Abstract
Kidney stones are a common problem for which inadequate prevention exists. We recruited ten recurrent kidney stone formers with documented calcium oxalate stones into a two phased study to assess safety and effectiveness of Cystone®, an herbal treatment for prevention of kidney stones. The first phase was a randomized double-blinded 12 week cross over study assessing the effect of Cystone® vs. placebo on urinary supersaturation. The second phase was an open label one year study of Cystone® to determine if renal stone burden decreased, as assessed by quantitative and subjective assessment of CT. Results revealed no statistically significant effect of Cystone® on urinary composition short (6 weeks) or long (52 weeks) term. Average renal stone burden increased rather than decreased on Cystone®. Therefore, this study does not support the efficacy of Cystone® to treat calcium oxalate stone formers. Future studies will be needed to assess effects on stone passage, or on other stone types.
Keywords: Computerized Tomography, Cystone®, herb, Kidney Calculi, supersaturation, nephrolithiasis, quantitative CT
Introduction
Kidney stones are a global affliction causing a great deal of morbidity and economic loss.1 The prevalence of nephrolithiasis increases as societies become industrialized.2,3,4 Therefore, the worldwide burden is likely to increase in future years. A method to prevent kidney stones would be an obvious benefit. Existing treatments with evidence that supports their long term efficacy to prevent calcium oxalate kidney stones include dietary and lifestyle changes, as well as chronic use of one of three medications (thiazides, potassium citrate, and allopurinol). No new treatments have been established in decades, and these that are available have inherent problems related to patient compliance, cost, effectiveness and side effects. Therefore, a preventive treatment that would be easy to take, low in cost, safe and effective would be highly desirable.
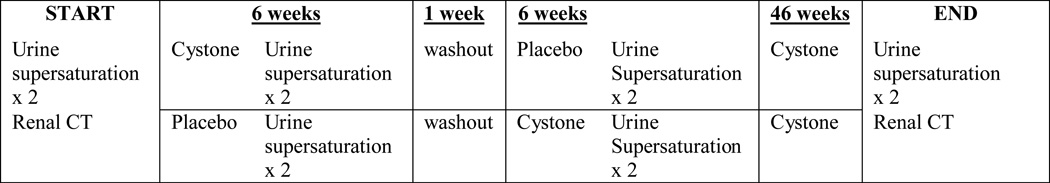
Cystone® tablets are an Ayurvedic treatment for stones, traditionally practiced in India. Many studies and long experience attest to the safety of this compound. It is also claimed that Cystone® decreases urinary supersaturation or micropulverizes and expels kidney stones, but existing studies have been limited by small patient numbers, weak methodology, and poor study design including lack of proper controls. Therefore, in this study we rigorously evaluated the ability of Cystone® to decrease urine supersaturation and to prevent new stone formation and growth of existing stones via a short-term randomized, placebo-controlled, double-blinded, cross over study (to evaluate effects on urinary chemistries), followed by an open label extension (to evaluate effects on stone burden) (Figure 1). Quantitative computerized tomography (CT) was used to assess changes in stone burden over the time of the study.
Fig. 1. Study Flow Chart.
Materials and Methods
Study Subjects
Ten patients (4 men, 6 women) with recurrent, analytically confirmed, calcium-containing kidney stones were recruited from the Mayo Stone Clinic. All were Caucasian adults and had passed at least one stone at entry into the study (Table 1). Metabolic activity prior to study entry was defined as an increase in stone size or number documented on a previous CT scan obtained within one year prior to study entry. Only two patients were metabolically active at entry into the study. Exclusions included age under 18 years, pregnancy, refusal to use an effective method of birth control during the study, chronic urinary infection, secondary causes of kidney stones, (e.g., bowel disease, renal tubular acidosis, primary hyperoxaluria) or mental incompetence to give informed written consent on a form approved by the institutional review board. All patients were allowed to continue their existing kidney stone treatment programs. All patients promptly began the study after consenting to be enrolled.
TABLE 1.
