Abstract
Black–white mortality disparities remain sizable in the United States. In this study, we use the concept of avoidable/amenable mortality to estimate cause-of-death contributions to the difference in life expectancy between whites and blacks by gender in the United States in 1980, 1993, and 2007. We begin with a review of the concept of “avoidable mortality” and results of prior studies using this cause-of-death classification. We then present the results of our empirical analyses. We classified causes of death as amenable to medical care, sensitive to public health policies and health behaviors, ischemic heart disease, suicide, HIV/AIDS, and all other causes combined. We used vital statistics data on deaths and Census Bureau population estimates and standard demographic decomposition techniques. In 2007, causes of death amenable to medical care continued to account for close to 2 years of the racial difference in life expectancy among men (2.08) and women (1.85). Causes amenable to public health interventions made a larger contribution to the racial difference in life expectancy among men (1.17 years) than women (0.08 years). The contribution of HIV/AIDS substantially widened the racial difference among both men (1.08 years) and women (0.42 years) in 1993, but its contribution declined over time. Despite progress observed over the time period studied, a substantial portion of black–white disparities in mortality could be reduced given more equitable access to medical care and health interventions.
Keywords: Health disparities, Life expectancy, Health Care, Amenable mortality, Demographic methods, Race
Introduction
This study aims to quantify the contribution of causes of death considered to be amenable to healthcare and other public health interventions to differences in life expectancy between white and black Americans over the past three decades. Our analyses complement previous comparisons of black–white (B–W) disparities in mortality (Elo and Drevenstedt 2004; Harper et al. 2007; Manton et al. 1987; Preston et al. 1996). In 2007, white life expectancy (78.4 years) exceeded that of blacks (73.6 years) by 4.8 years, with the gap being close to 2 years greater for men (75.9 vs. 70.0) than for women (80.8 vs. 76.8) (Xu et al. 2010). Black populations have higher death rates than whites from all major causes of adult and infant mortality, with suicide being the most notable exception (Harper et al. 2007; Rogers et al. 2000).
Several explanations have been offered for these disparities, including B–W differences in income, education, and wealth (Geruso 2012; Hayward et al. 2000; Rogers 1992; Williams and Sternthal 2010), experiences in utero and in early life, living conditions (Williams and Sternthal 2010), access to healthcare, and racial discrimination (Paradies 2006; Sanders-Phillips et al. 2009; Williams and Mohammed 2009). Among them, disparities in access to medical technologies and healthcare, including health insurance coverage, have emerged as significant factors (Lillie-Blanton and Hoffman 2005; Smedley et al. 2003; Sudano and Baker 2006; Tehranifar et al. 2009). Differential treatment in the healthcare system may further exacerbate disparities in access. Blacks have been, for example, less likely than whites to receive appropriate diagnostic tests and/or treatments for heart disease, certain cancers, kidney failure, and HIV/AIDS (Berndt et al. 2007; Berz et al. 2009; Levy et al. 2011; Mayberry et al. 2000; Salihu et al. 2010; Sonel et al. 2005; van Ryn et al. 2006). Although less is known about the role of individual preferences and patient–provider relationships, they may also affect treatment decisions and influence B–W differences in the timely and appropriate use of healthcare services (Ashton et al. 2003; Garrett and Yemane 2006). The persistence of these B–W disparities is a critical public health concern and an important consideration in the implementation of the Patient Protection and Affordable Care Act and other recent health reforms (Moy et al. 2011; Saenz 2010).
In this paper, we use the concept of avoidable/amenable mortality (AM) to estimate cause-of-death contributions to the racial gap in life expectancy at birth. Our objective is to quantify how much of this gap might reasonably be attributed to conditions that have been considered in the literature to be amenable to medical care or other health interventions as one indicator of how well the US has performed in reducing racial health disparities. This study is the first to assess the contribution of AM to differences in life expectancy between whites and blacks over time and the first to compare the relative contribution of avoidable with other causes of death to changes in the racial life expectancy gap in the US.
We begin with a review of the concept of avoidable mortality and results of prior studies in the US and elsewhere. This discussion is followed by a description of our data and methods and a presentation of our results. We end with a discussion and concluding comments.
The Concept and Measure(s) of Avoidable or Amenable Mortality (AM)
Rutstein et al. (1976) are credited with publishing the first list of about 90 conditions in the mid-1970s that represented “unnecessary” and “untimely” deaths [although there is some evidence that the concept dates back to the 1960s (Burgess et al. 1966)]. Rutstein’s list included sentinel events (e.g., deaths from appendicitis) when the occurrence of a single disease would justify asking why it occurred; conditions for which deaths pointed to problems with screening and secondary prevention (e.g., cervical cancer); and deaths from causes that could be influenced by public policy, social conditions, and/or behavior (e.g., smoking-related cancers). The purpose was to develop a list of causes that could be used to monitor and evaluate health system performance in the broadest sense. With the introduction of the 9th revision of the International Classification of Diseases (ICD), this list was updated in the early 1980s (Rutstein et al. 1980).
Several modifications have subsequently been made to Rutstein’s original list. The main distinction among subsequent compilations is how broadly the authors define AM, i.e., whether the list is limited to conditions for which effective medical treatment (e.g., infectious diseases) is available or whether conditions avoidable by behavior modification and policy (e.g., lung cancer and motor vehicle accidents) are also covered. In the early 1980s, Charlton et al. (1983) included 14 conditions designed to measure the impact of medical care quality on mortality. Conditions for which medical care could do little to prevent death (e.g., lung cancer) were excluded. At about the same time, Holland and others drawing on Rutstein’s and Charlton’s classifications made further modifications that led to the publication of the European Community Atlas of “Avoidable Death” (Holland 1988). The list of causes [updated in 1991 (Holland 1991) and 1993 (Holland 1993)] distinguished three types of AM: premature deaths that could be prevented with timely and appropriate medical care of good quality (e.g., infectious diseases); deaths that should not take place given adequate primary and secondary prevention (e.g., cervical cancer); and deaths sensitive to health policy (e.g., lung cancer, cirrhosis of the liver, and motor vehicle accidents). Several other variations of AM have also been used (Mackenbach 1996; Poikolainen and Eskola 1986; Simonato et al. 1998; Tobias and Jackson 2001; Westerling 1992).
A more recent list of “avoidable” mortality was published by Nolte and McKee (2004). Based on a comprehensive review of prior literature and consideration of recent advances in medical technology, the authors focused on causes of death that captured the impact of healthcare on mortality (primary care, hospital care, screening, and public health programs). Conditions influenced by public health policy and behavior (e.g., lung cancer) were excluded. The objective was to include conditions that could be used to assess the contribution of healthcare and to provide a standard list for international comparisons. This publication helped renew interest in the concept of avoidable mortality, and this list has been used in subsequent studies, including those focusing on cross-national comparisons of national health system performance (e.g., Nolte and McKee 2012).
Empirical Studies of Avoidable or Amenable Mortality
Following Rustein’s publications, the number of published studies of AM proliferated, initially covering Western Europe, but subsequently other countries as well (for a review of studies published prior to 2003 see Nolte and McKee (2004)). These studies, using primarily aggregate data, examined the contribution of medical care, primary or secondary prevention, and/or public policy to time trends in AM within countries, cross-country variations in AM, cross-sectional spatial variation in AM within countries, and variation in AM by area-level socioeconomic (SES) characteristics. Country-specific studies of time trends in AM have been conducted, for example, in Australia and New Zealand (Korda and Butler 2006; Piers et al. 2007), Canada (Pampalon 1993), Europe (Humblet et al. 2000), and Latin America (Abreu et al. 2007). These studies have typically shown AM to decline 2–5 times faster than all-cause mortality between the 1950s and early 1980s with some suggestion that the decline in AM has decelerated in the 1990s at least in some countries (Nolte and McKee 2008, 2011). Thus, reductions in AM appear to have made a sizable contribution to gains in life expectancy in the latter half of the 20th century. Mackenbach, for example, estimated that declines in death rates from causes amenable to medical care between the 1950s and 1990 in the Netherlands contributed 2.96 and 3.95 years to gains in male and female life expectancy, respectively (Mackenbach 1996; Mackenbach et al. 1988).
Declines in AM have also varied by areal level SES. For example, Korda et al. (2007) documented faster declines in mortality from causes of death amenable to medical care and those responsive to health policy (chronic liver disease and cirrhosis, lung cancer and accidents) at the highest area-level income quintile (5 % per annum) than at the lowest quintile (3.5 %) between 1986 and 2002 in Australia. These trends led to an increase in the relative but to a decline in the absolute area-level SES disparity in AM (see also Piers et al. 2007; Mackenbach et al. 1990; James et al. 2006).
Several recent studies have further tried to assess the relationship between avoidable mortality and features of the healthcare system. Heijink et al. (2012), for example, found a robust statistical association between increased healthcare spending and reduced rates of AM across a ten-year panel of OECD countries, including the US. Desai et al. (2011), examined trends in AM as an indicator of the performance of the British National Health Service (NHS) and showed that increased NHS funding corresponded with accelerated declines in AM in Great Britain. At the same time, some improvements were likely to have resulted from changes in prevention and early diagnosis related to public health and social changes rather than from increased NHS funding. In contrast, Plug et al. (2012) found that SES inequalities in AM in 14 OECD countries were not associated with indicators of healthcare access and utilization. The authors suggested that underlying social and behavioral factors (e.g., smoking) may have been responsible for mortality variation from both amenable and non-amenable causes of death.
In addition, AM has been used to study differences in mortality among population subgroups. In the Netherlands, immigrants were found to have an elevated risk (relative risk, RR = 1.13) of death from “avoidable” conditions compared to the native-born Dutch in 1995–2000, with the excess risk varying by cause of “avoidable” death and by immigrant subgroup (Stirbu et al. 2006). Individual demographic and socioeconomic characteristics largely explained the excess risks among immigrants (Stirbu et al. 2006). In Sweden, Westerling and Rosén found sizable differences in mortality between the native-born Swedish population and immigrants from other Nordic countries, former Yugoslavia and Eastern Europe but mainly for causes of death amenable to behavior or public health interventions (e.g., lung cancer and liver cirrhosis). Differences in mortality from causes considered amenable to medical care were small (Westerling and Rosén 2002). Ethnic differences in AM have also been documented in Singapore (Niti and Ng 2001), and an inverse association has also been found between individual-level SES and AM in New Zealand (Marshall et al. 1993) and South Korea (Song and Byeon 2000).
