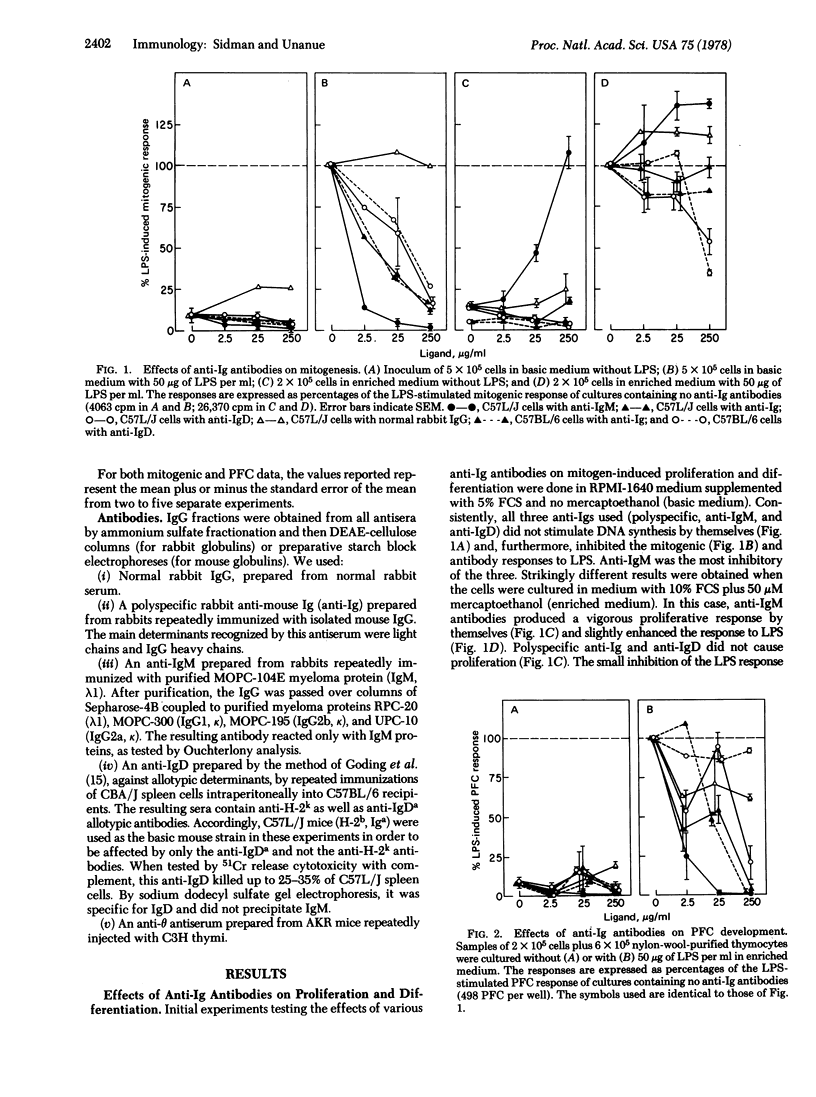
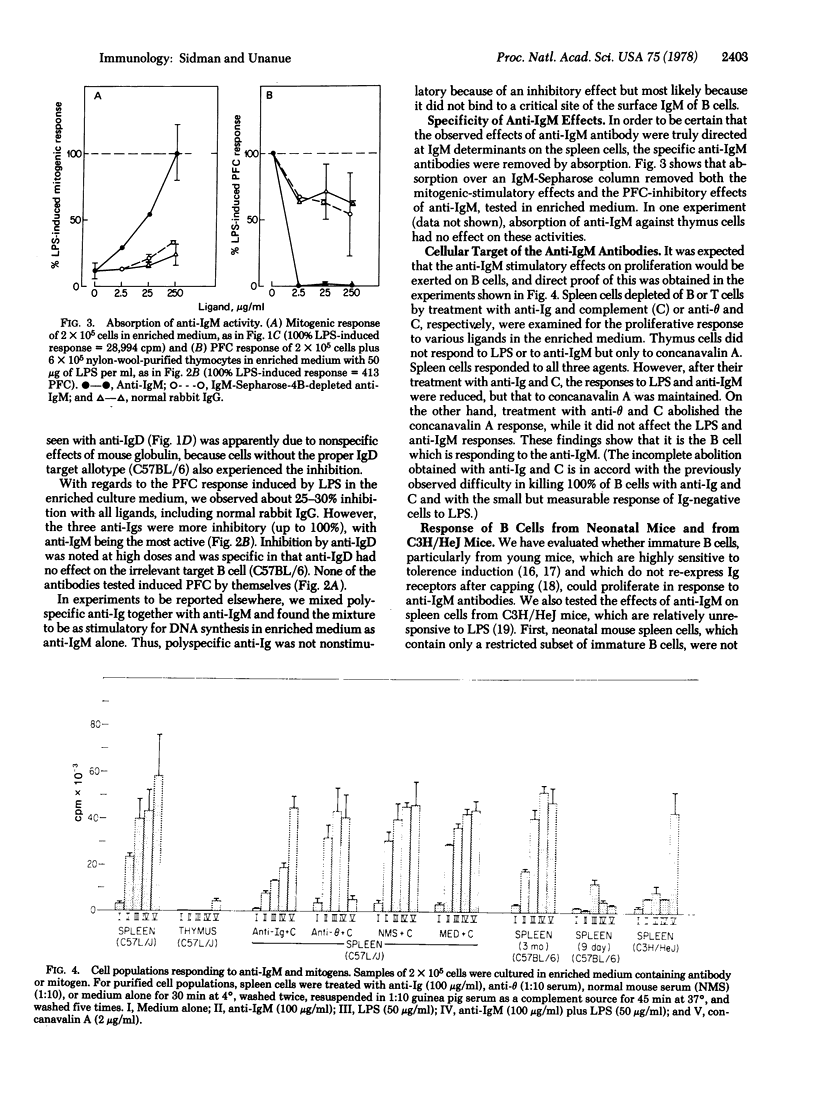
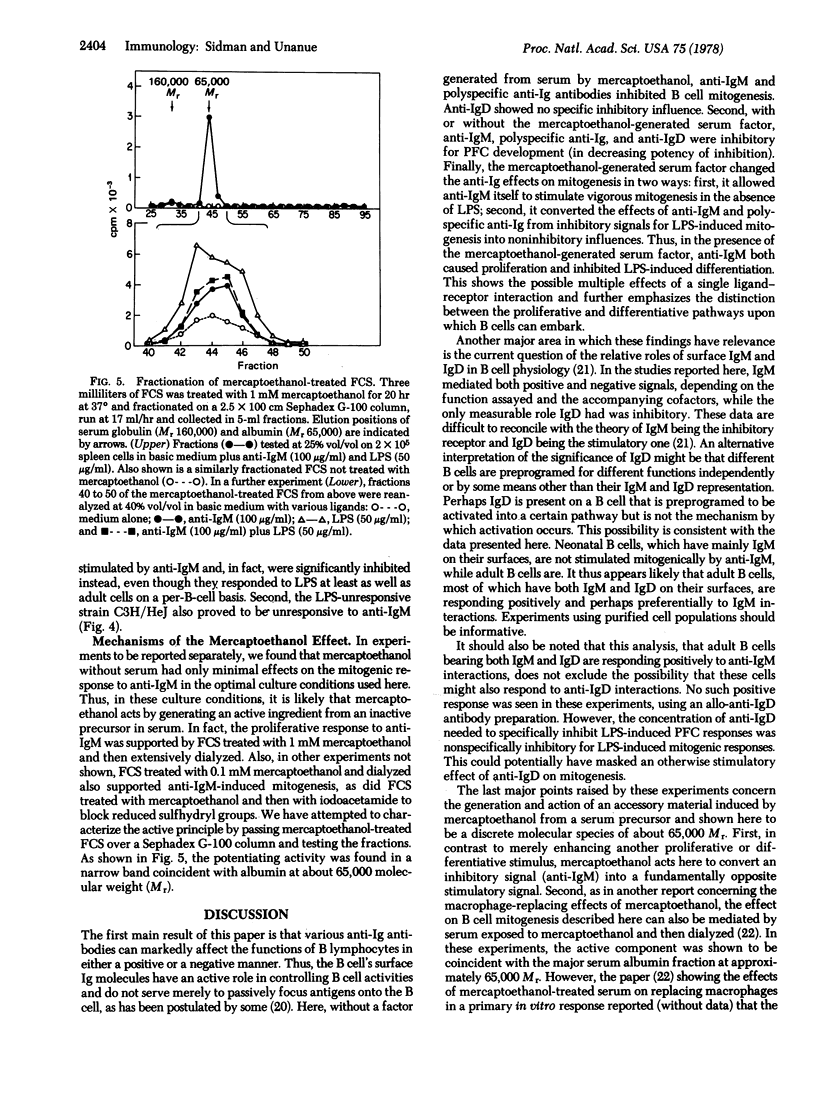
Abstract
The effects of various anti-Ig antibodies on different B lymphocyte functions were investigated. With the proper accessory cofactor(s) derived from serum, anti-IgM antibodies induced a vigorous proliferative response in normal adult murine B cells, while polyspecific anti-Ig and anti-IgD had no effect. Without the required cofactor, all three anti-Ig antibodies were inhibitory for mitogenic responses. All three anti-Ig antibodies were also inhibitory for mitogen-induced antibody responses with or without the cofactor. Even with the required cofactor, neonatal B cells as well as adult C3H/HeJ B cells were not triggered into proliferation by anti-IgM. Finally, the cofactor required for anti-IgM-triggered mitogenesis was shown to be generated from serum by 2-mercaptoethanol and to be approximately 65,000 in molecular weight. These results indicate that, for at least some responses, B lymphocyte surface IgM molecules are involved in both triggering and suppression, depending both on the developmental state of the B cell and the presence or absence of accessory influences. In these experiments, IgD gave evidence only of being suppressive.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Bullock W. W., Melchers F. Inhibition of mitogenic stimulation of mouse lymphocytes by anti-mouse immunoglobulin antibodies. I. Mode of action. Eur J Immunol. 1974 Nov;4(11):715–722. doi: 10.1002/eji.1830041103. [DOI] [PubMed] [Google Scholar]
