Abstract
Oropharyngeal squamous cell carcinoma (SCC) is frequently related to high risk human papillomavirus. This tumor expresses p16, frequently has a nonkeratinizing morphology, and has improved outcomes. Despite having a good prognosis, tumors can have focal or diffuse nuclear anaplasia or multinucleation, the significance of which is unknown. From a database of 270 oropharyngeal SCCs with known histologic typing (using our established system) and p16 immunohistochemistry, all surgically resected cases (149) were reviewed. Anaplasia was defined as any ×40 field with ≥ 3 tumor nuclei with diameters ≥ 5 lymphocyte nuclei (~25 μm), and multinucleation was defined as any ×40 field with ≥ 3 tumor cells with multiple nuclei. p16 was positive in 128 cases (85.9%), 64 cases (43.0%) showed anaplasia, and 71 (47.7%) showed multinucleation. Anaplasia and multinucleation were highly related (P < 0.001), and both also correlated with histologic type (P < 0.001 and P = 0.01, respectively), p16 status (P = 0.09 and 0.03, respectively), and partially with nodal extracapsular extension. There was no correlation with any of the other variables. In univariate analysis, cases showing anaplasia or multinucleation had worse overall, disease-specific, and disease-free survival (P < 0.006 for all). Higher T-stage, keratinizing histologic type, extracapsular extension, and smoking also all correlated with worse survival. In multivariate analysis, anaplasia and multinucleation both predicted worse disease-specific survival (hazard ratio 9.9, P = 0.04; and hazard ratio 11.9, P = 0.02, respectively) independent of the other variables. In summary, among surgically resectable oropharyngeal SCC (including among just the p16-positive cohort), tumor cell anaplasia and multinucleation independently correlated with disease recurrence and poorer survival.
Keywords: anaplasia, multinucleation, oropharyngeal squamous cell carcinoma, p16, human papillomavirus
Over the past several decades, a distinct type of oropharyngeal squamous cell carcinoma (SCC), which is associated with transcriptionally active, high-risk human papillomavirus (HPV), has been recognized and increasingly well characterized.1,4 It is now very clear that the incidence of these tumors is steadily increasing,23 whereas that of most other head and neck SCC types is decreasing, likely because of decreasing smoking rates.14,31,33 Because of its relationship to an infectious agent and its incidence pattern, HPV-related oropharyngeal SCC has been termed by many as constituting a cancer “epidemic.”21,23,33
The biology of HPV in these tumors and its association with clinical outcomes have also been increasingly well characterized over the past decade. On the basis of abundant retrospective20,25,37 and more recent prospective data,4,28 HPV-related oropharyngeal SCC is now recognized as a unique disease, having different patient demographics, a distinct molecular profile, and a significantly better prognosis,34 regardless of primary treatment type, whether primary surgery, induction chemotherapy, or definitive radiation or chemoradiation.10,20,27,36 The morphology is also distinct, with HPV-related SCCs usually classified as nonkeratinizing (NK).7,20
Many advocate for p16 immunohistochemistry as a screening test to be followed by, when positive, an HPV-specific test such as DNA in situ hybridization, polymerase chain reaction, or potentially both.6,24,29 There is an emerging view, however, that the strong risk stratification for patient survival provided by p16 immunohistochemistry in oropharyngeal SCC, and its practicality (being widely available clinically), make it a very suitable single marker for defining a patient's tumor as having the HPV biological signature and thus unique biology and prognosis.20,26,28,30,36 However, this is an area in which best practice is still being delineated.6
The therapies for head and neck SCC are associated with substantial morbidity (and occasionally even mortality). As the proportion of good prognosis HPV-related oropharyngeal SCCs among all head and neck SCCs has been increasing, many have been calling for changes and for instituting studies to investigate either decreasing the doses of therapy or using alternative therapies for these patients.2,24 As such, it is becoming clear that we need to be able to identify those patients under the larger umbrella of p16 positive/HPV-related oropharyngeal SCC who do not have a cancer of an indolent nature with good prognosis. Identifying prognostic subgroups among the HPV-related cases would allow for the lower-risk patients to be treated less intensively, whereas the higher-risk patients can be given the more traditional, more intense therapies that are appropriate to their cancers. However, very few factors have been shown to correlate with disease recurrence in just the p16 positive/HPV-related SCC patients. Clinical features such as T-stage and active smoking status do so, to an extent.4,27,36,12 Although many molecular changes have been associated with worse prognosis in oropharyngeal SCC, very few molecular changes in just the HPV-positive cohorts have been shown to stratify outcomes.9 It is clear that better markers are needed, and, in particular, we need a better understanding of the biological heterogeneity among p16 positive/HPV-related oropharyngeal SCC cases.
Recently, we have observed cases of NK SCC that have focal or diffuse nuclear anaplasia and/or tumor cell multinucleation. As NK SCC is typically associated with a favorable prognosis, the presence of such markedly atypical cells seems paradoxical. In many other malignancies, the presence of anaplasia is a major adverse prognostic indicator. Thus, we sought to explore whether this tumor cell anaplasia and multinucleation had any correlation with other clinical or pathologic features or with patient outcomes in oropharyngeal SCC, and specifically amongst just the subset of p16 positive/HPV-related cases.
MATERIALS AND METHODS
Cases of oropharyngeal SCC were identified from Human Research Protection Office-approved clinical databases from the Departments of Radiation Oncology and Otolaryngology Head and Neck Surgery at Washington University to develop a database of approximately 275 tumors.20 For the surgically treated patients, all cases were done so for primary disease, and none of them received radiation or any other cancer therapy before surgery. Also, none of the patients was treated differently based on their tumor HPV or p16 status. All patients who received radiation received intensity-modulated radiation therapy.
From this larger database, we included all patients who underwent surgical resection of their primary tumors and corresponding cervical nodal metastases (if present) and for whom all slides were available for us for review (to assure that we had histologically reviewed all, or at least the majority of, the patients’ tumor tissue). We excluded all patients who had undergone biopsy followed by definitive radiation and/or chemotherapy as we could not confirm the presence or absence of specific histologic features in the overall tumors when only evaluating a small biopsy specimen.