PATIENT CHARACTERISTICS
| PT | AGE | RACE | GENDER | STONE COMPOSTION |
PLACEBO DETECTION |
METABOLIC ACTIVITY |
RISK FACTORS |
RELEVANT MEDICATIONS |
|---|---|---|---|---|---|---|---|---|
| 1 | 36 | W | F | Ca Ox 60–70 % Ca P 30–40 % |
No | Active | MSK H Ca |
Self decreased Cystone to 1 b.i.d. |
| 2 | 71 | W | M | Ca Ox 100 % | No | Active | HTN | HCT 25 mg/day |
| 3 | 47 | W | F | Ca Ox 100 % | No | Indeterminate | FH Obesity |
None |
| 4 | 53 | W | M | Ca Ox 100 % | No | Active | HCa | None |
| 5 | 57 | W | M | Ca Ox | No | Indeterminate | FH HC Obese |
None |
| 6 | 47 | W | F | Ca Ox 10–20 % Ca P 80–90 % |
Yes | Active | HCa HCP Osteoporosis |
HCT 25 mg/day Calcium 1600 mg/day Fosamax 70 mg/week |
| 7 | 44 | W | F | Ca Ox 70–100 % Ca P 80–90 % |
No | Inactive | FH | Ammonium Chloride 500 mg q.i.d. |
| 8 | 75 | W | M | Ca Ox | No | Inactive | HC HO | None |
| 9 | 50 | W | F | Ca Ox 40–90 % Ca P 60–100 % |
No | Indeterminate | FH, HTN Obese, HCa |
None |
| 10 | 45 | W | F | Calcium | No | Indeterminate | LV, HC | None |
| LEGEND | |
|---|---|
| W-White | F-Female |
| M-Male | Ca Ox-Calcium oxalate |
| Ca P-Calcium phosphate | MSK-Medullary sponge kidney |
| HCa-Hypercalciuria | HCi-Hypercitraturia |
| HTN-Hypertension | FH-Family history |
| LV-Low urine volume | HO-Hyperoxaluria |
Study Design and Conduct
The protocol is illustrated in Figure 1. Patients were randomly assigned by Mayo Clinic Research Pharmacy to a 6 week treatment program with Cystone® tablets, 2 by mouth twice daily, or identical placebo. This is the dose recommended by the manufacturer. After a 1 week washout the patients crossed over to the alternate treatment for another 6 weeks. Patients provided two 24-hour urine collections shortly before starting the study, at the end of the first 6 weeks of treatment, and again at the end of the 6 week cross over, each of which were analyzed for determinants of urinary supersaturation in the Mayo Renal Function Laboratory and calculated using the Equil2 program.5 After completing both crossover arms, patients then immediately took Cystone® open label in the same dose for an additional 48 weeks, thus ensuring a 52 week total exposure to Cystone® during the 59 week study. Quantitative noncontrast multidetector CT exams were all performed on a 64-channel MDCT scanner (Sensation-64, Siemens Medical Solutions, Forschheim, Germany). For the 64-channel technique, patients were in the supine position on the CT table with arms above the head. An initial survey topogram was obtained for positioning purposes (80kVp, 300mA) from the top of the liver through the pubic symphysis. Subsequent CT images were obtained in a single breath-hold through the kidneys. A standardized acquisition protocol was used for all exams (collimation 64×0.6; gantry rotation time, 0.5seconds; table feed, 23mm/rotation (pitch of 1.2), quality reference 240 mAs; 120 kVp and the field of view was adjusted to patient size). Three reconstruction intervals were obtained from the raw data including: 5.0mm thickness at 5.0mm intervals (axial), 2.0mm thickness at 2.0mm intervals (axial) and 2.0mm thickness at 2.0 intervals (coronal adjusted to the long axis of the kidneys). The 2.0mm thickness at 2.0mm interval (axial) data set was also processed at a free standing 3D workstation (Vitrea, Vital Images, Inc., Minnetonka, MN) by dedicated 3D technologists to obtain quantitative calcium scoring data for each kidney. All scored images were reviewed to determine that the included calcifications were consistent with urolithiasis rather than renal arterial or parenchymal calcifications. The scoring programs are typically used for coronary artery calcification quantification and generate both an Agatston score (reported as Agatston Units, AU) and volumetric score (reported as mm3). In the algorithms positive calcification required a minimum density threshold of 130 Hounsfield Units and a minimum area threshold of 3 adjacent pixels of at least 130 Hounsfield Units. In addition to quantified scoring, all images including the axial and reconstructed coronal series were reviewed by the diagnostic radiology service and a clinical report was generated. These images were subsequently sent to a picture archiving and communication system and available for referring clinicians. In addition, all images were reviewed by a radiologist (TJV) in a blinded fashion to score each kidney as increased, no change, or decreased stone burden.