Avoidable or Amenable Mortality in the US
Despite the fact that the concept of “avoidable’” mortality originated in the US, it has not been put to a wide use in this country. Adler (1978) was first to show that Rutstein’s “sentinel health events,” a subset of causes from the full list, represented about 14 % of US deaths in 1970. Other studies have investigated AM in the US in comparison with other OECD countries. Kjellstrand et al. (1998) examined trends in six AM conditions1 at ages 5–64 in Australia, Canada, France, Germany, Italy, Japan, New Zealand, Sweden, the US, and the UK from 1980 to 1990. The US had the third lowest ranking in 1980 but it had declined to a fifth place by 1990 due to a slower decline in mortality from these causes in the US than in the other countries but one. In a more recent comparison of AM mortality in 19 OECD countries in 1998, the US ranked 16th with or without including 50 % of ischemic heart disease (IHD) deaths, which were considered partially avoidable (Nolte and McKee 2003). Higher US death rates from “avoidable” causes have also been implicated in the lower life expectancy in the US than in Canada among both blacks and whites (Kunitz and Pesis-Katz 2005; Manuel and Mao 2002). Between 1980 and 1996 the US also had higher mortality rates than Canada from 9 out of 11 cause-of-death groups2 considered amenable to medical care (with the exception of breast cancer and peptic ulcer) (Manuel and Mao 2002).
A comparison of 2007 rates of AM in the US with the UK, France, and Germany showed that the US rates were almost twice the rate of the country with the lowest rates (France) (Nolte and McKee 2012). Between 1999 and 2007, AM declined significantly for all four countries, but the decline was the smallest among American men (18.5 %) and women (17.5 %) with the largest declines seen among English men (37 %) and women (32 %). In terms of specific causes of death, the US had the lowest death rates from certain cancers (e.g., colon, skin, breast, cervix, and leukemia), whereas the US had the highest death rates from cerebrovascular disease and hypertension (Nolte and McKee 2012).
Within the US, amenable mortality has been shown to vary by geography and to contribute to SES and racial/ethnic mortality disparities. A recent study documented considerable state-level variation in mortality amenable to medical interventions, with the lowest rates found in Minnesota (70.2 per 100,000) and the highest in Washington, DC in 2002 (Commonwealth Fund 2007). Schoenbaum et al. (2011), in turn, documented a strong correlation between mortality amenable to healthcare among US states, especially among minority groups. State-level variation in mortality amenable to healthcare was additionally associated with hospital re-admissions and quality of diabetes care, which the authors attributed to state-level variation in healthcare system performance. In a cross-sectional study of large (over 100,000 population) US cities, higher income inequality and spending on police were positively associated with higher mortality from preventable causes, while spending on roads, education, health, and waste disposal (combined into a single measure) was negatively associated with mortality from preventable or immediate causes of death (e.g., accidents, asthma, diabetes, and homicide) (Ronzio et al. 2004).
Evidence that AM mortality in the US varies by SES comes from studies by Phelan et al. (2004) and Elo and Drevenstedt (2002), both based on the National Longitudinal Mortality Study (NLMS). Phelan et al. found that both education and family income had a significant negative association with mortality classified as highly “preventable” by two physician/epidemiologists; a list of causes closely correlated with Rutstein’s list of AM. For causes of death classified as having low probability of prevention (for which little is known about prevention or treatment), the mortality gradient by family income was less steep and their association with educational attainment was insignificant at ages 45 and above. In race- and gender-stratified analyses, SES was more strongly associated with mortality from highly preventable causes than from causes with low probability of prevention, except among black men. Elo and Drevenstedt (2002), in turn, found both absolute and relative measures of educational inequality in mortality to be smaller for medically amenable causes than for causes related to health behavior/policy at ages 25–44. At ages 45–64, both absolute and relative educational inequalities were more pronounced for medically amenable causes, including IHD, than causes related to health behavior/policy, although for the latter the education gradient was also present.
An early study of B–W disparities in AM found that age-adjusted mortality rates below age 65 from 12 of Rutstein’s sentinel causes3 were 4.5 times higher for blacks than for whites in the early 1980s (Schwartz et al. 1990). Similarly in Texas, medical care AM in the 1980s was more than three times higher among blacks than whites [standardized mortality ratio (SMR): 3.4]; the AM rate for Hispanics was only slightly above the white rate [SMR: 1.1]. At about the same time, Woolhandler et al. (1985) documented excess deaths among blacks compared to whites from medically AM in Alameda county, CA. Overall, 44 % of person-years of life lost (PYLL) for blacks was due to potentially preventable deaths, whereas for whites the respective estimate was 32 %. Elo and Drevenstedt (2006) further found that mortality was higher among blacks than whites from both medically amenable causes and causes related to health behavior/policy (e.g., lung cancer, liver cirrhosis, homicide, and accidents) during the 1980s. At ages 25–44, the two cause-of-death groups explained over 60 % of the B–W difference in age-adjusted death rates among males and females with behavioral causes being more important among men than women. At ages 45–64, medically amenable causes, together with heart disease and stroke, accounted for 53 % of the higher mortality of black men and 77 % of the higher mortality of black women. Causes related to health behavior/policy (e.g., smoking- and alcohol-related causes, and accidents) made smaller contributions at these older ages (27 % for men and 1.6 % for women). Adjustment for education, family income, marital status, and place of residence in sex-specific multivariate regression explained a larger fraction of the black excess risk in mortality from causes related to health behavior/policy than from causes amenable to medical care.
A more recent study examined trends in B–W disparities in age-adjusted death rates below age 65 between 1980 and 2005 from conditions amenable to medical care, those sensitive to public policy and/or behavior change, IHD, HIV, and residual causes of death (Macinko and Elo 2009). The authors found that medical care AM was the largest source of B–W mortality disparity in 2005, contributing 30 % of the B–W disparity in all-cause mortality among men and 42 % among women. At the same time, whereas the absolute B–W differences for most cause-of-death groups diminished over time, the relative differences measured by rate ratios exhibited little change, except in the case of HIV for which RRs increased substantially.
In this paper, we extend previous analyses of B–W disparities in mortality amenable to medical care and behavior/policy interventions by estimating their contribution to the difference in life expectancy at birth between whites and blacks and changes in this difference over time. We assess three periods (1980, 1993, and 2007), which reflect increasing B–W differences in life expectancy (1980–1993) and declining B–W disparities (1993–2007). Within each broad cause-of-death category, i.e., among medically amenable causes of death, we further distinguish among distinct cause-of-death groups (e.g., cancers and diabetes), to provide a more refined assessment of the role of AM in B–W mortality disparities. Among the behavior/policy interventions, we also distinguish among specific causes of death as their contributions to B–W disparities vary by sex and over time. Grouping all medically amenable causes and all causes related to behavior/policy interventions together can mask important variations in the contribution of more specific causes of death.
Data
We used data on deaths from Mortality Detail (1979–1991) and Multiple Cause of Death (1992–2007) files produced by the National Center for Health Statistics (NCHS). These data contain information on detailed causes of death, race, age, sex, and place of residence at the time of death. We classified causes of death based on the underlying cause of death reported on the death certificate. This approach is consistent with previous literature examining cause-specific mortality in the US (e.g., Geronimus et al. 1996; Harper et al. 2007). We used population estimates prepared by the Census Bureau available from the National Cancer Institute to construct age-specific death rates by age, race, and sex (National Cancer Institute 2011). The 85+ population was allocated into 5-year-age groups above age 85 based on census data (1980 and 1993) and tabulations obtained from NCHS for 2007.
Classification of Causes of Death
Our cause-of-death classification is based on prior studies of B–W mortality disparities (Elo and Drevenstedt 2004; Geronimus et al. 1996; Harper et al. 2007; Kochanek et al. 1994; Macinko and Elo 2009) and the literature on “amenable or avoidable mortality” (AM), particularly the most recent classification of AM by Nolte and McKee (AM) (Gay et al. 2011; Nolte and McKee 2004; Rutstein et al. 1976). We grouped causes of death into six categories: causes amenable to (1) medical care (deaths that could be prevented or reduced by timely medical care or primary or secondary prevention); (2) sensitive to public health policies and causes linked with health behaviors (e.g., smoking, excessive drinking, drunk driving, seatbelt use, access to and use of firearms); (3) IHD; (4) suicide (5); HIV; and (6) all other causes labeled “residual/less amenable” causes. Although Nolte and McKee categorized IHD as “partly amenable to healthcare” and partitioned 50 % of IHD deaths to medically amenable causes (Nolte and McKee 2004), we analyzed IHD separately because it represents an important cause of death and is amenable both to medical care and health behavior change. We also examined HIV separately because it emerged as a leading cause of death in young adulthood, made a sizable contribution to B–W mortality disparities (Levine et al. 2007), and was initially sensitive only to policy/behavior interventions before the advent of highly effective antiretroviral therapy (HAART) in the mid-1990s. Suicide, on the other hand, is one of the few causes that historically has been higher among whites than blacks so it was also included separately (Rogers 1992). In addition, to gain further insights regarding the contribution of specific conditions within these broader cause-of-death groups, we further distinguished among the most important causes within subcategories: medical care (infectious and respiratory diseases, cancers, circulatory diseases, birth related, diabetes, and other diseases); public health policy/behavior (lung cancer and cirrhosis, homicide, and traffic accidents); and 6 residual causes (cancers, heart disease, ill-defined causes, and all other less amenable causes). The ICD codes for each cause-of-death group are shown in the Appendix, Table 5.
We focus on mortality below age 75 for the following reasons. First, this age limit is consistent with prior studies of AM that have used age 75 as an upper age limit to classify causes of death (e.g., Nolte and McKee 2004), thus allowing our results to be compared with those obtained from previous studies. Second, mortality below age 75 accounted for over 90 % of the difference in male and female life expectancy between blacks and whites in 1980 and 2007 (authors’ calculations). Third, medical care and policy/behavior interventions are likely to be most effective in prolonging life at younger ages. Fourth, the identification of the underlying cause of death becomes increasingly problematic at the oldest ages when multiple diseases are present (Rosenberg 1999).
Methods
We first calculated age-, sex-, and race-specific death rates for all-cause mortality in five-year-age groups up to age 100 with the open-ended age interval beginning at age 100+ and estimated period life tables for blacks and whites by sex for 1980, 1993, and 2007 using standard life table procedures (Preston et al. 2001). Second, we estimated age- and cause-specific death rates in five-year-age groups below age 75. Third, we calculated cause-of-death contributions below age 75 to (1) the difference in life expectancy at birth between whites and blacks in 1980, 1993, and 2007 by sex; (2) the change in life expectancy at birth between 1980–1993 and 1993–2007 by race and sex, and (3) changes in the difference between whites and blacks between 1980–1993 and 1993–2007 by sex (Arriaga 1984; Beltrán-Sánchez et al. 2008).
In addition, we estimated the number of excess deaths (as the number of observed deaths minus the number of expected deaths) among blacks below age 75 by age, sex, and cause-of-death group in 2007. The estimated excess deaths represent deaths that would not have occurred if blacks had experienced the same age-, sex-, and cause-specific mortality rates as their white counterparts. Finally, we estimated standardized mortality ratios (SMRs) as the ratio of observed black deaths to the expected deaths for men and women and also computed their 95 % confidence intervals. Our methods for these calculations are detailed below.