- Cambier J. C., Kettman J. R., Vitetta E. S., Uhr J. W. Differential susceptibility of neonatal and adult murine spleen cells to in vitro induction of B-cell tolerance. J Exp Med. 1976 Jul 1;144(1):293–297. doi: 10.1084/jem.144.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho A., Möller G. Thymus-independent B-cell induction and paralysis. Adv Immunol. 1975;21:113–236. doi: 10.1016/s0065-2776(08)60220-5. [DOI] [PubMed] [Google Scholar]
- Elson C. J., Singh J., Taylor R. B. The effect of capping by anti-immunoglobulin antibody on the expression of cell surface immunoglobulin and on lymphocyte activation. Scand J Immunol. 1973;2(2):143–149. doi: 10.1111/j.1365-3083.1973.tb02025.x. [DOI] [PubMed] [Google Scholar]
- Goding J. W., Warr G. W., Warner N. L. Genetic polymorphism of IgD-like cell surface immunoglobulin in the mouse. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1305–1309. doi: 10.1073/pnas.73.4.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T., Miyake T., Nishizawa Y., Watanabe T., Yamamura Y. Triggering mechanism of B lymphocytes. I. Effect of anti-immunoglobulin and enhancing soluble factor on differentiation and proliferation of B cells. J Immunol. 1975 Nov;115(5):1179–1184. [PubMed] [Google Scholar]
- Lawton A. R., 3rd, Asofsky R., Hylton M. B., Cooper M. D. Suppression of immunoglobulin class synthesis in mice. I. Effects of treatment with antibody to -chain. J Exp Med. 1972 Feb 1;135(2):277–297. doi: 10.1084/jem.135.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonai P., McDevitt H. O. Genetic control of the immune response: in vitro stimulation of lymphocytes by (T,G)-A--L, (H,G)-A--L, and (Phe,G)-A--L. J Exp Med. 1974 Oct 1;140(4):977–994. doi: 10.1084/jem.140.4.