Study Drug
Cystone® is traditionally used for relief of a variety of urological problems including nephrolithiasis and is comprised of the following substances: shilapuspha (Didymocarpus pedicellata) 130mg, Pasanabheda (Saxifaga lgulata Syn. Bergenia ligulata/ciliata) 98 mg, Manjishtha (Rubia cordifolia) 32 mg, Nagarmusta (Cyperus scariosus) 32 mg, Apamarga (Achyranthes aspera) 32 mg, Gohija (Onosma bracteatum) 32 mg, Sahadevi (Vernonia cinerea) 32 mg, Shilajeet (Purified) 26 mg, and Hajrul yahood bhasma 32 mg. Its purported effect is to “prevent supersaturation of lithogenic substances, control oxamide (a substance that precipitates stone formation) from the intestine and correct the crystalloid-colloid imbalance. Cystone® inhibits calculogenesis by reducing stone-forming substances like oxalic acid, calcium hydroxyproline, etc, and causes their expulsion by micropulverization. Cystone® causes disintegration of the calculi and crystals by acting on the mucin, which binds the particles together. Cystone®’s antimicrobial activity is beneficial in the prevention of urinary tract infections associated with urinary stones and crystalluria. Cystone®’s antispasmodic and anti-inflammatory activities relieve ureteric colic and alleviate symptoms of painful and burning and micturition.” (http://himalayahealthcare.com/products/cystone.htm). Cystone® is manufactured and sold virtually world wide by Himalaya Health Care. In the United States, the product is known as Uricare®.
Statistics and Randomization
Randomization was accomplished using a table provided by the department of statistics to the study coordinator who was blinded as to whether the patients received placebo or Cystone®. Biochemical and supersaturation results were analyzed via a matched pair analysis using the JMP software package (SAS Instituted, Inc.); P values < 0.05 were deemed significant.
Results
Table 1 contains demographic and clinical characteristics of the study patients. Patients who consented to participate in the Cystone® study tended to have recurrent kidney stones inadequately controlled on their current program. This tended to select a more difficult-to-treat patient population. One patient thought that Cystone® could be discriminated from placebo by its “peppery” taste. The other study participants did not identify this difference.
Table 2 displays the 24 hour urinary supersaturation results. In a matched pair analysis of the initial crossover study no statistically significant differences for any parameter between Cystone® treatment as compared to placebo were present. Similarly, the 24-hour urine chemistries did not differ after one year on Cystone®, as compared to values on placebo during the initial crossover period. Therefore there was no evidence that Cystone® altered urinary chemistries after short term (6 weeks) or long term (1 year) usage.
TABLE 2.
URINARY SUPERSATURATIONS
| PT | BASELINE | CROSSOVER AFTER PLACEBO (6 WEEKS) |
CROSSOVER AFTER CYSTONE (6 WEEKS) |
END OPEN-LABEL CYSTONE (≥48 WEKS) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca-Ox | Br | HAP | UA | Ca-Ox | Br | HAP | UA | Ca-Ox | Br | HAP | UA | Ca-Ox | Br | HAP | UA | |
| 1 | 1.50 | 1.84 | 5.59 | 1.89 | 2.14* | 1.59* | 5.86* | 0.03 | 1.85* | 1.63* | 4.78* | 2.96 | 2.41* | 1.49* | 5.21* | 1.43 |
| 2 | 1.61 | 1.19 | 4.21 | −0.45 | 0.90 | −0.81 | 3.03 | −1.13 | 0.93 | −1.39 | 2.41 | −0.74 | 1.15 | −2.82 | −0.45 | 2.59 |
| 3 | 1.92 | −0.78 | 3.12 | 1.08 | 1.99 | 0.43 | 4.64 | 0.42 | 2.20 | 0.36 | 4.01 | 2.36 | 1.84 | 0.08 | 3.95 | 0.92 |