Histologic Review for Tumor Typing
Hematoxylin and eosin slides of the cases were previously histologically typed using our established system20,36 by a single study pathologist (J.S.L.). Keratinizing-type SCC (type 1) consists entirely of maturing squamous epithelium with no areas with NK or “basal” morphology (Fig. 1A). The cells have polygonal shapes with abundant, eosinophilic (keratinizing) cytoplasm, distinct cell borders, and intercellular bridges. The nests are usually angulated and irregular, and there is frequently marked stromal desmoplasia. Actual keratin formation is common but is not required as long as the cells have a prominent eosinophilic cytoplasm along with other features. NK SCC (type 3) consists of sheets, nests, or trabeculae of oval and frequently spindled, hyper-chromatic cells with indistinct cell borders and absence of prominent nucleoli (Fig. 1B). They have very little or only modest amounts of eosinophilic cytoplasm. Comedo-type necrosis and brisk mitotic activity are usually present. There is typically no (or minimal) stromal reaction to the invading tumor. Portions of the tumor can show squamous maturation, characterized by polygonal cells with mature, eosinophilic cytoplasm, distinct cell borders, intercellular bridges, and keratin pearls, but these mature areas must constitute <10% of the total surface area. NK SCC with maturation (“hybrid SCC” or type 2) is an intermediate group and consists of definitive areas with NK SCC morphology but also having maturing squamous differentiation comprising >10% of total surface area (Fig. 1C). These “maturing areas” have cells with more abundant, eosinophilic cytoplasm, nuclei with open chromatin and/or prominent nucleoli, irregular, angulated nests with stromal desmoplasia, or areas of frank keratinization. They also frequently show “reverse maturation” where the basal-appearing cells are central in the nests and the cells at the periphery show squamous maturation. Other rare histologic types such as basaloid, spindle cell, undifferentiated, and adenosquamous carcinoma were diagnosed on the basis of their published features and excluded.
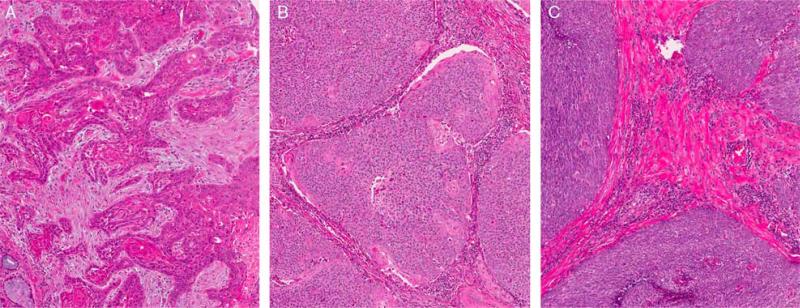
FIGURE 1.
Histologic types of oropharyngeal SCC, all lacking tumor cell anaplasia and multinucleation. A, Keratinizing-type SCC (type 1) showing angulated tumor nests in a desmoplastic stroma and with tumor cells showing abundant eosinophilic (keratinized) cytoplasm with patchy formation of actual keratin. B, NK SCC with maturation (“hybrid” or type 2) showing a tumor composed of large nests with rounded/smooth contours with little to no stromal reaction and composed predominantly of cells with little cytoplasm and hyperchromatic, round to oval nuclei with inconspicuous nucleoli. There is >10% maturing squamous differentiation with foci where the tumor cells have more abundant, eosinophilic cytoplasm. These maturing areas are predominantly at the periphery of the nests (so-called “reverse maturation”). C, NK SCC consisting of large nests of basophilic tumor cells with smooth borders and little to no stromal reaction. The tumor cells have round to oval, hyperchromatic nuclei with inconspicuous nucleoli and little cytoplasm. No significant maturing squamous differentiation is present (all images are at 200X magnification and hematoxylin and eosin stained).
Histologic Review for Tumor Cell Anaplasia and Multinucleation
All tumor-containing slides from each case were reviewed independently by both study pathologists (J.B.S. and J.S.L.) and classified as having nuclear anaplasia and/or tumor cell multinucleation using specific definitions. Nuclear anaplasia was defined as any ×400 magnification field (area = 0.2 mm2) with ≥ 3 nuclei with diameters equal to or wider than 5 lymphocyte nuclei (~25 μm) (Fig. 2). Tumor cell multinucleation was defined as any ×400 magnification field with ≥ 3 tumor cells clearly having multiple nuclei (Fig. 3). The location of the anaplasia or multinucleation focus (or foci) was recorded, whether within the primary tumor and/or in a nodal metastasis. The relative amount of the change present was also recorded using the classifiers of focal (scattered, rare foci meeting criteria), multifocal (many different foci meeting criteria), and diffuse (extensive areas meeting criteria). After independent review, all discrepant cases were resolved by consensus review by both pathologists together at the same microscope.
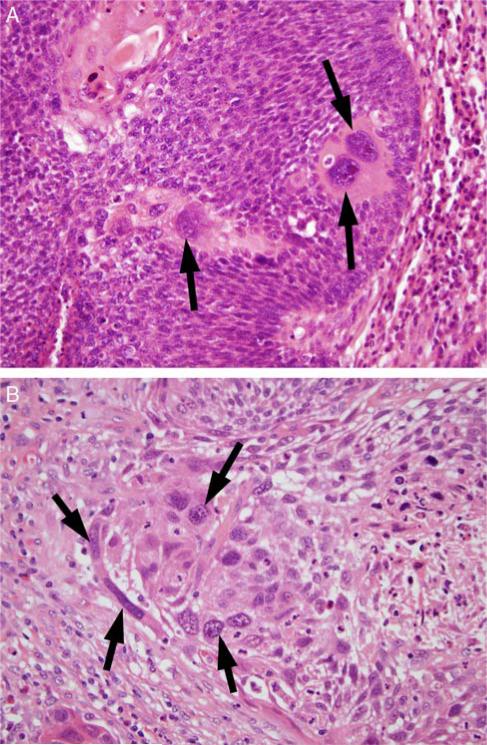
FIGURE 2.
Tumor cell anaplasia, defined as ≥ 3 tumor cell nuclei in one HPF, which are equal to or wider than 5 lymphocyte nuclei (~25 μm) in diameter. A, A single high-power field of NK SCC (type 3) with 3 anaplastic tumor nuclei (arrows) in a background of smaller tumor nuclei. B, A single high-power field of a keratinizing-type SCC (type 1) with >3 anaplastic tumor nuclei (arrows) (both images at 400X magnification and are hematoxylin and eosin stained).
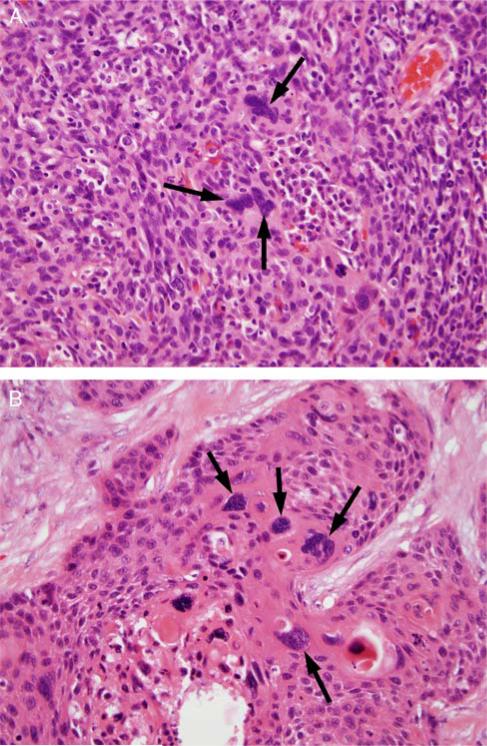
FIGURE 3.