Cause-Specific Contributions to the Difference in Life Expectancy Between Whites and Blacks and Their Contribution to the Change in Life Expectancy over Time by Race and Sex
Let and be the life expectancy at birth for whites and blacks, respectively, for sex k [k ∈ (men, women)], at time t [t ∈ (1980, 1993, 2007)]. Let be the difference in life expectancy at birth between whites and blacks for sex k at time t. Then can be decomposed as (Beltrán-Sánchez et al. 2008):
| (1) |
where , and represent the white probability of surviving from birth to age a, for sex k (k ∈ (men, women)) from cause of death i and cause −i (everything but cause i) at time t (t ∈ (1980, 1993, 2007)). Similar quantities for blacks are identified with the superscript B.
In discrete age intervals, and using life table notation, the above equation is equivalent to:
| (2) |
where l0 represents the life table radix, and and represent the white person-years lived between ages x and x + n for sex k (k ∈ (men, women)) at time t (t ∈ (1980, 1993, 2007)) in the life tables for cause i and cause −i, respectively. Similar quantities for blacks are identified with the superscript B. Equation 2 can also be used to estimate the cause-specific contributions to change in life expectancy among blacks and whites between two time points where B and W would now represent two time points. This method is equivalent to well-known decomposition methods of Pollard (1982) and Arriaga (1984) and is detailed in Beltrán-Sánchez et al. (2008).
Cause-Specific Contributions to the Change in the Difference in Life Expectancy Between Whites and Blacks over Time
Let be the change in the difference in life expectancy between whites and blacks at birth between time t1 and t2 which can be computed as:
| (3) |
Substituting Eq. 1 into Eq. 3 leads to
which is equivalent to
| (4) |
Thus, the cause-specific contributions to the change in the difference in life expectancy between whites and blacks over time can be estimated as the difference between the cause-specific contributions to this difference at time 1 and time 2 (Eq. 4). Each component of Eq. 4 can be estimated using Eqs. 1 (continuous form) and 2 (discrete form).
Excess Deaths and Standardized Mortality Ratios
Let and be the death rate for whites and blacks, respectively, between ages x and x + n for cause i, sex k in 2007 (k ∈ (men, women)) and be the black population between ages x and x + n for sex k in 2007. The estimated number of excess deaths by sex (k) for cause i in 2007, EDk,i (2007), are estimated as
The SMRs by cause of death and sex [SMRk,i (2007)] are then calculated as observed deaths divided by the expected deaths. If the ratio is greater than 1.0 then the number of observed deaths exceeds the number of expected deaths, and ratios below 1.0 indicate the reverse. The 95 % confidence interval is obtained as follows:
Results
Between 1980 and 2007, life expectancy increased by about 5 years for white men, from 70.8 to 75.9 years, and by about 6 years for black men, from 64.1 to 70.0 years (see Appendix, Table 4). The vast majority of this increase among black men occurred between 1993 and 2007, whereas the gains were more equally distributed between 1980–1993 and 1993–2007 among white men. Despite the more rapid mortality decline among black men between 1993 and 2007, their life expectancy in 2007 was only approaching what white males’ life expectancy had been 27 years earlier in 1980. Over this period, the racial gap in male life expectancy increased from about 7 years in 1980 to 8.5 years in 1993, but subsequently decline to 5.9 years in 2007.
Between 1980 and 2007, life expectancy increased by close to 3 years for white women, 78.2–80.8 years, and by close to 4 years for black women, from 72.9 to 76.8 years. As was the case with men, most of this increase among black women occurred between 1993 and 2007, whereas the gains were more equal in the two periods among white women. In 2007, the life expectancy of black women was also lower than that of white women in 1980 (see Appendix, Table 4). The gap in white and black female life expectancy increased from about 5.3 years in 1980 to 5.6 years in 1993, but subsequently declined to about 4.0 years in 2007.
Cause-Specific Contributions to Life Expectancy among Whites and Blacks and to the Difference in Life Expectancy Between Whites and Blacks
We next examine the contribution of the various cause-of-death groups to changes in life expectancy by race and sex and to the racial gap in male and female life expectancy. These results are presented in Tables 1 and 2 and in Fig. 1.
Table 1.
Cause-specific contributions to a change in life expectancy at birth by race and sex, 1980–1993 and 1993–2007 (years)
| Cause-of-death group | White males | Black males | White females | Black females |
|---|---|---|---|---|
| Δe0 1980–1993 | ||||
| Medically amenable causes | 0.49 | 0.63 | 0.39 | 0.62 |
| Infectious and respiratory diseases | 0.03 | 0.11 | −0.14 | −0.05 |
| Cancer | 0.06 | −0.03 | 0.15 | 0.00 |
| Circulatory diseases | 0.16 | 0.26 | 0.19 | 0.40 |
| Birth related | 0.25 | 0.29 | 0.19 | 0.27 |
| Diabetes | −0.04 | −0.09 | −0.03 | −0.07 |
| Other diseases | 0.03 | 0.09 | 0.02 | 0.06 |
| Ischemic Heart Disease | 1.12 | 0.60 | 0.48 | 0.51 |
| HIV | −0.44 | −1.28 | −0.05 | −0.44 |
| Suicide | −0.01 | −0.04 | 0.03 | 0.01 |
| Behavior/policy | 0.46 | 0.17 | −0.07 | −0.06 |
| Lung cancer | 0.09 | 0.11 | −0.15 | −0.06 |
| Homicide | 0.04 | −0.09 | 0.00 | 0.00 |
| Traffic accidents | 0.33 | 0.15 | 0.08 | 0.00 |
| Residual causes | 0.43 | 0.87 | 0.27 | 0.62 |
| Cancer | 0.04 | 0.08 | 0.06 | 0.12 |
| Heart disease | 0.05 | 0.05 | 0.00 | 0.01 |
| Ill-defined causes | 0.04 | 0.14 | 0.03 | 0.15 |
| All other non-amenable | 0.30 | 0.59 | 0.17 | 0.34 |
| Mortality above age 75 | 0.34 | −0.03 | 0.40 | 0.03 |
| Change in life expectancy | 2.34 | 0.90 | 1.40 | 1.30 |
| Δe0 1993–2007 | ||||
| Medically amenable causes | 0.30 | 0.70 | 0.36 | 0.78 |
| Infectious and respiratory diseases | 0.06 | 0.21 | −0.02 | 0.08 |
| Cancer | 0.17 | 0.18 | 0.26 | 0.26 |
| Circulatory diseases | 0.06 | 0.16 | 0.08 | 0.26 |
| Birth related | 0.05 | 0.20 | 0.04 | 0.17 |
| Diabetes | −0.03 | −0.01 | 0.02 | 0.09 |
| Other diseases | −0.01 | −0.03 | −0.02 | −0.06 |
| Ischemic Heart Disease | 0.88 | 0.78 | 0.43 | 0.68 |
| HIV | 0.41 | 1.02 | 0.04 | 0.20 |
| Suicide | 0.03 | 0.09 | −0.01 | 0.01 |
| Behavior/policy | 0.37 | 1.23 | 0.13 | 0.35 |
| Lung cancer | 0.29 | 0.49 | 0.07 | 0.11 |
| Homicide | 0.08 | 0.69 | 0.03 | 0.20 |
| Traffic accidents | 0.00 | 0.06 | 0.03 | 0.04 |
| Residual causes | 0.17 | 1.32 | −0.02 | 0.68 |
| Cancer | 0.10 | 0.31 | 0.11 | 0.18 |
| Heart disease | 0.17 | 0.27 | 0.09 | 0.24 |
| Ill-defined causes | 0.07 | 0.24 | 0.04 | 0.13 |
| All other non-amenable | −0.18 | 0.49 | −0.26 | 0.12 |
| Mortality above age 75 | 0.91 | 0.56 | 0.66 | 0.51 |
| Change in life expectancy | 3.1 | 5.7 | 1.6 | 3.2 |
Source Authors’ analysis of data from Mortality Detail and Multiple Cause of Death files
Cause-specific mortality rates operate from birth to age 75. A positive (negative) value indicates that the cause of death contributed to an increase (decrease) in life expectancy between two time points. Numbers may not add up due to rounding
Table 2.
Cause-specific contributions to the difference in life expectancy at birth between whites and blacks by sex, 1980, 1993, and 2007 and to change in this difference between 1980–1993 and 1993–2007 (years)
| Cause-of-death group | Contributions to W–B difference in e0
|
Contributions to the change in W–B difference in e0
|
||||||
|---|---|---|---|---|---|---|---|---|
| 1980 | 1993 | 2007 | 1980–1993 | 1993–2007 | ||||
| Males | ||||||||
| Medically amenable causes | 2.33 | 2.24 | 2.08 | −0.09 | −0.16 | |||
| Infectious and respiratory diseases | 0.49 | 0.43 | 0.30 | −0.06 | −0.13 | |||
| Cancer | 0.11 | 0.23 | 0.22 | 0.11 | −0.01 | |||
| Circulatory diseases | 0.83 | 0.71 | 0.72 | −0.11 | 0.01 | |||
| Birth related | 0.56 | 0.50 | 0.37 | −0.06 | −0.13 | |||
| Diabetes | 0.12 | 0.21 | 0.24 | 0.09 | 0.03 | |||
| Other diseases | 0.22 | 0.16 | 0.23 | −0.06 | 0.07 | |||
| Ischemic Heart Disease | 0.31 | 0.58 | 0.53 | 0.27 | −0.05 | |||
| HIV | 0.00 | 1.08 | 0.42 | 1.08 | −0.65 | |||
| Suicide | −0.14 | −0.11 | −0.21 | 0.02 | −0.10 | |||
| Behavior/policy | 1.70 | 2.01 | 1.17 | 0.31 | −0.84 | |||
| Lung cancer and cirrhosis | 0.47 | 0.47 | 0.22 | 0.00 | −0.24 | |||
| Homicide | 1.32 | 1.49 | 0.95 | 0.18 | −0.55 | |||
| Traffic accidents | −0.08 | 0.06 | 0.00 | 0.14 | −0.05 | |||
| Residual causes | 2.83 | 2.42 | 1.41 | −0.41 | −1.01 | |||
| Cancer | 0.54 | 0.52 | 0.33 | −0.01 | −0.19 | |||
| Heart disease | 0.55 | 0.58 | 0.51 | 0.03 | −0.07 | |||
| Ill-defined causes | 0.41 | 0.30 | 0.13 | −0.12 | −0.17 | |||
| All other non-amenable | 1.34 | 1.03 | 0.45 | −0.31 | −0.58 | |||
| Mortality above age 75 | −0.05 | 0.28 | 0.44 | 0.33 | 0.16 | |||
| White e0 (t) – Black e0 (t) | 6.99 | 8.50 | 5.85 | 1.51 | −2.66 | |||
| Females | ||||||||
| Medically amenable causes | 2.39 | 2.19 | 1.85 | −0.20 | −0.34 | |||
| Infectious & respiratory diseases | 0.29 | 0.24 | 0.15 | −0.05 | −0.09 | |||
| Cancer | 0.17 | 0.31 | 0.30 | 0.14 | −0.01 | |||
| Circulatory diseases | 0.93 | 0.70 | 0.55 | −0.23 | −0.15 | |||
| Birth related | 0.56 | 0.47 | 0.35 | −0.09 | −0.12 | |||
| Diabetes | 0.26 | 0.32 | 0.28 | 0.07 | −0.04 | |||
| Other diseases | 0.19 | 0.15 | 0.22 | −0.04 | 0.07 | |||
| Ischemic Heart Disease | 0.85 | 0.75 | 0.45 | −0.10 | −0.30 | |||
| HIV | 0.00 | 0.42 | 0.27 | 0.42 | −0.15 | |||
| Suicide | −0.08 | −0.07 | −0.09 | 0.02 | −0.02 | |||
| Behavior/policy | 0.29 | 0.32 | 0.08 | 0.03 | −0.24 | |||
| Lung cancer and cirrhosis | 0.10 | 0.04 | −0.02 | −0.06 | −0.06 | |||
| Homicide | 0.30 | 0.31 | 0.13 | 0.01 | −0.17 | |||
| Traffic accidents | −0.11 | −0.03 | −0.04 | 0.08 | −0.01 | |||
| Residual causes | 2.04 | 1.70 | 1.06 | −0.34 | −0.64 | |||
| Cancer | 0.34 | 0.28 | 0.21 | −0.06 | −0.07 | |||
| Heart disease | 0.50 | 0.52 | 0.37 | 0.02 | −0.15 | |||
| Ill-defined causes | 0.33 | 0.20 | 0.11 | −0.13 | −0.09 | |||
| All other non-amenable | 0.87 | 0.70 | 0.38 | −0.17 | −0.32 | |||
| Mortality above age 75 | −0.02 | 0.31 | 0.38 | 0.32 | 0.07 | |||
| White e0 (t) − Black e0 (t) | 5.48 | 5.63 | 4.00 | 0.15 | −1.62 | |||
Source Authors’ analysis of data from Mortality Detail and Multiple Cause of Death files
Cause-specific mortality rates operate from birth to age 75. A positive (negative) value indicates that the cause of death contributed to an increase (decrease) in W–B difference in life expectancy at a given point in time or between two time points. Numbers may not add up due to rounding
Fig. 1.