| 4 | 1.97 | −0.57 | 2.83 | 1.31 | 2.37 | 0.58 | 5.15 | −3.71 | 2.36 | 0.14 | 4.50 | −2.81 | 1.97 | −0.09 | 4.31 | −3.69 |
| 5 | 2.05 | −1.45 | 0.69 | 2.30 | 1.75 | 1.59 | 5.40 | 1.01 | 1.99 | 0.16 | 3.80 | 0.92 | ||||
| 6 | 1.98 | 1.23 | 5.90 | −1.43 | 1.43 | 0.73 | 6.69 | −5.60 | 2.21 | 1.75 | 8.01 | −5.11 | 2.22 | 1.46 | 7.25 | −5.56 |
| 7 | 1.90 | 0.69 | 6.04 | −2.87 | 2.19 | 1.18 | 5.79 | −0.08 | 1.71* | 1.05* | 7.31* | −5.48 | 1.13 | −1.12 | 5.05 | −3.47 |
| 8 | 2.24 | 0.98 | 7.59 | −6.62 | 1.90 | −0.49 | 2.90 | 1.67 | 2.04 | −0.03 | 4.11 | −0.22 | 2.17 | −0.25 | 4.23 | −1.82 |
| 9 | 1.45 | −0.14 | 3.09 | 2.07 | 1.79 | 1.25 | 5.13 | 0.31 | 1.59 | 0.05 | 2.95 | 1.89 | 1.78 | 0.06 | 3.00 | 2.83 |
| 10 | 2.08 | 0.55 | 3.44 | 3.39 | 1.92 | 1.25 | 5.61 | −0.08 | 1.38 | 0.96 | 4.25 | 3.07 | ||||
| LEGEND | |
|---|---|
| Ca-Ox | Calcium oxalate |
| Br | Calcium phosphate (Brushite) |
| HAP | Calcium Phosphate (hydroxyapatite) |
| UA | Uric acid |
Table 3 contains results of CT studies at baseline and one year. The blinded radiologist’s opinion of changes in kidney stone burden generally agreed with the quantitative data, with the exception of both kidneys in patient 4. We have no explanation for this discrepancy. Patient 4 was excluded from CT analysis because of bilateral stone removal surgery during the study. Therefore, we evaluated 18 of 20 kidneys for stone burden. Spontaneous stone passage occurred from 4 of 18 kidneys during the study. Nevertheless, the official radiology interpretation was that 8 kidneys had increased stone material, 8 kidneys were unchanged, and only 2 kidneys had decreased stone material. Quantitative scoring of renal calcium content in 18 kidneys from these same 9 patients revealed that mean total stone burden per kidney increased over this time period as assessed by the volumetric scoring system (93 to 114 mm3; P=0.07 by matched pairs analysis) or the Agatston scoring system (108 to 136 AU; P=0.10 by matched pairs analysis). These results did not support an overall positive effect off Cystone® on stone burden.
Table 3.
CT SCORES
| RIGHT KIDNEYS |
PATIENT | BASELINE VOLUME |
BASELINE AGATSTON |
1 YEAR VOLUME |
1 YEAR AGATSTON |
RADIOLOGIST IMPRESSION |
CLINICAL EVENTS |
|---|---|---|---|---|---|---|---|
| 1-001 | 3494 | 4564 | 4078 | 5286 | NC | NONE | |
| 1-003 | 13 | 11 | 8 | 5 | NC | NONE | |
| 1-004 | 0 | 0 | 0 | 0 | NC | NONE | |
| 1-006 | 0 | 0 | 19 | 6 | NC | NONE | |
| 1-007 | 9955 | 13187 | 9211 | 12221 | NC | NONE | |
| 1-009 | 29 | 28 | 130 | 144 | I | NONE | |
| 1-011 | 21 | 14 | 19 | 17 | NC | NONE | |
| 1-012 | 914 | 1167 | 1631 | 1974 | I | NONE | |
| 1-014 | 0 | 0 | 0 | 0 | NC | NONE | |
|
Mean SD P vs. Baseline |
1602 3335 |
2107 4417 |
1677 3139 0.96 |
2183 4157 0.97 |
|||
| LEFT KIDNEYS |
1-001 | 71 | 64 | 2673 | 3484 | I | SURG |
| 1-003 | 0 | 0 | 0 | 0 | NC | NONE | |
| 1-004 | 83 | 105 | 88 | 109 | NC | PASS | |
| 1-006 | 310 | 380 | 1086 | 1407 | I | PASS+SURG | |
| 1-007 | 260 | 331 | 6305 | 8390 | I | NONE | |
| 1-009 | 95 | 92 | 64 | 50 | D | SURG | |
| 1-011 | 31 | 31 | 235 | 299 | I | PASS | |
| 1-012 | 1333 | 1755 | 1901 | 2138 | I | ? | |
| 1-014 | 530 | 688 | 282 | 332 | D | SURG | |
|
Mean SE P vs. baseline |
301 422 |
383 560 |
2064 688 0.13 |
1801 2741 0.15 |
|||
| LEGEND | |
| I | Increased Stones |
| D | Decreased Stones |
| NC | No Change in Stones |
| PASS | Passed Stone |
| SURG | Stone Removed |
| ? | Unknown |
No patients described any side effects attributable to Cystone®, consistent with previous studies.