Tumor cell multinucleation, defined as ≥ 3 tumor cell nuclei in one HPF which are definitively multinucleated. A, A single high-power field of NK SCC (type 3) with 3 multinucleated tumor cells (arrows). B, A single high-power field of a maturing area of a NK SCC with maturation (type 2) with >3 multinucleated tumor cells (arrows) (both images are at 400X magnification and hematoxylin and eosin stained).
The node-positive cases had also been characterized for extracapsular extension in previous studies using an established classification system as: no extracapsular extension, simple extracapsular extension, and soft tissue metastasis (masses of tumor with no residual nodal tissue or architecture such as discrete lymphoid tissue with germinal centers or a subcapsular sinus). For soft tissue metastasis, we included any size of deposit, large or small, that was discrete, irregular, and without residual nodal architecture. All cases were classified on the basis of worst extent, and analysis was divided binarily into those with no extracapsular extension versus any pattern of extracapsular extension or those with no or simple extracapsular extension versus soft tissue metastasis, as previous analysis had shown these to be the only clinically significant cutoffs in oropharyngeal SCC.25
p16 Immunohistochemistry
p16 immunohistochemistry had been performed on all cases as previously published20,36 on representative 4-mm-thick sections cut from formalin-fixed, paraffin-embedded tissue blocks using a monoclonal antibody to p16 (MTM Laboratories; monoclonal; 1:1 dilution) on a Ventana Benchmark LT automated immunostainer (Ventana Medical Systems Inc., Tucson, AZ) according to standard protocols. Antigen retrieval, standard on the machine, used the Ventana CC1, EDTA-Tris, pH 8.0 solution. A known p16-expressing head and neck SCC case was used as the positive control, and sections of normal tonsil were used for negative controls with each run. Staining was nuclear and cytoplasmic in all cases and was graded binarily as positive if staining was present in >50% of the tumor cells and negative if no staining was present or if it was present in <50% of the tumor cells. This cutoff was chosen on the basis of the literature showing that only cases with extensive p16 expression have transcriptionally active HPV19 and improved prognosis.4
Statistical Analyses
We examined associations between anaplasia, multinucleation, and other clinical and pathologic variables of interest using the Pearson χ2 or Fisher exact tests as appropriate. The primary interest of the study was to investigate prognostic ability of variables for survival endpoints. Overall survival, disease-free survival, and disease-specific survival were defined as the time interval between the date of surgical resection of the patient's tumor and the date of death due to any cause, the date of death due to any cause or the date of first tumor recurrence, and the date of death for patients with known recurrent cancer, respectively. Empirical survival probabilities were estimated using the Kaplan-Meier product limit method and illustrated by Kaplan-Meier curves, whereas log-rank tests were used to examine survival differences, indicating the significance of a variable being prognostic for a survival endpoint. We also performed multivariate Cox proportional hazard models to investigate the independent prognostic ability of variables of interest after accounting for classic clinical variables, indicated by hazard ratios (HRs), associated 95% confidence intervals, and Wald test P-values. Proportional hazard assumption was examined by diagnostic graphics and tests. For the survival analyses, 6 years after surgery was used as the end of study time because of too few patients with at least 6 years of follow-up and to obtain reliable estimates of survival probability 6 years after surgery. All tests were 2 sided with the level for significance set at 0.05. All analyses were carried out using statistical software R 2.13.1 (http://www.r-project.org).
RESULTS
Demographic features of the 149 cases are presented in Table 1. p16 immunohistochemistry was positive in 125 cases (85.9%). After consensus review, 64 cases (43.0%) met criteria for nuclear anaplasia and 71 (47.7%) for multinucleation. Anaplasia and multinucleation were highly related (P < 0.001; Supplementary Table 1, Supplemental Digital Content http://links.lww.com/PAS/A119), with 85 of the 149 cases (57.0%) showing either anaplasia or multinucleation and 50 of these 85 cases (58.8%) showing both. The distribution of anaplasia and multinucleation is presented in Supplementary Table 2, Supplemental Digital Content http://links.lww.com/PAS/A119. When present, both features were usually present in both the primary tumor and nodal metastases (> 75% for each), although there was a significant minority of cases in which they were present only in one component or the other. Both anaplasia and multinucleation were multifocal or diffuse in the majority of cases (76.2% and 59.2% of the cases that were positive, respectively; Supplementary Table 2, Supplemental Digital Content http://links.lww.com/PAS/A119) as well.
TABLE 1.
Demographic, Clinical, and Pathologic Characteristics by Group
| Group (#) | All (149) | Anaplasia Positive (64) |
Anaplasia Negative (85) |
P † | Multinucleation Positive (71) |
Multinucleation Negativef (78) |
P † |
|---|---|---|---|---|---|---|---|
| Age (mean ± SD) | 56.6 ± 8.6 | 56.8 ± 9.3 | 56.5 ± 8.0 | 0.85† | 57.2± 9.4 | 57.0 ± 10.4 | 0.43 |
| Sex (%) | 0.56 | 0.90 | |||||
| Male | 137 (91.9) | 60 (93.8) | 85 (91.4) | 65 (91.5) | 72 (92.3) | ||
| Female | 12 (8.1) | 4 (6.2) | 8 (8.6) | 6 (8.5) | 6 (7.7) | ||
| Race (%) | 0.41 | 0.32 | |||||
| White | 134 (90.5) | 56 (87.5) | 78 (92.9) | 62 (87.3) | 72 (93.5) | ||
| Other | 14 (9.5) | 8 (12.5) | 6 (7.1) | 9 (12.7) | 5 (6.5) | ||
| Smoking (%) | 0.056 | 0.35 | |||||
| Yes (current or former) | 95 (66.4) | 45 (76.3) | 50 (59.5) | 47 (71.2) | 48 (62.3) | ||
| No (never) | 48 (33.6) | 14 (23.7) | 34 (40.5) | 19 (28.8) | 29 (37.7) | ||
| T-stage (%) | 0.39 | 0.96 | |||||
| T1/T2 | 100 (67.1) | 40 (62.5) | 60 (70.5) | 47 (66.2) | 53 (67.9) | ||
| T3/T4 | 49 (32.9) | 24 (37.5) | 25 (29.5) | 24 (33.8) | 25 (32.1) | ||
| N-stage (%) | 0.84 | 0.32 | |||||
| N0 | 16 (10.9) | 7 (11.1) | 9 (10.7) | 10 (14.3) | 6 (7.8) | ||
| N1-3 | 131 (89.1) | 56 (88.9) | 75 (89.3) | 60 (85.7) | 71 (92.2) | ||
| Extracapsular extension | 0.10 | 0.004 | |||||
| None | 29 (34.9) | 11 (25.5) | 18 (45.0) | 8 (19.0) | 21 (51.2) | ||
