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudas L. J., Pardee A. B. Model for regulation of Escherichia coli DNA repair functions. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2330–2334. doi: 10.1073/pnas.72.6.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD-FLANDERS P., THERIOT L. A method for selecting radiation-sensitive mutants of Escherichia coli. Genetics. 1962 Sep;47:1219–1224. doi: 10.1093/genetics/47.9.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertman I., Luria S. E. Transduction studies on the role of a rec+ gene in the ultraviolet induction of prophage lambda. J Mol Biol. 1967 Jan 28;23(2):117–133. doi: 10.1016/s0022-2836(67)80021-4. [DOI] [PubMed] [Google Scholar]
- Holloman W. K., Radding C. M. Recombination promoted by superhelical DNA and the recA gene of Escherichia coli. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3910–3914. doi: 10.1073/pnas.73.11.3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss R. D. Models of genetic recombination. Annu Rev Microbiol. 1974;28(0):445–468. doi: 10.1146/annurev.mi.28.100174.002305. [DOI] [PubMed] [Google Scholar]
- Lin P. F., Bardwell E., Howard-Flanders P. Initiation of genetic exchanges in lambda phage--prophage crosses. Proc Natl Acad Sci U S A. 1977 Jan;74(1):291–295. doi: 10.1073/pnas.74.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd R. G., Low B., Godson G. N., Birge E. A. Isolation and characterization of an Escherichia coli K-12 mutant with a temperature-sensitive recA- phenotype. J Bacteriol. 1974 Oct;120(1):407–415. doi: 10.1128/jb.120.1.407-415.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEntee K., Hesse J. E., Epstein W. Identification and radiochemical purification of the recA protein of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3979–3983. doi: 10.1073/pnas.73.11.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meselson M. S., Radding C. M. A general model for genetic recombination. Proc Natl Acad Sci U S A. 1975 Jan;72(1):358–361. doi: 10.1073/pnas.72.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim A. B., Riley M. Covalent union of parental DNA's following conjugation in Escherichia coli. J Mol Biol. 1967 Sep 28;28(3):503–511. doi: 10.1016/s0022-2836(67)80100-1. [DOI] [PubMed] [Google Scholar]
- Radding C. M. Molecular mechanisms in genetic recombination. Annu Rev Genet. 1973;7:87–111. doi: 10.1146/annurev.ge.07.120173.000511. [DOI] [PubMed] [Google Scholar]
- Rupp W. D., Howard-Flanders P. Discontinuities in the DNA synthesized in an excision-defective strain of Escherichia coli following ultraviolet irradiation. J Mol Biol. 1968 Jan 28;31(2):291–304. doi: 10.1016/0022-2836(68)90445-2. [DOI] [PubMed] [Google Scholar]
- Stonington O. G., Pettijohn D. E. The folded genome of Escherichia coli isolated in a protein-DNA-RNA complex. Proc Natl Acad Sci U S A. 1971 Jan;68(1):6–9. doi: 10.1073/pnas.68.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worcel A., Burgi E. On the structure of the folded chromosome of Escherichia coli. J Mol Biol. 1972 Nov 14;71(2):127–147. doi: 10.1016/0022-2836(72)90342-7. [DOI] [PubMed] [Google Scholar]
- Yoakum G. H., Cole R. S. Role of ATP in removal of psoralen cross-links from DNA of Escherichia coli permeabilized by treatment with toluene. J Biol Chem. 1977 Oct 25;252(20):7023–7030. [PubMed] [Google Scholar]