Discussion
Current non-surgical therapies of kidney stones take 3 different approaches. Prevention, either primary or secondary is preferred. Indeed, evidence exists that supports the prescription of specific dietary measures and/or drugs for this purpose. Chemolysis (dissolution) of existing stones may be possible with uric acid and some cystine stones. No scientific data supports the feasibility of calcium stone chemolysis, to our knowledge. Expulsion therapy to help pass stones that have moved into the ureter (but not stones resident in the kidney) is, however, supported by recent controlled trials.6
Randomized controlled studies exist to support the efficacy of thiazides,7 allopurinol,8 and potassium magnesium citrate9 for secondary prevention of calcium oxalate kidney stones. Side effects, cost, and imperfect prevention make the ready availability of cheap, safe and effective stone prevention therapy highly desirable.
Current treatments for stone prevention typically decrease urinary supersaturation by affecting urinary composition (e.g., decrease calcium excretion). No agent is known that can be safely taken and enter the urine to decrease calcium oxalate crystallization, or perhaps even better dissolve calcium oxalate stones and/or crystals. If such a compound were found, it would represent a new class of treatment for renal stones. Several herbs have been purported to decrease stone risk, or hasten stone passage. However, hard scientific evidence regarding their efficacy is scanty. The Chinese Kampou medicine has been used to treat disease for centuries, including for prevention and treatment of urinary calculi. An experimental study suggested an inhibitory effect of Kampou extracts on in vitro CaOx crystallization.10 In this report, the two species from Kampou (Takusya and Kagosou) also were effective for preventing renal crystallization in a rat nephrolithiasis model; similar results were obtained in a second report.11 Chorey-to, another Chinese medicine which contains Takusya, also exhibited a protective effect in rats rendered hyperoxaluric with ethylene glycol, even though urinary citrate levels fell.12
Many stone patients in Brazil take a tea made from the annual herb Phyllanthus niuri that grows in the tropical indigenous area and does not cause side effects.12 This natural product has been called "break stone" because it has been used for generations to eliminate gallstones and kidney stones.12 Diverse classes of potentially active compounds have been identified from genus Phyllanthus, including alkaloids, flavonoids, lactones, steroids, terpenoids, lignans, and tannins. Some researchers have demonstrated antispasmodic and analgesic activities in Phyllanthus niuri which could explain the popular use of the plant for kidney and bladder stones.12,13 The alkaloid extract caused smooth muscle relaxation specific to the urinary and biliary tract which could facilitate the expulsion of both kind of stones.14 Phyllanthus niuri has also been shown to inhibit CaOx endocytosis by renal tubular cells,15 another mechanism by which this agent could decrease crystal retention in the kidney, and in a small clinical trial appeared to reduce urinary calcium excretion amongst hypercalciuric stone formers.16 No toxicity was apparent in the latter study. A Moroccan herb Hernaria hirsuta has similarly been evaluated for effects on CaOx crystallization, including by our group.17 Interestingly, Phyllanthus niuri is purported to act by promoting nucleation of more crystals that achieve a smaller size.
A major shortcoming of prevention trials to date is the lack of adequate end points. Typically, the hard end point in most trials is stone passage rate, even though there has not been any data to suggest that any current treatment prevents passage of preformed stones. This formulation presumes relatively tight correlation between stone burden and stone passage rates. Although it is true that one cannot pass a stone unless it has developed and grown, the time between stone development and passage appears to be variable and unpredictable. Therefore, the ability to accurately measure stone size in vivo over time in vivo could represent a valuable surrogate end point for clinical trials in the future. Stone risk, composition, and risk of recurrence all correlate with urinary supersaturation, as calculated using the iterative computer program Equil2.18 Therefore, urinary supersaturation is a second potential surrogate endpoint for clinical trials. In this study we assessed the effect of Cystone®, a common stone prevention treatment outside of Europe and the United States, on both urinary supersaturation and radiographically assessed stone burden.