| Present | 54 (65.1) | 32 (74.5) | 22 (55.0) | 34 (81.0) | 20 (48.8) | ||
| Extracapsular extension | 0.66 | 0.13 | |||||
| None or limited | 53 (63.9) | 26 (60.4) | 27 (67.5) | 23 (54.8) | 30 (73.2) | ||
| Extensive (STM) | 30 (36.1) | 17 (39.6) | 13 (32.5) | 19 (45.2) | 11 (26.8) | ||
| Resection margins (%) | 0.44 | 0.20 | |||||
| Negative | 125 (86.8) | 55 (90.2) | 70 (84.3) | 62 (82.7) | 63 (91.3) | ||
| Positive | 19 (13.2) | 6 (9.8) | 13 (15.7) | 13 (17.3) | 6 (8.7) | ||
| IMRT (%) | 0.50 | 0.31 | |||||
| Yes | 139 (93.9) | 59 (92.2) | 80 (95.2) | 65 (91.5) | 74 (96.1) | ||
| No | 9 (6.1) | 5 (78.1) | 4 (4.8) | 6 (8.5) | 3 (3.9) | ||
| Chemotherapy (%) | 0.91 | 0.57 | |||||
| Yes | 58 (45.3) | 32 (56.1) | 38 (53.5) | 36 (58.1) | 34 (51.5) | ||
| No | 70 (54.7) | 25 (43.9) | 33 (46.5) | 26 (41.9) | 32 (48.5) | ||
| Histologic type | < 0.0001 | 0.01 | |||||
| K SCC (type 1) | 22 (14.7) | 15 (23.4) | 7 (8.2) | 16 (22.5) | 6 (7.7) | ||
| NK with maturation (type 2) | 33 (22.1) | 20 (31.2) | 13 (15.3) | 18 (25.6) | 15 (19.2) | ||
| NK SCC (type 3) | 94 (63.1) | 29 (45.3) | 65 (76.5) | 37 (52.1) | 57 (73.1) | ||
| p16 IHC* | 0.10 | 0.03 | |||||
| Positive | 128 (85.9) | 51 (79.7) | 77 (90.6) | 56 (78.9) | 72 (92.3) | ||
| Negative | 21 (14.1) | 13 (20.3) | 8 (9.4) | 15 (21.1) | 6 (7.7) |
Values in bold are statistically significant (P < 0.05)
Defined as positive if >50% of tumor cells stained and negative if no staining or < 50% of tumor cells stained.
P-values indicate correlation between demographic, clinical, or pathologic variables with anaplasia and multinucleation. The P-values are derived from a 2-sample t test for age and Χ2 or Fisher exact tests as appropriate for others.
IHC indicates immunohistochemistry; IMRT, intensity-modulated radiation therapy; K SCC, keratinizing-type SCC; STM, soft tissue metastasis.
Correlations between anaplasia and multinucleation and the major clinical and pathologic variables are presented in Table 1. Anaplasia and multinucleation rates were statistically significantly different in keratinizing-type SCC (type 1) and NK SCC with maturation (type 2) compared with NK SCC (P < 0.001 for anaplasia and P = 0.01 for multinucleation). Anaplasia was focal in 4 of 16 (25.0%) of the keratinizing-type (type 1) SCCs that showed it, was focal in 2 of 18 (11.1%) of the NK SCCs with maturation (type 2), and was focal in 16 of 37 (43.2%) of the NK (type 3) SCCs. On comparing the rates between the keratinizing-type SCC and NK SCC with maturation tumors with NK SCC, the NK SCC were statistically significantly more likely to be only focal for anaplasia and multinucleation (P = 0.039).
Among the other variables, anaplasia was more likely to be present in tumors from current or former smokers than in those from lifetime nonsmokers, although only borderline for statistical significance (P = 0.056). Among the cases with nodal metastases, tumor cell multinucleation was slightly more prevalent in cases with extracapsular extension than in those without (P = 0.004). Anaplasia and multinucleation were not correlated with any of the other major variables, most notably not with T-stage (as T1-2 vs. T3-4), N-stage (as N0 vs. N1-3), resection margin status, or postoperative treatment type (either radiation therapy or chemotherapy).
Survival Analysis
For the entire cohort, there was excellent patient survival with low rates of recurrent disease. The study population had an average clinical follow-up time of 4.1 years (range, 0.4 to 9.7 y; median 3.9 y) for surviving patients. Only 18 of the 149 patients (12.1%) suffered disease recurrence of any kind after surgery and postoperative therapy (if administered), and only 9 patients (6.1%) developed distant metastases. Patients whose tumors showed anaplasia suffered recurrence of their cancers in 10 of 64 cases (15.6%) compared with only 4 of 85 patients (4.7%) lacking anaplasia (P = 0.044). Patients whose tumors showed multinucleation suffered recurrence of their cancers in 13 of 71 cases (18.3%) compared with only 5 of 78 patients (6.4%) whose tumors lacked multinucleation (P = 0.042). Patients whose tumors showed only focal anaplasia and/or multinucleation had slightly lower rates of disease recurrence compared with patients with multifocal or diffuse anaplasia and/or multinucleation, but the differences were not statistically significant (P > 0.6 for each). Of the 9 patients who developed distant metastases, 7 (77.8%) had tumors with either anaplasia, multinucleation, or both.
Because the distribution/extent of the findings did not correlate significantly with disease recurrence, for the survival analysis we simply considered anaplasia and multinucleation as binary variables, either present or absent. Among the entire cohort, patients whose tumors did not have anaplasia had overall, disease-free, and disease-specific survival rates of 85%, 81%, and 99%, respectively, compared with 51%, 52%, and 81%, respectively, for those with tumors that had it. Patients whose tumors did not have multinucleation had overall, disease-free, and disease-specific survival rates of 84%, 81%, and 99%, respectively, compared with 53%, 51%, and 83%, respectively, for those with tumors that had it.
We also considered outcomes in just the p16-positive cohort (128 patients) because one of the major questions in current practice is how to determine which patients among this “best prognosis” group have cancers that are more likely to recur. There were 68 (53.1%) with anaplasia or multinucleation and 60 (46.9%) without. Those whose tumors did not have anaplasia had overall, disease-free, and disease-specific survival rates of 88%, 83%, and 99%, respectively, compared with 61%, 62%, and 86% for those whose tumors had it. Patients with p16-positive tumors that did not show multinucleation had overall, disease-free, and disease-specific survival rates of 88%, 85%, and 99%, respectively, compared with 61%, 59%, and 87%, respectively, for those whose tumors had it. This shows a consistent drop in survival rates when tumor cell anaplasia or multinucleation is present, both within the larger group and also amongst just the p16-positive tumors. Specifically for those patients with tumors lacking anaplasia and multinucleation, disease recurrence was minimal. Only 2 of the 54 patients (3.7%) with tumors lacking these features suffered disease recurrence of any kind (1 regional nodal recurrence and 1 distant metastasis). Both of these patients were p16 positive and NK histologic type (type 3).