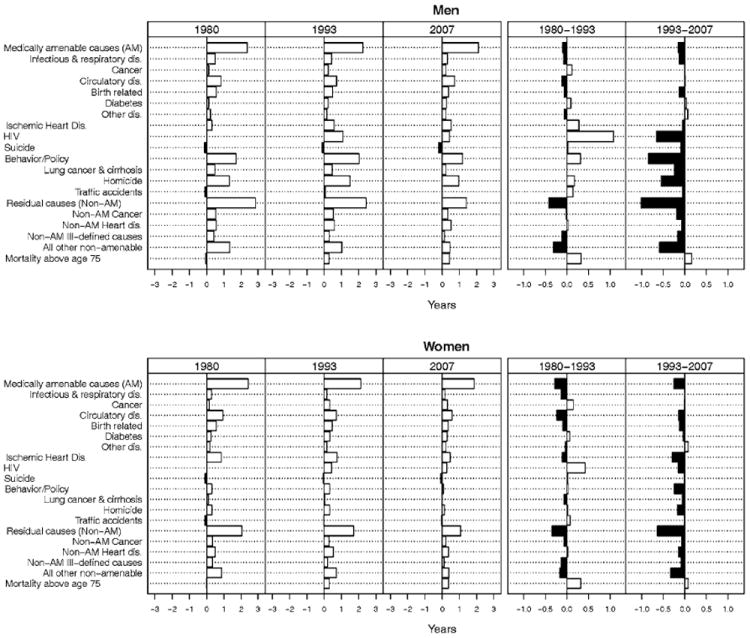
Cause-specific contributions to the difference in life expectancy between whites and blacks at birth by sex, 1980, 1993, and 2007 and to change in this difference between 1980–1993 and 1993–2007 (years). Source See Table 1. A positive (negative) value indicates that the cause of death contributed to an increase (decrease) in B–W difference in life expectancy at a given point in time or between two time points
Medically Amenable Causes of Death
As shown in Table 1, medically amenable causes of death taken together made a larger contribution to gains in life expectancy among both black men (1.33 years) and black women (1.40 years) than among white men (0.79 years) or white women (0.75 years) between 1980 and 2007. As a result of these race-specific trends, the contribution of medically amenable causes of death to the difference in white and black male and female life expectancy declined slightly over time; from 2.3 years in 1980 to 2.1 in 2007 among men and from 2.4 years to 1.9 years among women (Table 2). However, despite the decline in the overall contribution of medically amenable causes, their percentage contribution fluctuated over time due to mortality trends from other causes of death. Among men, the percentage contributions of medically AM to the racial gap in life expectancy was 33 % in 1980, 26 % in 1993, and 36 % in 2007; the respective percentages for women were 44, 39, and 46 %.
Among the medically amenable causes, mortality trends were more favorable for blacks than for whites for all causes other than diabetes and cancers between 1980 and 1993 and “other medically amenable causes” between 1993 and 2007. Among these causes, cardiovascular diseases made the largest contribution to the racial gap in life expectancy at each time point (1980, 1993, and 2007) among both men (0.83, 0.71, and 0.72 years) and women (0.93, 0.70, and 0.55 years).
Causes of Death in the Behavior-Policy Category, including Suicide
Mortality from causes in the behavior/policy group made a much larger contribution to the racial difference in male than female life expectancy (Table 2). Together they accounted for 1.7 years (24 %) of the 7.0-year gap in male life expectancy in 1980, about 2.0 years (24 %) of the 8.5-year gap in 1993, before declining to 1.2 years (20 %) of the 5.9-year gap in 2007. The cause of death largely responsible for this pattern was homicide mortality among black men, which increased between 1980 and 1993 but subsequently declined between 1993 and 2007. Among women, this cause-of-death group accounted for only 0.29 years (5 %) of the 5.5-year gap in life expectancy in 1980, 0.32 years (6 %) of the 5.6-year gap in 1993 and 0.08 years (2 %) of the 4.0-year difference in 2007. As was the case with men, the most important contributor to the higher mortality among black women compared to white women was homicide.
In addition, declining mortality from lung cancer/cirrhosis contributed to an increase in life expectancy among both white and black men (Table 1). In contrast, increasing lung cancer/cirrhosis mortality in the earlier period dampened life expectancy gains among both white and black women (Table 1), reflecting gender differences in cohort smoking patterns in the US (Ho and Elo 2012; Preston and Wang 2006). Nevertheless, the contribution of these causes to the racial difference in life expectancy among both men and women declined over time (Table 2). The contribution of traffic accidents to the racial gap in male and female life expectancy was small, and suicide mortality was higher among whites than blacks (Table 2).
Ischemic Heart Disease
Nolte and McKee (2004) partitioned half of the deaths from IHD to medically AM. We chose to examine this cause separately because both access to medical care and health behaviors (e.g., smoking, diet, and exercise) can contribute to variation in mortality from this cause. It is also a leading cause of death in the US. Between 1980 and 2007, the contribution of IHD to gains in life expectancy varied by race and sex. Declines in IHD death rates added 2.0 years to life expectancy for white males, 1.4 years for black males, 0.91 years for white females, and 1.2 years for black females (Table 1). Among men, the contribution of IHD to the racial gap in life expectancy increased from 0.31 years in 1980 to 0.58 years in 1993; but subsequently declined to 0.53 years in 2007. In contrast, due to the more rapid decline in IHD mortality among black women than white women, IHD’s contribution to the gap in white and black female life expectancy declined over time, from 0.85 years in 1980, to 0.75 years in 1993, to 0.45 years in 2007.
Human Immunodeficiency Virus (HIV)
The emergence of HIV as a leading cause of death among young adults during the 1980s offset mortality declines from other causes of death between 1980 and 1993, especially among black men and to lesser extent among white men and black women, whereas it played a much smaller role among white women (Table 1). These patterns reversed after 1993 and mortality declines from HIV made the largest contribution to gains in life expectancy among black men, followed by white men and black women. In 1993, HIV accounted for 1.1 years of the difference in white and black male life expectancy, but its contribution had declined to 0.42 years by 2007. HIV made a smaller contribution to the difference in white and black female life expectancy in both 1993, 0.42 years, and in 2007, 0.27 years.
Residual Causes of Death
Causes of death included among the residual category made a much larger contribution to gains in life expectancy among blacks than whites between 1980 and 2007, contributing about 2 years among black men, 0.6 years among white men, 1.3 years among black women, and 0.25 years among white women. Between 1980 and 2007, the contribution of these causes to the difference in male life expectancy was cut in half, from 2.8 to 1.4 years; the respective contributions among women were 2.0 years in 1980 and 1.1 years in 2007. Among these causes, the largest contributions were made by a very heterogeneous group of causes, other than heart disease and cancer (Table 1).
Excess Deaths and Standardized Mortality Ratios
We estimated the number of excess deaths among black men and black women for 2007 by calculating the difference between observed deaths by cause of death and the deaths that would have occurred had blacks experienced the age-, sex-, and cause-specific death rates of white men and white women. That is, if racial disparities in mortality were eliminated how many deaths among black men and black women would have been avoided in 2007. Standardized mortality ratios in turn provide an estimate of the relative excess mortality among blacks compared to whites by cause of death. We present these results in Table 3.
Table 3.