The current results did not document any beneficial effect of Cystone® on the urinary composition. However, the failure to find statistically significant change in urinary supersaturation does not rule out a beneficial effect. Equil2 only calculates SS based upon the inorganic composition of urine,5 and does not take into account the potential effect of potential macromolecular inhibitors such as Tamm-Horsfall protein or osteopontin,19 or smaller molecules such as phytate.20 Furthermore, Cystone® could exert effects on other ion pairs that can form in urine and influence growth of calcium oxalate crystals, but are not included in the Equil2 calculations.21
Cystone® is purported to promote stone passage. However, on average stone burden increased rather than decreased in our study. It is important to note that stone formers in this study tended to be those who had failed standard therapy, which may have influenced the end point of stone formation and passage. It is also possible an effect may have been apparent with longer follow up.
No patient reported any side effects from Cystone®. This is in accord with previously published studies.
Conclusion
This short term study does not suggest that Cystone® affects those urinary chemistries commonly measured and known to influence calcium oxalate stone formation, nor does decrease renal calcium stone burden over a 1 year period. It is possible elements of the urine were affected that are not typically measured (e.g., glycoprotein inhibitors). A longer term study with more patients would be necessary to detect changes in stone events or enhanced stone passage, or effects on other stone types. In any new study of Cystone, the botanical authenticity of each individual herb will need to be documented by the manufacturer using high pressure liquid chromatography. This short term trial failed to find evidence that Cystone® prevents kidney stone formation and growth in recurrent calcium oxalate stone formers.
Acknowledgment
The authors wish to thank Beverly Tietje, study coordinator; and Kathy Laabs and Joni Langowski, secretaries, for their invaluable assistance in performing the study and preparing the manuscript, respectively. Funding of the research aspects of the study (2 of the 4 duplicate urine supersaturations, pregnancy testing, and statistical analysis and all Cystone® and placebo tablets) were provided by the Mayo Foundation and Himalaya Health Care. The entire design of the study, its supervision, data analysis, manuscript preparation and decision to publish were entirely the work of the authors. None of the authors have any financial interest in Himalaya Health Care. Investigators on this study (J.C. Lieske, T.J. Vrtiska) were supported by the Mayo Clinic O’Brien Urology Research Center (P50 DK083007)
Cystone® tablets and partial study funding were supplied by Himalaya Health Care
Abbreviations
- AU
Agatston Units
- CT
Computerized Tomography
- mm3
cubic millimeter
Literature Citations
- 1.Saigal CS, Joyce G, Timilsina AR. Direct and indirect costs of nephrolithiasis in an employed population: Opportunity for disease management? Kidney Int. 2005;68:1808. doi: 10.1111/j.1523-1755.2005.00599.x. [DOI] [PubMed] [Google Scholar]
- 2.Goldfarb DS. Increasing prevalence of kidney stones in the United States. Kidney Int. 2003;63:1951. doi: 10.1046/j.1523-1755.2003.00942.x. [DOI] [PubMed] [Google Scholar]
- 3.Stamatelou KK, Francis ME, Jones CA, et al. Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int. 2003;63:1817. doi: 10.1046/j.1523-1755.2003.00917.x. [DOI] [PubMed] [Google Scholar]
- 4.Sowers MR, Jannausch M, Wood C, et al. Prevalence of renal stones in a population-based study with dietary calcium, oxalate, and medication exposures. Am J Epidemiol. 1998;147:914. doi: 10.1093/oxfordjournals.aje.a009381. [DOI] [PubMed] [Google Scholar]