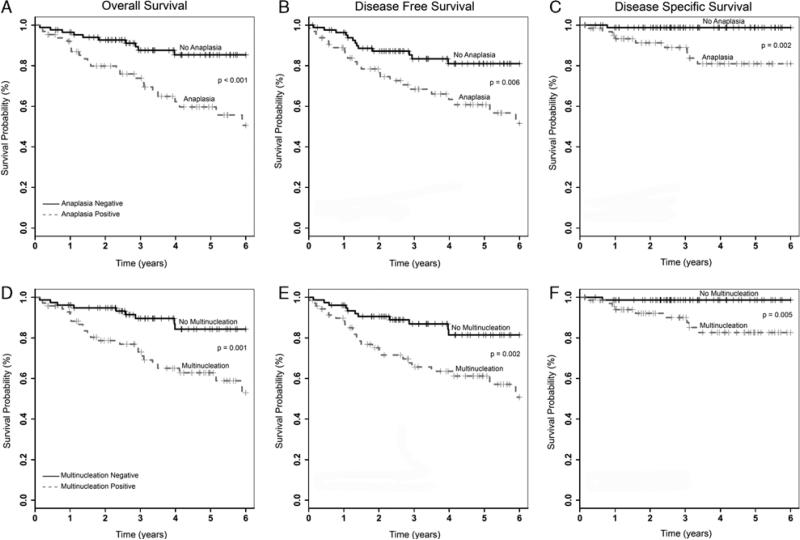
Univariate survival analysis for the entire cohort (149 patients) is presented in Table 2. Patients with tumors showing anaplasia had worse overall, disease-free, and disease-specific survival (P < 0.001, P = 0.006, and P = 0.002, respectively; Fig. 4). Patients with tumors showing multinucleation also had worse overall, disease-free, and disease-specific survival (P = 0.001, 0.002, and 0.005, respectively; Fig. 4). Patients with tumors showing either anaplasia or multinucleation or both also had worse overall, disease-free, and disease-specific survival (P = 0.003, 0.007, and 0.005, respectively). Among the other major clinical and pathologic variables, patients with higher T-stage tumors had statistically significant worse overall, disease-free, and disease-specific survival. Binary p16 immunohistochemical status also correlated strongly with worse overall and disease-free survival and showed a strong trend toward worse disease-specific survival. Current or former smoking status correlated with worse overall and disease-free, but not disease-specific, survival. Among the node-positive cases, extracapsular extension, considered binarily as no extracapsular extension versus any pattern of extracapsular extension or as no or simple extracapsular extension versus soft tissue metastasis, correlated strongly with worse overall, disease-free, and disease-specific survival as well. None of the other clinical or pathologic features showed any statistical correlations with survival.
TABLE 2.
Univariate Survival Analysis for the Entire Patient Cohort (n = 149) by Respective Clinical or Pathologic Variable
| Variable | Overall Survival, P | Disease-Free Survival, P | Disease-Specific Survival, P |
|---|---|---|---|
| Sex† | 0.98 | 0.79 | 0.34 |
| Race (White vs. other) | 0.74 | 0.83 | 0.28 |
| Smoking (ever vs. never) | 0.006 | 0.021 | 0.29 |
| Treatment (surgery alone vs. postoperative radiation) | 0.75 | 0.66 | 0.42 |
| Chemotherapy (yes or no) | 0.75 | 0.52 | 0.50 |
| T-stage (%) | < 0.001 | < 0.001 | < 0.001 |
| T1-T2 | |||
| T3-T4 | |||
| N-stage (%) | 0.21 | 0.89 | 0.06 |
| N0 vs. N1-N3 | |||
| Extracapsular extension | 0.047 | 0.018 | 0.075 |
| None vs. present | |||
| Extracapsular extension | 0.004 | < 0.001 | 0.019 |
| None or limited vs. extensive | |||
| Resection margin status | 0.93 | 0.71 | 0.97 |
| p16 immunohistochemistry* | < 0.001 | < 0.001 | 0.094 |
| Histologic type | < 0.001 | < 0.001 | 0.67 |
| Tumor cell anaplasia | < 0.001 | 0.006 | 0.002 |
| Tumor cell multinucleation | 0.001 | 0.002 | 0.005 |
| Tumor cell anaplasia or multinucleation | 0.003 | 0.007 | 0.005 |
Defined as positive if >50% of tumor cells stained and negative if no staining or < 50% of tumor cells stained.
P-values calculated from log-rank tests.
Values in bold are statistically significant (P < 0.05).
FIGURE 4.
Kaplan-Meier univariate survival analysis for tumor cell anaplasia—(A) overall survival; (B) disease-free survival; (C) disease-specific survival; and for tumor cell multinucleation—(D) overall survival; (E) disease-free survival; (F) disease-specific survival.
Univariate survival analysis in only the p16-positive cohort (128 patients) showed that the presence of anaplasia still statistically significantly correlated with worse overall and disease-specific survival (P = 0.008 and 0.01, respectively) and showed a strong trend toward worse disease-free survival (P = 0.057). Multinucleation still correlated with worse overall, disease-free, and disease-specific survival (P = 0.005, 0.010, and 0.017, respectively). All of the remaining variables other than T-stage and extracapsular extension lacked any statistically significant correlation with outcomes in this select cohort. Higher T-stage correlated with worse overall, disease-free, and disease-specific survival (P = 0.004, 0.005, and 0.012, respectively). Among the node-positive cases, extracapsular extension of any degree correlated with worse overall and disease-free survival (P = 0.019 and 0.007, respectively). The presence of soft tissue metastasis correlated with worse overall, disease-free, and disease-specific survival (P = 0.002, P < 0.001, and P = 0.004, respectively).
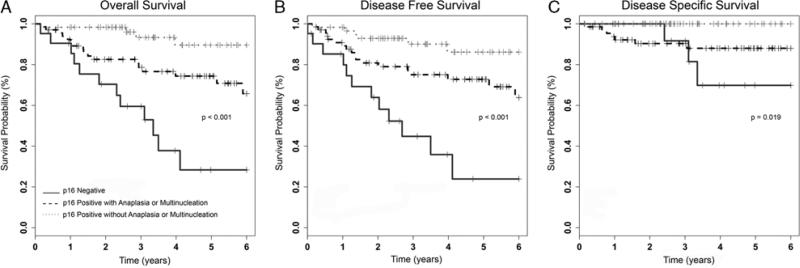
Univariate survival analysis comparing the 21 cases that were p16 negative with the 60 p16-positive cases without anaplasia or multinucleation and the 68 p16-positive cases with anaplasia or multinucleation (Fig. 5) showed statistically significantly better overall, disease-free, and disease-specific survival for the p16-positive tumors without anaplasia or multinucleation compared with the p16-negative cohort (P < 0.001 for all). Overall and disease-free survival rates were also statistically significantly better for the p16-positive cases with anaplasia or multinucleation than for the p16-negative cases (P = 0.002 and 0.003, respectively). Interestingly, however, disease-specific survival rates were not significantly different (P = 0.51).
FIGURE 5.