Estimated number of excess deaths and standardized mortality ratios (SMRs), Black men and Black women below age 75, 2007
| Cause-of-death group | Deaths | % | SMR | 95 % CI |
|---|---|---|---|---|
| Males | ||||
| Medically amenable causes | 16,722 | 38.3 | 2.04 | 2.02–2.07 |
| Infectious and respiratory diseases | 2,496 | 5.7 | 1.50 | 1.47–1.53 |
| Cancer | 1,982 | 4.5 | 1.47 | 1.43–1.50 |
| Circulatory diseases | 6,357 | 14.5 | 3.31 | 3.24–3.37 |
| Birth related | 1,844 | 4.2 | 2.38 | 2.30–2.47 |
| Diabetes | 2,099 | 4.8 | 2.19 | 2.12–2.26 |
| Other diseases | 1,944 | 4.4 | 3.02 | 2.91–3.13 |
| Ischemic Heart Disease | 4,662 | 10.7 | 1.51 | 1.48–1.53 |
| HIV | 3,617 | 8.3 | 7.91 | 7.67–8.16 |
| Suicide | −1,704 | −3.9 | 0.48 | 0.45–0.50 |
| Behavior/policy | 8,489 | 19.4 | 1.77 | 1.74–1.79 |
| Lung cancer and cirrhosis | 2,027 | 4.6 | 1.34 | 1.31–1.37 |
| Homicide | 6,393 | 14.6 | 7.07 | 6.91–7.23 |
| Traffic accidents | 70 | 0.2 | 1.02 | 0.99–1.05 |
| Residual causes | 11,906 | 27.2 | 1.53 | 1.51–1.55 |
| Cancer | 2,989 | 6.8 | 1.45 | 1.42–1.47 |
| Heart disease | 4,317 | 9.9 | 2.24 | 2.19–2.29 |
| Ill-defined causes | 858 | 2.0 | 1.91 | 1.82–2.00 |
| All other non-amenable | 3,742 | 8.6 | 1.33 | 1.31–1.35 |
| Total | 43,692 | 1.70 | 1.69–1.71 | |
| Females | ||||
| Medically amenable causes | 14,316 | 50.3 | 1.87 | 1.85–1.89 |
| Infectious and respiratory diseases | 1,114 | 3.9 | 1.23 | 1.20–1.26 |
| Cancer | 2,578 | 9.1 | 1.46 | 1.42–1.49 |
| Circulatory diseases | 4,790 | 16.8 | 2.97 | 2.90–3.04 |
| Birth related | 1,608 | 5.6 | 2.41 | 2.32–2.50 |
| Diabetes | 2,367 | 8.3 | 2.70 | 2.62–2.79 |
| Other diseases | 1,860 | 6.5 | 3.04 | 2.92–3.15 |
| Ischemic Heart Disease | 3,939 | 13.8 | 1.91 | 1.87–1.95 |
| HIV | 2,121 | 7.4 | 15.94 | 15.3–16.6 |
| Suicide | −682 | −2.4 | 0.33 | 0.30–0.37 |
| Behavior/policy | 468 | 1.6 | 1.07 | 1.04–1.09 |
| Lung cancer and cirrhosis | −120 | −0.4 | 0.98 | 0.95–1.00 |
| Homicide | 844 | 3.0 | 3.12 | 2.95–3.29 |
| Traffic accidents | −254 | −0.9 | 0.85 | 0.80–0.89 |
| Residual causes | 8,315 | 29.2 | 1.47 | 1.45–1.49 |
| Cancer | 1,806 | 6.3 | 1.29 | 1.26–1.32 |
| Heart disease | 3,090 | 10.9 | 2.18 | 2.12–2.24 |
| Ill-defined causes | 613 | 2.2 | 1.92 | 1.81–2.02 |
| All other non-amenable | 2,805 | 9.9 | 1.34 | 1.31–1.36 |
| Total | 28,476 | 1.61 | 1.60–1.62 |
Source Authors’ analysis of data from Mortality Detail and Multiple Cause of Death files. Cause-specific mortality rates operate from birth to age 75
Excess deaths are estimated as the number of actual deaths minus the number of estimated deaths if blacks had the age- and cause-specific death rates of whites. Numbers may not add up due to rounding
We estimate that there were over 46,000 excess deaths among black men below age 75 in 2007, 38 % of which were due to medically amenable causes, i.e., causes of death from which mortality could be reduced with access to high-quality preventive and curative healthcare. Although not a direct measure of access to healthcare among blacks and whites, it provides some indication of the potential role of heath disparities in access to healthcare (Desai et al. 2011; Mackenbach 1996). Among medically amenable causes cardiovascular diseases made the largest contribution accounting for close to 15 % of all excess black male deaths. The contribution of other medically amenable causes was in the 4–6 % range. The behavior/policy category in turn made up about 19 % of all excess deaths among black men, with homicide being the most important. IHD (11 %) and HIV (8 %) together contributed close to 20 %. The number of deaths from suicide was higher among whites, whereas the number of excess deaths from traffic accidents among black men was estimated to be only 70. The residual cause-of-death group made up 27 % of the excess deaths.
Among black women, the estimated number of excess deaths was over 28,000 of which medically amenable causes of death accounted for about 50 %. As was the case for black men, among these causes cardiovascular diseases were the most important, making up close to 17 % of all excess deaths. In contrast to men, the policy/behavior category accounted for less than 2 % of the excess deaths among black women, whereas excess deaths from IHD (14 %) made up a larger proportion and HIV made up a slightly smaller proportion (7 %) than was the case among black men. Mortality from lung cancer and cirrhosis, traffic accidents, and suicide was somewhat higher among white women than black women.
The relative mortality differentials, as measured by the SMRs, exhibited a somewhat different pattern. The SMR was highest for HIV among both men (7.91; 95 % CI 7.67–8.16) and women (15.94; 95 % CI 15.3–16.6). It was similarly high for homicide among men (7.07; 95 % CI 6.91–7.23), but less so among women (3.12; 95 % CI 2.95–3.29). For most other causes of death, the SMRs ranged from 1.5 to slightly over 3.00. They were below 1.00 for suicide for which whites have higher mortality than blacks.
Discussion
Our results are consistent with prior studies that have documented a widening of the racial gap in life expectancy in the 1980s and early 1990s and its subsequent narrowing after 1993 (Elo and Drevenstedt 2004; Harper et al. 2007; Kochanek et al. 1994). They are also consistent with the expectation that mortality from conditions that are amenable to medical care make a large contribution to this difference.
Medically AM below age 75 accounted close to 2 years of the racial gap in male and female life expectancy in 1980, 1993, and 2007, although its overall contribution declined somewhat over time. In 2007, the number of excess deaths from these causes among blacks was high, accounting for more than a third (38 %) of all excess deaths among black men and a half (50 %) among black women. The relative difference as measured by the SMR was close to 2 for both men and women. Thus, there appears to be considerable room for narrowing the B–W mortality gap by known preventive and curative medical technologies.
At the same time, however, our results suggest the need for caution in making generalizations about all medically amenable conditions based on the results for the medically amenable category taken as a whole. When analyzed separately, we showed that mortality from some conditions contributed to the widening of the B–W gap in life expectancy (e.g., cancers and diabetes) whereas others contributed to its narrowing (e.g., infectious and respiratory diseases, birth-related causes, and circulatory diseases) over the study period. These results are consistent with evidence showing higher mortality among blacks than whites from cancers for which screening and/or treatment are available, e.g., breast, colorectal, and prostate cancer (SEER 2011; Soneji et al. 2010), and prior findings showing that B–W disparities in mortality are wider for cancers that are more amenable to medical interventions (Tehranifar et al. 2009). Although there is a debate about the effectiveness of some cancer screening (e.g., prostate cancer screening), especially among younger age groups, our results are consistent with other evidence suggesting that whites compared to blacks obtain higher rates of recommended screenings and treatments at earlier stages of cancer presentation, which are associated with higher 5-year survival rates (Virnig et al. 2009). Other evidence shows that adherence to complex treatment regimes for diabetes is related to SES (Bailey and Kodack 2011) and thus may also be related to B–W differences in diabetes mortality. In addition, B–W differences in environmental exposures may place blacks at a greater risk of diabetes than whites (LaVeist et al. 2009).
In 2007, IHD mortality accounted for 10.7 % of black male and 13.8 % of black female excess deaths below age 75 and close to half a year of the difference in male and female life expectancy between whites and blacks. The relative differences were also higher for women (SMR: 1.9) than for men (SMR: 1.5). In the last three decades, coronary heart disease mortality has declined substantially in the US. It has been estimated that about 47 % of the overall decline in CVD between 1980 and 2000 was due to treatment and about 44 % to a change in risk factors (Ford et al. 2007; Ford and Capewell 2011). We found that IHD mortality decline was more pronounced among white men than black men especially between 1980 and 1993, whereas among women, IHD contributed to the narrowing of the B–W difference over time (Table 2). Thus, in the early period white men appeared to have benefited more from treatments and risk factor reductions than black men (Petersen et al. 2002; Peterson et al. 1997), but in subsequent years these differences appear to have diminished.
Among the causes of death considered amenable to behavior modification and public health interventions, homicide was most important among men. It also made a large contribution to the widening of the racial gap in male life expectancy between 1980 and 1993 and to its subsequent narrowing. In contrast, homicide made only a minor contribution among women. High rates of homicide among black men are especially evident in poor urban areas (Geronimus et al. 1999, 2011), and they are tied to high levels of economic segregation and poverty concentration (Eitle et al. 2006; Krivo and Peterson 2000). Public health interventions to restrict access to fire arms and policies to reduce racial residential segregation may help curb high levels of violence in urban America (Lizotte 1986; Massey 1995; McDowall et al. 1995).
Similarly, lung cancer and cirrhosis accounted for a larger share of the racial difference in male than female life expectancy with lung cancer being the most important. Between 1993 and 2007, lung cancer contributed to the narrowing of the racial gap in both male and female life expectancy. As noted previously, lung cancer mortality has been closely tied to smoking duration, intensity, and cohort smoking histories (Ho and Elo 2012; King et al. 2004; Siahpush et al. 2010). Deaths from motor vehicle accidents and suicide played a relatively small role in the racial gap in life expectancy. A number of factors have been implicated in fatalities from traffic accidents: including characteristics of the vehicles, driver behavior including drunk driving and seat belt use, quality of medical care and miles driven, and factors that are also likely to play a role in racial disparities in mortality from traffic accidents (Transportation Research Board 2010). The lower mortality from suicide among blacks than whites has raised questions about the possibility that the quality of reporting is worse among blacks than whites thus underestimating suicide mortality among blacks (Rockett et al. 2006).
During the study period, HIV emerged as a one of the leading causes of death in young adulthood and by 1993 it accounted for 12.7 and 7.5 % of the difference in male and female life expectancy between whites and blacks, respectively. After the introduction of HAART, HIV death rates declined quickly among both blacks and whites leading to a reduction in the absolute B–W difference in mortality from HIV (Levine et al. 2007; Rubin et al. 2010). Thus by 2007, the contribution of HIV to the racial gap in male and female life expectancy had declined, as had the number of excess deaths due to HIV. At the same time, however, the relative mortality disparities were now very large. Therefore, although the introduction of HAART led to a large reduction in death rates from HIV and thus a decline in the absolute racial difference in mortality from HIV, the relative racial mortality disparities have become exceptionally pronounced (Levine et al. 2007; Rubin et al. 2010).
It is also of interest to note that the residual/less amenable cause-of-death category made a substantial contribution to the narrowing of the B–W gap in life expectancy over time among both men and women. Within this category, the largest contribution was made by the heterogeneous group of causes, although mortality from cancers and heart disease also contributed. It may be that a secular decline from many of these causes occurred earlier among whites than blacks. In any case, given the heterogeneous nature of this category, multiple factors must have played a role.