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning D. D., Jutila J. W. Immunosuppression of mice injected with heterologous anti-immunoglobulin heavy chain antisera. J Exp Med. 1972 Jun 1;135(6):1316–1333. doi: 10.1084/jem.135.6.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchers F., Braun V., Galanos C. The lipoprotein of the outer membrane of Escherichia coli: a B-lymphocyte mitogen. J Exp Med. 1975 Aug 1;142(2):473–482. doi: 10.1084/jem.142.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf E. S., Klinman N. R. In vitro tolerance induction of neonatal murine B cells. J Exp Med. 1976 Jun 1;143(6):1327–1340. doi: 10.1084/jem.143.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opitz H. G., Opitz U., Lemke H., Hewlett G., Schreml W., Flad H. D. The role of fetal calf serum in the primary immune response in vitro. J Exp Med. 1977 Apr 1;145(4):1029–1038. doi: 10.1084/jem.145.4.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker D. C. Stimulation of mouse lymphocytes by insoluble anti-mouse immunoglobulin. Nature. 1975 Nov 27;258(5533):361–363. doi: 10.1038/258361a0. [DOI] [PubMed] [Google Scholar]
- SELL S., GELL P. G. STUDIES ON RABBIT LYMPHOCYTES IN VITRO. I. STIMULATION OF BLAST TRANSFORMATION WITH AN ANTIALLOTYPE SERUM. J Exp Med. 1965 Aug 1;122:423–440. doi: 10.1084/jem.122.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader J. W. Antagonism of B lymphocyte mitogenesis by anti-immunoglobulin antibody. J Immunol. 1975 Aug;115(2):323–326. [PubMed] [Google Scholar]
- Scribner D. J., Weiner H. L., Moorhead J. W. Surface immunoglobulin and Fc receptors on murine B lymphocytes: loss of receptor interaction after cell activation. J Immunol. 1977 Dec;119(6):2084–2088. [PubMed] [Google Scholar]