Kaplan-Meier univariate survival analysis for cases by tumor p16 immunohistochemical status, with the p16-positive cases further divided into those with the presence or absence of anaplasia and multinucleation—(A) overall survival; (B) disease-free survival; (C) disease-specific survival.
Multivariate Cox proportional hazard model analysis was carried out for the entire cohort to determine whether the correlations for anaplasia (Table 3) and tumor cell multinucleation (Table 4) with outcomes were independent of the other variables. After controlling for T-stage, p16 immunohistochemical status, smoking status, and histopathologic tumor type, the presence of anaplasia still correlated significantly with worse disease-specific survival (P = 0.036; HR 9.9). There were strong trends for worse overall (P = 0.13; HR 1.9) and disease-free (P = 0.25; HR 1.6) survival as well. After controlling for tumor stage, p16 immunohistochemical status, smoking status, and histopathologic tumor type, the presence of multinucleation still correlated significantly with worse disease-specific survival (P = 0.021; HR 11.9). There were strong trends toward worse overall survival (P = 0.073; HR 2.2) and disease-free survival (P = 0.070; HR 2.1) with multinucleation as well.
TABLE 3.
Multivariate Cox Model Analysis for the Entire Patient Cohort (n = 149) Including Tumor Cell Anaplasia and the Major Clinical or Pathologic Variables
|
P HR (95%
CI) |
|||
|---|---|---|---|
| Variable | Overall Survival | Disease-Free Survival | Disease-Specific Survival |
| Smoking (ever vs. never) | 0.067 | 0.17 | 0.38 |
| 2.8 (0.93-8.94) | 2.0 (0.75-5.15) | 2.7 (0.29-24.06) | |
| T-stage (T1/T2 vs. T3/T4) | 0.011 | 0.011 | 0.009 |
| 2.9 (1.27-6.40) | 2.6 (1.25-5.55) | 8.6 (1.70-43.19) | |
| Histologic type 1 vs. type 2 | 0.90 | 0.57 | 0.18 |
| 0.8 (0.13-4.35) | 0.6 (0.12-3.17) | 8.3 (0.37-185.17) | |
| Histologic type 1 vs. type 3 | 0.91 | 0.96 | 0.28 |
| 0.9 (0.15-5.54) | 1.0 (0.18-5.18) | 5.9 (0.24-141.68) | |
| p16 IHC* (positive vs. negative) | 0.42 | 0.31 | 0.39 |
| 0.49 (0.09-2.82) | 0.43 (0.08-2.17) | 0.30 (0.02-4.68) | |
| Anaplasia (positive vs. negative) | 0.13 | 0.26 | 0.036 |
| 1.9 (0.83-4.45) | 1.6 (0.72-3.43) | 9.9 (1.16-83.80) | |
Defined as positive if >50% of tumor cells stained and negative if no staining or < 50% of tumor cells stained.
CI indicates confidence interval; IHC, immunoliistocliemistry.
Values in bold are statistically significant (P < 0.05).
TABLE 4.
Multivariate Cox Model Analysis for the Entire Patient Cohort (n = 149) Including Tumor Cell Multinucleation and Major Clinical or Pathologic Variables
|
P HR (95%
CI) |
|||
|---|---|---|---|
| Variable | Overall Survival | Disease-Free Survival | Disease-Specific Survival |
| Smoking (ever vs. never) | 0.037 | 0.11 | 0.21 |
| 3.2 (1.07-9.76) | 2.1 (0.84-5.42) | 3.7 (0.47-29.19) | |
| T-stage (T1/T2 vs. T3/T4) | 0.002 | 0.004 | 0.003 |
| 3.4 (1.58-7.48) | 2.9 (1.40-6.08) | 11.6 (2.31-58.47) | |
| Histologic type (2 vs. 1) | 0.79 | 0.65 | 0.12 |
| 0.8 (0.11-5.21) | 0.5 (0.09-2.89) | 11.8 (0.54-258.31) | |
| Histologic type (3 vs. 1) | 0.83 | 0.95 | 0.29 |
| 0.8 (0.11-5.75) | 1.0 (0.17-5.45) | 6.5 (0.20-207.96) | |
| p16 IHC* (positive vs. negative) | 0.66 | 0.46 | 0.48 |
| 0.65 (0.10-4.42) | 0.52 (0.09-2.89) | 0.34 (0.02-6.53) | |
| Multinucleation (positive vs. negative) | 0.07 | 0.07 | 0.021 |
| 2.2 (0.93-5.15) | 2.1 (0.94-4.52) | 11.9 (1.44-98.10) | |
Defined as positive if >50% of tumor cells stained and negative if no staining or < 50% of tumor cells stained.
Values in bold are statistically significant (P < 0.05).
CI indicates confidence interval; IHC, immunoliistocliemistry.
Although we did not specifically control for extracapsular extension in the multivariate analysis (as it is only applicable to the node-positive cases), a separate analysis of only those node-positive patients with extracapsular extension data showed that there was still correlation with poorer outcome for patients with anaplasia and/or multinucleation (data not presented).
DISCUSSION
As we begin the important search for prognostic variables within the large (and rapidly growing) HPV-related oropharyngeal SCC population, the results of this study may be an important first step. Merely on the basis of the pathologist-reviewed histologic slides we have identified features that herald more aggressive cancers within the larger group. One would assume that not all p16-positive/HPV-related oropharyngeal SCCs are the same, and these study findings clearly support this notion. The concepts of anaplasia and multinucleation in oropharyngeal SCC are completely novel for this tumor type. As most have graded oropharyngeal SCC by the traditional well, moderately, and poorly differentiated terminology (which is also still that recommended by the World Health Organization since 200517), NK SCC would be classified by most as “poorly differentiated” or “high grade.” As such, any nuclear anaplasia or multinucleation would go unnoticed as being just a part of the spectrum of the tumors’ grade and differentiation. We specifically avoided applying a tumor “grade” or “differentiation” in our previously described, 3-tiered histologic typing system20 to avoid the paradoxical connotation that such labels provide to the clinician about NK SCC, for they actually have the best prognosis of the 3 histologic types.
We did find an interesting correlation between the presence of anaplasia and multinucleation and the degree of squamous maturation in the tumors. These features were present in increasing amounts from NK, to NK with maturation, to keratinizing-type SCC, and the overall amount/distribution of the anaplasia and multinucleation was different, with NK SCC more frequently showing these features only focally compared with the other types. What this means with regard to the biology of the tumors is not clear. However, as some have speculated that NK SCCs are actually very well differentiated and are just differentiating into the mature, but basal-appearing, cells of the normal tonsillar crypt, perhaps as the tumors progress they lose this “lock” on differentiation and gain anaplasia and/or multinucleation as they acquire more maturing squamous differentiation.