Limitations
We should also note several limitations of our study. First, because we use the underlying cause of death, we may underestimate the contribution of some causes (e.g., diabetes) often listed as a contributing rather than an underlying cause on the death certificate. Second, causes of death classified as “ill-defined” contributed to a decline in the racial differences in life expectancy gap, leading to some potential bias due to improvements in the classification of causes of death among blacks over time. Third, due to inconsistencies in the coding of Hispanics and other race/ethnicities in these data sources, we limited our study to blacks and whites. Fourth, although differences in age-reporting accuracy between blacks and whites are a concern, it is less important at ages below 75 than at the highest ages. Fifth, we did not show age-specific death rates by cause of death, which could reveal different age patterns of mortality by race and sex.
Finally, it is important to note that there is no consensus regarding the list of conditions that are considered amenable to medical care and public health interventions, and the use of different definitions can lead to differing conclusions (Beltrán-Sánchez 2011; Wheller et al. 2007). For this reason, we included subcategories within the medically amenable and behavior/policy classifications and examined trends separately from IHD and HIV. We also included all other causes of death in a residual/less amenable cause-of-death category. All subcategories reveal considerable heterogeneity in mortality trends by subgroup and thus grouping all causes considered medically amenable, those falling into the behavior/policy category, or to the residual group of causes together would have missed important heterogeneity in cause-specific mortality trends over time.
Conclusions
Persistent B–W health disparities are a critical public health concern and an important consideration in the implementation of recent health reforms (Moy et al. 2011; Saenz 2010). Mortality differentials below age 75, when healthcare and policy/behavior interventions are likely to have their largest impact, continue to explain over 90 % of the difference in life expectancy between whites and blacks. The results presented here suggest that despite the narrowing of the racial gap in life expectancy since the mid-1990s, a substantial portion of racial mortality disparities continue to result from causes that could be reduced given more equitable access to healthcare and more targeted public health interventions.
These conclusions also underscore the complex interplay of medical care with socioeconomic status. The disparities that we identified, although considered to be amenable to existing interventions, are also undoubtedly related to differences in SES between blacks and whites. In the US, more highly educated and wealthy individuals tend to have lower mortality. In addition, access to healthcare is related to income, employment, and educational attainment in that individuals with higher incomes and educational attainment and full-time employment are more likely to have access to health insurance.
Acknowledgments
We thank anonymous reviewers for their comments and Ye Wang for programming assistance. Irma T. Elo and James Macinko were supported by a grant (R21 HD060175-01A1) from Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institute of Health. Hiram Beltran-Sanchez acknowledges financial support from the National Institute on Aging (T32AG000037), the Harvard Center for Population and Development Studies and the Center for Demography, Health and Aging at the University of Wisconsin-Madison.
Appendix
Table 4.
Life expectancy by race and sex, US 1980, 1993, and 2007
| Life expectancy at birth
|
White
|
Black
|
|||
|---|---|---|---|---|---|
| Year | Source | Male | Female | Male | Female |
| 1980 | Our estimate | 70.8 | 78.2 | 63.8 | 72.7 |
| Officiala | 70.8 | 78.2 | 64.1 | 72.9 | |
| 1993 | Our estimate | 73.2 | 79.6 | 64.7 | 74.0 |
| Officialb | 73.1 | 79.5 | 64.6 | 73.7 | |
| 2007 | Our estimate | 76.3 | 81.2 | 70.4 | 77.2 |
| Officialc | 75.9 | 80.8 | 70.0 | 76.8 | |
In the above table, we compare our estimates of life expectancies at birth to official U.S. life tables. The differences in the estimates in 2007 come mainly from differences in how mortality is estimated at the oldest ages, which has no appreciable impact on the conclusions drawn from the cause-specific results reported in this paper. Furthermore, the methodology used to estimate life expectancies in this paper is the same in all years
Vital Statistics of the United States, 1985. U.S. Decennial Life Tables for 1979–1981. Volume I, Number 1
Vital Statistics of the United States, 1993. Life Tables, Reprint of Volume II, Part A, Section 6 (pp. 8, 10)
National Vital Statistics Report, 2010. Deaths: Final Data for 2007. Volume 58, No. 19 (p. 12, Table 7)
Table 5.
Definitions of cause-of-death categories
| Category | ICD-9 | ICD-10 |
|---|---|---|
| Medical care avoidable mortality (AM) | ||
| AM—infectious and respiratory diseases | ||
| Intestinal infections, tuberculosis, Zoonotic bacterial diseases, other bacterial diseases, Septicemia, Poliomyelitis, Measles, Rubella, Infectious hepatitis, Ornithosis, Rickettsioses/arthropod-borne, Syphilis (all forms), Yaws, respiratory diseases, Influenza and Pneumonia, Chronic lower respiratory diseases | 001–009, 010–018, 32, 33, 37, 137, 020–027,38, 45, 55–56,70, 73, 080–082, 087, 090–099, 102, 460–479, 500–519, 480–488, 490–496 | A00–A09, A16–A19, B90, A20–A26, A28, A32, A33, A35, A36, A37, A40–A41, A80, B05–B06, B15–B19, A70, A68, A75, A77, A50–A64, A66, J00–J08, J20–J39, J60–J99, J09–J18, J40–J47 |
| AM—cancers | ||
| Malignant neoplasm of colon, skin, breast, cervix, prostate, testis, bladder, kidney—Wilm’s tumor only, eye, Thyroid carcinoma, Hodgkin’s disease, | 153–154, 172–173, 174, 180, 185, 186, 188–189, 190, 193, 201, 204–208 | C18–C21, C43–C44, C50, C53, C61, C62, C67, C64, C69, C73, C81, C91–C95 |
| Leukemia | ||
| AM—circulatory | ||
| Active/acute rheumatic fever, chronic rheumatic heart disease, hypertensive disease, cerebrovascular disease | 390–392, 393–398, 401–405, 430–438 | I00–I02, I05–I09, I10–I13, I15, I60–I69 |
| AM—birth | ||
| Maternal deaths (all), congenital cardiovascular anomalies, perinatal deaths (excluding stillbirths) | 630–676, 745–747, 760–779 | O00–O99, Q20–Q28, P00–P96 |
| AM—diabetes | 250 | E10–E14 |
| AM—Other | ||
| Disease of thyroid, Epilepsy, Peptic ulcer, Appendicitis, Abdominal hernia, Cholelithiasis & cholecystitis, Nephritis, Benign prostatic hyperplasia, Misadventures to patients during surgical or medical care | 240–246, 345, 531–533, 540–543, 550–553, 574–575.1, 580–589, 600, E870–E876, E878–E879 | E00–E07, G40–G41, K25–K27, K35–K38, K40–K46, K80–K81, N00–N07, N17–N19, N25–N27, N40, Y60–Y69, Y83–Y84 |
| IHD | 410–414, 429.2 | I20–I25 |
| HIV | 279.1a, 042–044 | B20–B24 |
| Suicide and self-inflicted injuries | E950–E959 | U03, X60–X84, Y87.0 |
| Policy AM | ||
| Lung cancer and cirrhosis | 162, 571.1–571.3 | C33–C34, K70 |
| Homicide | E960–E969 | X85–Y09 |
| Road traffic accidents | E810–E819 | V01–V99, |
| Residual causes | ||
| Other cancers | 140–239 | C00–D48 |
| Other heart disease | 390–459 if not listed above | I00–I99 if not listed above |
| Ill-defined causes | 780–799 | R00–R99 |
| All other non-amenable causes (residual) | All other codes not listed above | All other codes not listed above |
Classified as 279.1 before 1986
Footnotes
Tuberculosis, cervical cancer, rheumatic heart disease, hypertension, stroke, and appendicitis.
Maternal and perinatal mortality, Hodgkin’s disease, cervical and breast cancer, tuberculosis, asthma, appendicitis, cholelithiasis, cholecystitis and abdominal hernia, ischemic heart disease, hypertension and cerebrovascular disease, and peptic ulcers.
Tuberculosis, cervical cancer, Hodgkin’s disease, rheumatic heart disease, hypertensive disease, acute respiratory disease, pneumonia and bronchitis, influenza, asthma, appendicitis, hernias, and cholecystitis.
Contributor Information
Irma T. Elo, Email: popelo@pop.upenn.edu, Population Studies Center, University of Pennsylvania, 3718 Locust Walk, Philadelphia, PA 19104, USA.
Hiram Beltrán-Sánchez, Email: beltrans@ssc.wisc.edu, Center for Demography and Ecology, University of Wisconsin, 4329 Sewell Social Science, Madison, WI, USA.
James Macinko, Email: jmj5@nyu.edu, New York University, 411 Lafayette Street 5th Floor, New York, NY 10003, USA.