- Sidman C. L., Unanue E. R. Control of B-lymphocyte function. I. Inactivation of mitogenesis by interactions with surface immunoglobulin and Fc-receptor molecules. J Exp Med. 1976 Oct 1;144(4):882–896. doi: 10.1084/jem.144.4.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman C. L., Unanue E. R. Receptor-mediated inactivation of early B lymphocytes. Nature. 1975 Sep 11;257(5522):149–151. doi: 10.1038/257149a0. [DOI] [PubMed] [Google Scholar]
- Sieckmann D. G., Asofsky R., Mosier D. E., Zitron I. M., Paul W. E. Activation of mouse lymphocytes by anti-immunoglobulin. I. Parameters of the proliferative response. J Exp Med. 1978 Mar 1;147(3):814–829. doi: 10.1084/jem.147.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vischer T. L., Jaquet C. Effect of antibodies against immunoglobulins and the theta antigen on the specific and non-specific stimulation of mouse spleen cells in vitro. Immunology. 1972 Feb;22(2):259–266. [PMC free article] [PubMed] [Google Scholar]
- Vitetta E. S., Uhr J. W. IgD and B cell differentiation. Immunol Rev. 1977;37:50–88. doi: 10.1111/j.1600-065x.1977.tb00245.x. [DOI] [PubMed] [Google Scholar]
- Watson J., Riblet R. Genetic control of responses to bacterial lipopolysaccharides in mice. I. Evidence for a single gene that influences mitogenic and immunogenic respones to lipopolysaccharides. J Exp Med. 1974 Nov 1;140(5):1147–1161. doi: 10.1084/jem.140.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner H. L., Moorhead J. W., Claman H. N. Anti-immunoglobulin stimulation of murine lymphocytes. I. Age dependency of the proliferative response. J Immunol. 1976 Jun;116(6):1656–1661. [PubMed] [Google Scholar]