It is interesting to speculate about what anaplasia and multinucleation in these cancers actually represent biologically. These features are typically associated with genetic complexity or progression. Most carcinomas that have overt anaplasia or tumor multinucleation are not specifically singled out as being different from other overall groups of high-grade or poorly differentiated cancers. Obvious examples in which anaplasia is specifically important, however, include Wilms tumors and medulloblastomas. Among carcinomas, anaplastic thyroid, head and neck spindle cell,8,35 and lung sarcomatoid, giant cell, and pleomorphic carcinomas have anaplasia and multinucleation,11,22 but these are by definition diffuse and very obvious, and markers of cellular differentiation are frequently absent on immunohistochemical analysis or other testing in such tumors.5,35 This is clearly different from that seen in oropharyngeal SCC in which it must be emphasized that patients with p16-positive tumors showing anaplasia or multinucleation otherwise show the same histologic patterns of differentiation as those without and still have relatively favorable outcomes (which remain better than that of p16-negative tumors). Also, there is no apparent reduction or loss of p16 expression by these overtly abnormal cells.
Given that HPV-related oropharyngeal SCC has been shown to be less genetically complex than non-HPV–related SCC,3,32 the histologic features identified here might be highlighting a subset of tumors that have genetic progression. It is quite obvious just by microscopic examination of the anaplastic nuclei in these cancers (Figs. 2 and 3) that they are genetically complex cells. As the presence and amount of anaplasia and multinucleation increased as the amount of keratinization increased and as the rates of p16 positivity decreased, one could speculate that the tumors develop this change as part of losing HPV (or of never having HPV at all). The data, however, do not support this notion, for there are large numbers of p16-positive SCC cases that have anaplasia or multinucleation, and the correlation with disease recurrence and poorer survival holds even amongst just the p16-positive patients (Fig. 5) and in multivariate analysis of the whole cohort controlling for p16 status.
There are other findings in p16-positive/HPV-related oropharyngeal SCC that predict worse prognosis. These include T-stage, current smoking status, and in some studies N-stage, although not in all.4,12,20,27 Recent studies have also shown that major extracapsular extension in lymph nodes is also a modest, but significant, predictor of worse survival in the p16-positive/HPV-related cohort of oropharyngeal SCC.18 A few molecular markers have been suggested in recent studies, such as higher cyclin D1, higher epidermal growth factor receptor, and lower p21 expression,9,13,15,16,26 but these are findings that are not strongly prognostic in the HPV-related patient cohort alone. In addition, they are findings on a continuous spectrum and are method dependent as well; hence, they may not be well suited for clinical application.
The anaplasia and multinucleation observed here were based on specific criteria/cutoffs for the necessary size and number of cells in a single high-power field. Although this seems like a very tight definition, it would probably be difficult to apply in routine clinical practice. We do not know what the reproducibility of the classification of tumors as positive or negative for anaplasia or multinucleation would be amongst different pathologists. Also, we specifically reviewed only surgically resected oropharyngeal SCC cases to ensure that we were seeing all (or at least a large representation) of the overall tumor. Small biopsy specimens cannot rule out the presence of anaplasia or multinucleation for tumors, and, actually, given the focal and somewhat patchy nature of the change even in the multifocal cases, would almost certainly have poor negative predictive value for these features. As such, classifying tumors as positive or negative for these changes would only be useful in those patients undergoing primary surgery.
In summary, surgically treated oropharyngeal SCC patients whose tumors have the simple morphologic features of tumor cell anaplasia and multinucleation have almost 3 times as frequent disease recurrence and have lower disease-specific survival rates independent of all other clinical and pathologic features. As such, this appears to represent a unique pattern of tumor progression and could potentially be considered as part of a system of tumor “grading.” Future studies will need to investigate these findings in a prospective cohort of patients, address the interobserver variability amongst pathologists in classifying them as present, and, most importantly, address the underlying cellular mechanisms, particularly the genetics, of such tumors.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Sue Pagano and Mary Madden for their support in acquiring and organizing the block and slide materials and for entering and collating spreadsheet data. The authors thank Rebecca D. Chernock, MD, for her review of the manuscript and insightful comments.
Supported by the Biostatistics Core, Siteman Comprehensive Cancer Center, and NCI Cancer Center Support Grant P30 CA091842.
Footnotes
Presented at the 100th United States and Canadian Academy of Pathology Annual Meeting in February, 2011.
Conflicts of Interest and Source of Funding: The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.ajsp.com.
REFERENCES
- 1.Adelstein DJ, Ridge JA, Gillison ML, et al. Head Neck; Head and neck squamous cell cancer and the human papillomavirus: summary of a National Cancer Institute State of the Science Meeting; Washington, DC.. November 9-10, 2008; 2009. pp. 1393–1422. [DOI] [PubMed] [Google Scholar]
- 2.Adelstein DJ, Rodriguez CP. Human papillomavirus: changing paradigms in oropharyngeal cancer. Curr Oncol Rep. 2010;12:115–120. doi: 10.1007/s11912-010-0084-5. [DOI] [PubMed] [Google Scholar]
- 3.Agrawal N, Frederick MJ, Pickering CR, et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 2011;333:1154–1157. doi: 10.1126/science.1206923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bishop JA, Sharma R, Westra WH. PAX8 immunostaining of anaplastic thyroid carcinoma: a reliable means of discerning thyroid origin for undifferentiated tumors of the head and neck. Hum Pathol. 2011;42:1873–1877. doi: 10.1016/j.humpath.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Cantley RL, Gabrielli E, Montebelli F, et al. Ancillary studies in determining human papillomavirus status of squamous cell carcinoma of the oropharynx: a review. Pathol Res Int. 2011;2011:138469. doi: 10.4061/2011/138469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chernock RD, El-Mofty SK, Thorstad WL, et al. HPV-related nonkeratinizing squamous cell carcinoma of the oropharynx: utility of microscopic features in predicting patient outcome. Head Neck Pathol. 2009;3:186–194. doi: 10.1007/s12105-009-0126-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellis GL, Corio RL. Spindle cell carcinoma of the oral cavity. A clinicopathologic assessment of fifty-nine cases. Oral Surg Oral Med Oral Pathol. 1980;50:523–533. doi: 10.1016/0030-4220(80)90436-3. [DOI] [PubMed] [Google Scholar]