References
- Abreu DMX, Cesar CC, France EB. The relationship between deaths that are avoidable with adequate health care and the implementation of the unified health system in Brazil. Revista Panamericana de Salud Publica. 2007;21:282–291. doi: 10.1590/s1020-49892007000400003. [DOI] [PubMed] [Google Scholar]
- Adler GS. Measuring the quality of medical care. New England Journal of Medicine. 1978;298:574. doi: 10.1056/NEJM197803092981020. [DOI] [PubMed] [Google Scholar]
- Arriaga E. Measuring and explaining the change in life expectancies. Demography. 1984;21:83–96. [PubMed] [Google Scholar]
- Ashton CM, Haidet P, Paterniti DA, Collins TC, Gordon HS, O’Malley K, et al. Racial and ethnic disparities in the use of health services: Bias, preferences, or poor communication? Journal of General Internal Medicine. 2003;18:146–152. doi: 10.1046/j.1525-1497.2003.20532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CJ, Kodack M. Patient adherence to medication requirements for therapy of type 2 diabetes. International Journal of Clinical Practice. 2011;65:314–322. doi: 10.1111/j.1742-1241.2010.02544.x. [DOI] [PubMed] [Google Scholar]
- Beltrán-Sánchez H. Avoidable mortality: A review. In: Rogers RR, Crimmins EM, editors. International handbook of adult mortality. Dordrecht: Springer; 2011. pp. 491–508. [Google Scholar]
- Beltrán-Sánchez H, Preston SH, Canudas-Romo V. An integrated approach to cause-of-death analysis: Cause-deleted life tables and decompositions of life expectancy. Demographic Research. 2008;19:1323–1350. doi: 10.4054/DemRes.2008.19.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt SI, Carter HB, Schoenberg MP, Newschaffer CJ. Disparities in treatment and outcome for renal cell cancer among older black and white patients. Journal of Clinical Oncology. 2007;25:3589–3595. doi: 10.1200/JCO.2006.10.0156. [DOI] [PubMed] [Google Scholar]
- Berz JP, Johnston K, Backus B, Doros G, Rose AJ, Pierre S, et al. The influence of black race on treatment and mortality for early-stage breast cancer. Medical Care. 2009;47:986–992. doi: 10.1097/MLR.0b013e31819e1f2b. [DOI] [PubMed] [Google Scholar]
- Burgess AM, Jr, Colton T, Peterson OL. Avoidable mortality. Some practical aims for regional medical programs. Archives of Environmental Health. 1966;13:794–798. doi: 10.1080/00039896.1966.10664666. [DOI] [PubMed] [Google Scholar]
- Charlton JR, Hartley RM, Silver R, Holland WW. Geographical variation in mortality from conditions amenable to medical intervention in England and Wales. Lancet. 1983;1:691–696. doi: 10.1016/s0140-6736(83)91981-5. [DOI] [PubMed] [Google Scholar]
- Commonwealth Fund. Aiming higher: Results from a state scorecard on health system performance. Washington, DC: Commonwealth Fund; 2007. http://www.commonwealthfund.org/publications/publications_show.htm?doc_id=494551. [Google Scholar]
- Desai M, Nolte E, Karanikolos M, Khoshaba B, McKee M. Measuring NHS performance 1990-2009 using amenable mortality: Interpret with care. Journal of the Royal Society of Medicine. 2011;104:370–379. doi: 10.1258/jrsm.2011.110120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitle D, D’Alessio SJ, Stolzenberg L. Economic segregation, race, and homicide. Social science quarterly. 2006;87:638–657. [Google Scholar]
- Elo IT, Drevenstedt GL. Yearbook of population research in Finland. Helsinki: The Population Research Institute; 2002. Educational differences in cause-specific mortality in the United States; pp. 37–54. [Google Scholar]
- Elo IT, Drevenstedt GL. Cause-specific contributions to black-white differences in adult male mortality between 1960 and 1995. Demographic Research-Special Collection Determinants of Diverging Trends in Mortality. 2004;2:255–276. [Google Scholar]
- Elo IT, Drevenstedt GL. Black-white differentials in cause-specific mortality in the United States in the 1980s: The role of medical care and health behaviors. 2006 PSC Working Paper Series PSC 06-02. [Google Scholar]
- Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, et al. Explaining the decrease in us deaths from coronary disease, 1980–2000. New England Journal of Medicine. 2007;356:2388–2398. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- Ford ES, Capewell S. Proportion of the decline in cardiovascular mortality disease due to prevention versus treatment: Public health versus clinical care. Annual Review of Public Health. 2011;32:5–22. doi: 10.1146/annurev-publhealth-031210-101211. [DOI] [PubMed] [Google Scholar]
- Garrett B, Yemane A. Racial and ethnic differences in insurance coverage and health care access and use: A synthesis of findings from the assessing the new federalism project. Washington, DC: The Urban Institute; 2006. [Google Scholar]
- Gay JG, Paris V, Devaux M, de Looper M. Mortality amenable to health care in 31 OECD countries estimates and methodological issues. Paris: OECD Publishing; 2011. OECD Health Working Papers. [Google Scholar]
- Geronimus AT, Bound J, Colen CG. Excess black mortality in the United States and in selected black and white high-poverty areas, 1980–2000. American Journal of Public Health. 2011;101:720–729. doi: 10.2105/AJPH.2010.195537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus AT, Bound J, Waidmann TA. Poverty, time, and place: Variation in excess mortality across selected U.S. populations, 1980-1990. Journal of Epidemiology and Community Health. 1999;53:325–334. doi: 10.1136/jech.53.6.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus AT, Bound J, Waidmann TA, Hillemeier MM, Burns PB. Excess mortality among blacks and whites in the United States. New England Journal of Medicine. 1996;335:1552–1558. doi: 10.1056/NEJM199611213352102. [DOI] [PubMed] [Google Scholar]
- Geruso M. Black-white disparities in life expectancy: How much can the standard SES variables explain. Demography. 2012;49:553–574. doi: 10.1007/s13524-011-0089-1. [DOI] [PubMed] [Google Scholar]
- Harper S, Lynch J, Burris S, Smith GD. Trends in the black-white life expectancy gap in the United States, 1983–2003. Journal of the American Medical Association. 2007;297:1224–1232. doi: 10.1001/jama.297.11.1224. [DOI] [PubMed] [Google Scholar]
- Hayward MD, Crimmins EM, Miles TP, Ang Y. The significance of socioeconomic status in explaining the racial gap in chronic health conditions. American Sociological Review. 2000;65:910–930. [Google Scholar]
- Heijink R, Koolman X, Westert GP. Spending more money, saving more lives? The relationship between avoidable mortality and healthcare spending in 14 countries. European Journal of Health Economics. 2012;14(3):527–538. doi: 10.1007/s10198-012-0398-3. [DOI] [PubMed] [Google Scholar]
- Ho JY, Elo IT. The contribution of smoking to black-white differences in mortality. Demography. 2012;50(2):545–568. doi: 10.1007/s13524-012-0159-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland WW, editor. The European community atlas of “avoidable death”. Oxford: Oxford University Press; 1988. [Google Scholar]
- Holland WW, editor. European community atlas of ‘avoidable death’. Oxford: Oxford University Press; 1991. [Google Scholar]
- Holland WW, editor. European community atlas of ‘avoidable death’. Oxford: Oxford University Press; 1993. [Google Scholar]
- Humblet PC, Lagasse R, Leveque A. Trends in Belgian premature avoidable deaths over a 20 year period. Journal of Epidemiology and Community Health. 2000;54:687–691. doi: 10.1136/jech.54.9.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James PD, Manuel DG, Mao Y. Avoidable mortality across Canada from 1975 to 1999. BMC Public Health. 2006;6:137. doi: 10.1186/1471-2458-6-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G, Polednak A, Bendel RB, Vilsaint MC, Nahata SB. Disparities in smoking cessation between African Americans and whites: 1990–2000. American Journal of Public Health. 2004;94:1965–1971. doi: 10.2105/ajph.94.11.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjellstrand CM, Kovithavongs C, Szabo E. On the success, cost and efficiency of modern medicine: An international comparison. Journal of Internal Medicine. 1998;243:3–14. doi: 10.1046/j.1365-2796.1998.00248.x. [DOI] [PubMed] [Google Scholar]
- Kochanek KD, Maurer JD, Rosenberg HM. Why did black life expectancy decline from 1984 through 1989 in the United States? American Journal of Public Health. 1994;84:938–944. doi: 10.2105/ajph.84.6.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korda RJ, Butler JR. Effect of healthcare on mortality: Trends in avoidable mortality in Australia and comparisons with Western Europe. Public Health. 2006;120:95–105. doi: 10.1016/j.puhe.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Korda RJ, Butler JR, Clements MS, Kunitz SJ. Differential impacts of health care in Australia: Trend analysis of socioeconomic inequalities in avoidable mortality. International Journal of Epidemiology. 2007;36:157–165. doi: 10.1093/ije/dyl282. [DOI] [PubMed] [Google Scholar]
- Krivo LJ, Peterson RD. The structural context of homicide: Accounting for racial differences in process. American Sociological Review. 2000;65:547–559. [Google Scholar]
- Kunitz SJ, Pesis-Katz I. Mortality of white Americans, African Americans, and Canadians: The causes and consequences for health of welfare state institutions and policies. Milbank Quarterly. 2005;83:5–39. doi: 10.1111/j.0887-378X.2005.00334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVeist TA, Thorpe RJ, Galarraga JE, Bower KM, Gary-Webb TL. Environmental and socio-economic factors as contributors to racial disparities in diabetes prevalence. Journal of General Internal Medicine. 2009;24:1144–1148. doi: 10.1007/s11606-009-1085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RS, Briggs NC, Kilbourne BS, King WD, Fry-Johnson Y, Baltrus PT, et al. Black white mortality from HIV in the United States before and after introduction of highly active antiretroviral therapy in 1996. American Journal of Public Health. 2007;97:1884–1892. doi: 10.2105/AJPH.2005.081489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DE, Byfield SD, Comstock CB, Garber JE, Syngal S, Crown WH, et al. Underutilization of BRCA1/2 testing to guide breast cancer treatment: Black and hispanic women particularly at risk. Genetics in Medicine. 2011;13:349–355. doi: 10.1097/GIM.0b013e3182091ba4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie-Blanton M, Hoffman C. The role of health insurance coverage in reducing racial/ethnic disparities in health care. Health Affairs. 2005;24:398–408. doi: 10.1377/hlthaff.24.2.398. [DOI] [PubMed] [Google Scholar]
- Lizotte AJ. The costs of using gun control to reduce homicide. Bulletin of the New York Academy of Medicine. 1986;62:539–549. [PMC free article] [PubMed] [Google Scholar]
- Macinko J, Elo IT. Black–white differences in avoidable mortality in the USA, 1980–2005. Journal of Epidemiology and Community Health. 2009;63:715–721. doi: 10.1136/jech.2008.081141. [DOI] [PubMed] [Google Scholar]
- Mackenbach JP. The contribution of medical care to mortality decline: Mckeown revisited. Journal of Clinical Epidemiology. 1996;49:1207–1213. doi: 10.1016/s0895-4356(96)00200-4. [DOI] [PubMed] [Google Scholar]
- Mackenbach JP, Looman CW, Kunst AE, Habbema JD, van der Maas PJ. Post-1950 mortality trends and medical care: Gains in life expectancy due to declines in mortality from conditions amenable to medical intervention in the Netherlands. Social Science and Medicine. 1988;27:889–894. doi: 10.1016/0277-9536(88)90278-x. [DOI] [PubMed] [Google Scholar]
- Mackenbach JP, Bouvier-Colle MH, Jougla E. “Avoidable” mortality and health services: A review of aggregate data studies. Journal of Epidemiology and Community Health. 1990;44:106–111. doi: 10.1136/jech.44.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manton KG, Patrick CH, Johnson KW. Health differentials between blacks and whites: Recent trends in mortality and morbidity. Milbank Quarterly. 1987;65:129–199. [PubMed] [Google Scholar]
- Manuel DG, Mao Y. Avoidable mortality in the United States and Canada, 1980–01996. American Journal of Public Health. 2002;92:1481–1484. doi: 10.2105/ajph.92.9.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall SW, Kawachi I, Pearce N, Borman B. Social class differences in mortality from diseases amenable to medical intervention in New Zealand. International Journal of Epidemiology. 1993;22:255–261. doi: 10.1093/ije/22.2.255. [DOI] [PubMed] [Google Scholar]
- Massey DS. Getting away with murder: Segregation and violent crime in urban America. University of Pennsylvania Law Review. 1995;143:1203–1232. [Google Scholar]
- Mayberry RM, Mili F, Ofili E. Racial and ethnic differences in access to medical care. Medical Care Research Review. 2000;57:108–145. doi: 10.1177/1077558700057001S06. [DOI] [PubMed] [Google Scholar]
- McDowall D, Loftin C, Wiersema B. Easing concealed firearms laws: Effects on homicide in three states. The Journal of Criminal Law and Criminology. 1995;86:193–206. [Google Scholar]
- Moy B, Polite BN, Halpern MT, Stranne SK, Winer EP, Wollins DS, et al. American society of clinical oncology policy statement: Opportunities in the patient protection and affordable care act to reduce cancer care disparities. Journal of Clinical Oncology. 2011;29:3816–3824. doi: 10.1200/JCO.2011.35.8903. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute. U.S. population data 1969–2009. 2011 Retrieved January 2012, from http://seer.cancer.gov/popdata/download.html.