- 5.Werness PJ, Brown CM, Smith LH, et al. EQUIL2: a BASIC computer program for the calculation of urinary saturation. J Urol. 1985;134:1242. doi: 10.1016/s0022-5347(17)47703-2. [DOI] [PubMed] [Google Scholar]
- 6.Hollingsworth JM, Rogers MAM, Kaufman SR, et al. Medical therapy to facilitate urinary stone passage: a meta-analysis. Lancet. 2006;368:1171. doi: 10.1016/S0140-6736(06)69474-9. [DOI] [PubMed] [Google Scholar]
- 7.Ettinger B, Citron JT, Livermore B, et al. Chlorthalidone reduces calcium oxalate calculous recurrence but magnesium hydroxide does not. J Urol. 1988;139:679. doi: 10.1016/s0022-5347(17)42599-7. [DOI] [PubMed] [Google Scholar]
- 8.Ettinger B, Tang A, Citron JT, et al. Randomized trial of allopurinol in the prevention of calcium oxalate calculi. N Engl J Med. 1986;315:1386. doi: 10.1056/NEJM198611273152204. [DOI] [PubMed] [Google Scholar]
- 9.Ettinger B, Pak CY, Citron JT, et al. Potassium-magnesium citrate is an effective prophylaxis against recurrent calcium oxalate nephrolithiasis. J Urol. 1997;158:2069. doi: 10.1016/s0022-5347(01)68155-2. [DOI] [PubMed] [Google Scholar]
- 10.Koide T, Yamaguchi S, Utsunomiya M, et al. The inhibitory effect of kampou extracts on in vitro calcium oxalate crystallization and in vivo stone formation in an animal model. Int J Urol. 1995;2:81. doi: 10.1111/j.1442-2042.1995.tb00429.x. [DOI] [PubMed] [Google Scholar]
- 11.Yasui T, Fujita K, Sato M, et al. The effect of Takusya, a Kampou medicine, on renal stone formation and osteopontin expression in rat urolithiasis model. Urol Res. 1999;27:194. doi: 10.1007/s002400050109. [DOI] [PubMed] [Google Scholar]
- 12.Calixto JB, Santos AR, Cechinel Filho V, et al. A review of the plants of the genus Phyllanthus: their chemistry, pharmacology, and therapeutic potential. Med Res Rev. 1998;18:225. doi: 10.1002/(sici)1098-1128(199807)18:4<225::aid-med2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 13.Santos AR, Filho VC, Niero R, et al. Analgesic effects of callus culture extracts from selected species of Phyllanthus in mice. J Pharm Pharmacol. 1994;46:755. doi: 10.1111/j.2042-7158.1994.tb03897.x. [DOI] [PubMed] [Google Scholar]
- 14.Calixto JB, Yunes RA, Neto AS, et al. Antispasmodic effects of an alkaloid extracted from Phyllanthus sellowianus: a comparative study with papaverine. Braz J Med Biol Res. 1984;17:313. [PubMed] [Google Scholar]
- 15.Campos AH, Schor N. Phyllanthus niruri inhibits calcium oxalate endocytosis by renal tubular cells: its role in urolithiasis. Nephron. 1999;81:393. doi: 10.1159/000045322. [DOI] [PubMed] [Google Scholar]
- 16.Nishiura JL, Campos AH, Boim MA, et al. Phyllanthus niruri normalizes elevated urinary calcium levels in calcium stone forming (CSF) patients. Urol Res. 2004;32:362. doi: 10.1007/s00240-004-0432-8. [DOI] [PubMed] [Google Scholar]
- 17.Atmani F, Slimani Y, Mimouni M, et al. Effect of aqueous extract from Herniaria hirsuta L. on experimentally nephrolithiasic rats. J Ethnopharmacol. 2004;95:87. doi: 10.1016/j.jep.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 18.Parks JH, Coward M, Coe FL. Correspondence between stone composition and urine supersaturation in nephrolithiasis. Kidney Int. 1997;51:894. doi: 10.1038/ki.1997.126. [DOI] [PubMed] [Google Scholar]
- 19.Kumar V, Pena de la Vega L, Farell G, et al. Urinary macromolecular inhibition of crystal adhesion to renal epithelial cells is impaired in male stone formers. Kidney Int. 2005;68:1784. doi: 10.1111/j.1523-1755.2005.00595.x. [DOI] [PubMed] [Google Scholar]
- 20.Grases F, March JG, Prieto RM, et al. Urinary phytate in calcium oxalate stone formers and healthy people. Scand J Urol Nephrol. 2000;34:162. doi: 10.1080/003655900750016526. [DOI] [PubMed] [Google Scholar]
- 21.Rodgers A, Allie-Hamdulay S, Jackson G. Therapeutic action of citrate in urolithiasis explained by chemical speciation: increase in pH is the determinant factor. Nephrol Dial Transplant. 2006;21:361. doi: 10.1093/ndt/gfi211. [DOI] [PubMed] [Google Scholar]