- 9.Fischer CA, Jung M, Zlobec I, et al. Co-overexpression of p21 and Ki-67 in head and neck squamous cell carcinoma relative to a significantly poor prognosis. Head Neck. 2011;33:267–273. doi: 10.1002/hed.21440. [DOI] [PubMed] [Google Scholar]
- 10.Fischer CA, Zlobec I, Green E, et al. Is the improved prognosis of p16 positive oropharyngeal squamous cell carcinoma dependent of the treatment modality? Int J Cancer. 2010;126:1256–1262. doi: 10.1002/ijc.24842. [DOI] [PubMed] [Google Scholar]
- 11.Franks TJ, Galvin JR. Sarcomatoid carcinoma of the lung: histologic criteria and common lesions in the differential diagnosis. Arch Pathol Lab Med. 2010;134:49–54. doi: 10.5858/2008-0547-RAR.1. [DOI] [PubMed] [Google Scholar]
- 12.Hafkamp HC, Manni JJ, Haesevoets A, et al. Marked differences in survival rate between smokers and nonsmokers with HPV 16-associated tonsillar carcinomas. Int J Cancer. 2008;122:2656–2664. doi: 10.1002/ijc.23458. [DOI] [PubMed] [Google Scholar]
- 13.Hafkamp HC, Mooren JJ, Claessen SM, et al. P21 Cip1/WAF1 expression is strongly associated with HPV-positive tonsillar carcinoma and a favorable prognosis. Mod Pathol. 2009;22:686–698. doi: 10.1038/modpathol.2009.23. [DOI] [PubMed] [Google Scholar]
- 14.Hammarstedt L, Lindquist D, Dahlstrand H, et al. Human papillomavirus as a risk factor for the increase in incidence of tonsillar cancer. Int J Cancer. 2006;119:2620–2623. doi: 10.1002/ijc.22177. [DOI] [PubMed] [Google Scholar]
- 15.Hong A, Dobbins T, Lee CS, et al. Relationships between epidermal growth factor receptor expression and human papillomavirus status as markers of prognosis in oropharyngeal cancer. Eur J Cancer. 2010;46:2088–2096. doi: 10.1016/j.ejca.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 16.Hong AM, Dobbins TA, Lee CS, et al. Human papillomavirus predicts outcome in oropharyngeal cancer in patients treated primarily with surgery or radiation therapy. Br J Cancer. 2011;103:1510–1517. doi: 10.1038/sj.bjc.6605944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson N, Francheschi S, Ferlay J, et al. Squamous cell carcinoma of the oral cavity and oropharynx. In: Barnes EL, Eveson JW, Reichart P, et al., editors. World Health Organization Classification of Tumours - Pathology and Genetics Head and Neck Tumours. IARC Press; Lyon, France: 2005. pp. 168–175. [Google Scholar]
- 18.Lewis JS, Jr, Carpenter DH, Thorstad WL, et al. Extracapsular extension is a poor predictor of disease recurrence in surgically treated oropharyngeal squamous cell carcinoma. Mod Pathol. 2011;24:1413–1420. doi: 10.1038/modpathol.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis JS, Jr, Chernock RD, Ma X, et al. Partial p16 staining in oropharyngeal squamous cell carcinoma: extent and pattern correlate with human papillomavirus RNA status. Mod Pathol. 2012 doi: 10.1038/modpathol.2012.79. (In press) [DOI] [PubMed] [Google Scholar]
- 20.Lewis JS, Jr, Thorstad WL, Chernock RD, et al. p16 positive oropharyngeal squamous cell carcinoma:an entity with a favorable prognosis regardless of tumor HPV status. Am J Surg Pathol. 2010;34:1088–1096. doi: 10.1097/PAS.0b013e3181e84652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marur S, D'Souza G, Westra WH, et al. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010;11:781–789. doi: 10.1016/S1470-2045(10)70017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakajima M, Kasai T, Hashimoto H, et al. Sarcomatoid carcinoma of the lung: a clinicopathologic study of 37 cases. Cancer. 1999;86:608–616. [PubMed] [Google Scholar]
- 23.Nasman A, Attner P, Hammarstedt L, et al. Incidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: an epidemic of viral-induced carcinoma? Int J Cancer. 2009;125:362–366. doi: 10.1002/ijc.24339. [DOI] [PubMed] [Google Scholar]
- 24.Olthof NC, Straetmans JM, Snoeck R, et al. Next-generation treatment strategies for human papillomavirus-related head and neck squamous cell carcinoma: where do we go? Rev Med Virol. 2012;22:88–105. doi: 10.1002/rmv.714. [DOI] [PubMed] [Google Scholar]
- 25.Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer. 2007;121:1813–1820. doi: 10.1002/ijc.22851. [DOI] [PubMed] [Google Scholar]
- 26.Reimers N, Kasper HU, Weissenborn SJ, et al. Combined analysis of HPV-DNA, p16 and EGFR expression to predict prognosis in oropharyngeal cancer. Int J Cancer. 2007;120:1731–1738. doi: 10.1002/ijc.22355. [DOI] [PubMed] [Google Scholar]
- 27.Rich JT, Milov S, Lewis JS, Jr, et al. Transoral laser microsurgery (TLM) +/– adjuvant therapy for advanced stage oropharyngeal cancer: outcomes and prognostic factors. Laryngoscope. 2009;119:1709–1719. doi: 10.1002/lary.20552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rischin D, Young RJ, Fisher R, et al. Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02.02 phase III trial. J Clin Oncol. 2010;28:4142–4148. doi: 10.1200/JCO.2010.29.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson M, Sloan P, Shaw R. Refining the diagnosis of oropharyngeal squamous cell carcinoma using human papillomavirus testing. Oral Oncol. 2010;46:492–496. doi: 10.1016/j.oraloncology.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 30.Shi W, Kato H, Perez-Ordonez B, et al. Comparative prognostic value of HPV16 E6 mRNA compared with in situ hybridization for human oropharyngeal squamous carcinoma. J Clin Oncol. 2009;27:6213–6221. doi: 10.1200/JCO.2009.23.1670. [DOI] [PubMed] [Google Scholar]
- 31.Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma: increasing trends in the U.S. population ages 20-44 years. Cancer. 2005;103:1843–1849. doi: 10.1002/cncr.20998. [DOI] [PubMed] [Google Scholar]
- 32.Stransky N, Egloff AM, Tward AD, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333:1157–1160. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sturgis EM, Cinciripini PM. Trends in head and neck cancer incidence in relation to smoking prevalence: an emerging epidemic of human papillomavirus-associated cancers? Cancer. 2007;110:1429–1435. doi: 10.1002/cncr.22963. [DOI] [PubMed] [Google Scholar]
- 34.Syrjanen S. Human papillomavirus (HPV) in head and neck cancer. J Clin Virol. 2005;32(suppl 1):S59–S66. doi: 10.1016/j.jcv.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 35.Thompson LD, Wieneke JA, Miettinen M, et al. Spindle cell (sarcomatoid) carcinomas of the larynx: a clinicopathologic study of 187 cases. Am J Surg Pathol. 2002;26:153–170. doi: 10.1097/00000478-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Ukpo OC, Flanagan JJ, Ma XJ, et al. High risk human papillomavirus E6/E7 mRNA detection by a novel in situ hybridization assay strongly correlates with p16 expression and patient outcomes in oropharyngeal squamous cell carcinoma. Am J Surg Pathol. 2011;35:1343–1350. doi: 10.1097/PAS.0b013e318220e59d. [DOI] [PubMed] [Google Scholar]
- 37.Weinberger PM, Yu Z, Kountourakis P, et al. Defining molecular phenotypes of human papillomavirus-associated oropharyngeal squamous cell carcinoma: validation of three-class hypothesis. Otolaryngol Head Neck Surg. 2009;141:e381. doi: 10.1016/j.otohns.2009.04.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.