- Niti M, Ng TP. Temporal trends and ethnic variations in amenable mortality in Singapore 1965-1994: The impact of health care in transition. International Journal of Epidemiology. 2001;30:966–973. doi: 10.1093/ije/30.5.966. [DOI] [PubMed] [Google Scholar]
- Nolte E, McKee M. Measuring the health of nations: Analysis of mortality amenable to health care. British Medical Journal. 2003;327:1129. doi: 10.1136/bmj.327.7424.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte E, McKee M. Does healthcare save lives? Avoidable mortality revisited. London: The Nuffield Trust; 2004. [Google Scholar]
- Nolte E, McKee CM. Measuring the health of nations: Updating an earlier analysis. Health Affairs. 2008;27:58–71. doi: 10.1377/hlthaff.27.1.58. [DOI] [PubMed] [Google Scholar]
- Nolte E, McKee M. Variations in amenable mortality—Trends in 16 high-income nations. Health Policy. 2011;103:47–52. doi: 10.1016/j.healthpol.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Nolte E, McKee CM. In amenable mortality—Deaths avoidable through health care—Progress in the US lags that of three European countries. Health Affairs. 2012;31:2114–2122. doi: 10.1377/hlthaff.2011.0851. [DOI] [PubMed] [Google Scholar]
- Pampalon R. Avoidable mortality in Quebec and its regions. Social Science and Medicine. 1993;37:823–831. doi: 10.1016/0277-9536(93)90376-f. [DOI] [PubMed] [Google Scholar]
- Paradies Y. A systematic review of empirical research on self-reported racism and health. International Journal of Epidemiology. 2006;35:888–901. doi: 10.1093/ije/dyl056. [DOI] [PubMed] [Google Scholar]
- Petersen LA, Wright SM, Peterson ED, Daley J. Impact of race on cardiac care and outcomes in veterans with acute myocardial infarction. Medical Care. 2002;40:86–96. doi: 10.1097/00005650-200201001-00010. [DOI] [PubMed] [Google Scholar]
- Peterson ED, Shaw LK, DeLong ER, Pryor DB, Califf RM, Mark DB. Racial variation in the use of coronary-revascularization procedures—Are the differences real? Do they matter? New England Journal of Medicine. 1997;336:480–486. doi: 10.1056/NEJM199702133360706. [DOI] [PubMed] [Google Scholar]
- Phelan JC, Link BG, Diez-Roux A, Kawachi I, Levin B. “Fundamental causes” of social inequalities in mortality: A test of the theory. Journal of Health and Social Behavior. 2004;45:265–285. doi: 10.1177/002214650404500303. [DOI] [PubMed] [Google Scholar]
- Piers LS, Carson NJ, Brown K, Ansari Z. Avoidable mortality in Victoria between 1979 and 2001. Australian and New Zealand Journal of Public Health. 2007;31:5–12. doi: 10.1111/j.1753-6405.2007.00002.x. [DOI] [PubMed] [Google Scholar]
- Plug I, Hoffmann R, Artnik B, Bopp M, Borrell C, Costa G, et al. Socioeconomic inequalities in mortality from conditions amenable to medical interventions: Do they reflect inequalities in access or quality of health care? BMC Public Health. 2012;12:346. doi: 10.1186/1471-2458-12-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poikolainen K, Eskola J. Mortality from causes amenable to health services intervention. Lancet. 1986;1:1386–1387. doi: 10.1016/s0140-6736(86)91697-1. [DOI] [PubMed] [Google Scholar]
- Pollard JH. The expectation of life and its relationship to mortality. Journal of Institute of Actuaries. 1982;109:225–240. [Google Scholar]
- Preston SH, Elo IT, Rosenwaike I, Hill M. African American mortality at older ages: Results of a matching study. Demography. 1996;33:193–209. [PubMed] [Google Scholar]
- Preston SH, Heuveline P, Guillot M. Demography. Measuring and modeling population processes. Malden, MA: Blackwell; 2001. [Google Scholar]
- Preston SH, Wang H. Sex mortality differences in the United States: The role of cohort smoking patterns. Demography. 2006;43:631–646. doi: 10.1353/dem.2006.0037. [DOI] [PubMed] [Google Scholar]
- Rockett IRH, Samora JB, Coben JH. The black–white suicide paradox: Possible effects of misclassification. Social Science and Medicine. 2006;63:2165–2175. doi: 10.1016/j.socscimed.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Rogers RG. Living and dying in the U.S.A: Sociodemographic determinants of death among blacks and whites. Demography. 1992;29:287–303. [PubMed] [Google Scholar]
- Rogers RG, Hummer RA, Nam CB. Living and dying in the U S A: Behavioral, health, and social differentials of adult mortality. New York: Academic Press; 2000. [Google Scholar]
- Ronzio CR, Pamuk E, Squires GD. The politics of preventable deaths: Local spending, income inequality, and premature mortality in US cities. Journal of Epidemiology and Community Health. 2004;58:175–179. doi: 10.1136/jech.2003.008672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg HM. Cause of death as a contemporary problem. Journal of the History of Medicine. 1999;54:133–153. doi: 10.1093/jhmas/54.2.133. [DOI] [PubMed] [Google Scholar]
- Rubin MS, Colen CG, Link BG. Examination of inequalities in HIV/AIDS mortality in the United States from a fundamental cause perspective. American Journal of Public Health. 2010;100:1053–1059. doi: 10.2105/AJPH.2009.170241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutstein DD, Berenberg W, Chalmers TC, Child CG, 3rd, Fishman AP, Perrin EB. Measuring the quality of medical care. A clinical method. New England Journal of Medicine. 1976;294:582–588. doi: 10.1056/NEJM197603112941104. [DOI] [PubMed] [Google Scholar]
- Rutstein DD, Berenberg W, Chalmers TC, Fishman AP, Perrin EB, Zuidema GD. Measuring the quality of medical care: Second revision of tables of indexes. New England Journal of Medicine. 1980;302:1146. doi: 10.1056/nejm198005153022012. [DOI] [PubMed] [Google Scholar]
- Saenz M. Health disparities and the affordable care act. NCSL Legisbrief. 2010;18:1–2. [PubMed] [Google Scholar]
- Salihu HM, Stanley KM, Mbah AK, August EM, Alio AP, Marty PJ. Disparities in rates and trends of HIV/AIDS during pregnancy across the decade, 1998–2007. Journal of Acquired Immune Deficiency Syndromes. 2010;55:391–396. doi: 10.1097/QAI.0b013e3181f0cccf. [DOI] [PubMed] [Google Scholar]
- Sanders-Phillips K, Settles-Reaves B, Walker D, Brownlow J. Social inequality and racial discrimination: Risk factors for health disparities in children of color. Pediatrics. 2009;124(Suppl 3):S176–S186. doi: 10.1542/peds.2009-1100E. [DOI] [PubMed] [Google Scholar]
- Schoenbaum SC, Schoen C, Nicholson JL, Cantor JC. Mortality amenable to health care in the United States: The roles of demographics and health systems performance. Journal of Public Health Policy. 2011;32:407–429. doi: 10.1057/jphp.2011.42. [DOI] [PubMed] [Google Scholar]
- Schwartz E, Kofie VY, Rivo M, Tuckson RV. Black/white comparisons of deaths preventable by medical intervention: United States and the District of Columbia 1980–1986. International Journal of Epidemiology. 1990;19:591–598. doi: 10.1093/ije/19.3.591. [DOI] [PubMed] [Google Scholar]
- SEER. SEER*Stat Database: Incidence—SEER 17 REGS Research Data + Hurricane Katrina impacted Louisiana cases, Nov 2010 sub (1973–2008 varying)—Linked to county attributes—Total U S, 1969–2009 counties. National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch: National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch; 2011. Released April 2011 (updated 2010/2028/2011), based on the November 2010 submission) [Google Scholar]
- Siahpush M, Singh G, Jones P, Timsina L. Racial/ethnic and socioeconomic variations in duration of smoking: Results from 2003, 2006 and 2007 tobacco use supplement of the current population survey. Journal of Public Health. 2010;32:210–218. doi: 10.1093/pubmed/fdp104. [DOI] [PubMed] [Google Scholar]
- Simonato L, Ballard T, Bellini P, Winkelmann R. Avoidable mortality in Europe 1955–1994: A plea for prevention. Journal of Epidemiology and Community Health. 1998;52:624–630. doi: 10.1136/jech.52.10.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smedley BD, Stith AY, Nelson AR. Unequal treatment: Confronting racial and ethnic disparities in health care. Washington, DC: National Academy Press; 2003. [PubMed] [Google Scholar]
- Soneji S, Iyer SS, Armstrong KBA, Asch DA. Racial disparities in stage-specific colorectal cancer mortality: 1960–2005. American Journal of Public Health. 2010;100:1912–1916. doi: 10.2105/AJPH.2009.184192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonel AF, Good CB, Mulgund J, Roe MT, Gibler WB, Smith SC, Jr, et al. Racial variations in treatment and outcomes of black and white patients with high-risk non-st-elevation acute coronary syndromes: Insights from crusade (can rapid risk stratification of unstable angina patients suppress adverse outcomes with early implementation of the ACC/AHA guidelines?) Circulation. 2005;111:1225–1232. doi: 10.1161/01.CIR.0000157732.03358.64. [DOI] [PubMed] [Google Scholar]
- Song YM, Byeon JJ. Excess mortality from avoidable and non-avoidable causes in men of low socioeconomic status: A prospective study in Korea. Journal of Epidemiology and Community Health. 2000;54:166–172. doi: 10.1136/jech.54.3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirbu I, Kunst AE, Bos V, Mackenbach JP. Differences in avoidable mortality between migrants and the native Dutch in the Netherlands. BMC Public Health. 2006;6:78. doi: 10.1186/1471-2458-6-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudano JJ, Baker DW. Explaining us racial/ethnic disparities in health declines and mortality in late middle age: The roles of socioeconomic status, health behaviors, and health insurance. Social Science and Medicine. 2006;62:909–922. doi: 10.1016/j.socscimed.2005.06.041. [DOI] [PubMed] [Google Scholar]
- Tehranifar P, Neugut AI, Link BG, Liao Y, Terry MB, Phelan JC, et al. Medical advances and racial/ethnic disparities in cancer survival. Cancer Epidemiology, Biomarkers and Prevention. 2009;18:2701–2708. doi: 10.1158/1055-9965.EPI-09-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias M, Jackson G. Avoidable mortality in New Zealand, 1981–97. Australia and New Zealand Journal of Public Health. 2001;25:12–20. doi: 10.1111/j.1467-842x.2001.tb00543.x. [DOI] [PubMed] [Google Scholar]
- Transportation Research Board. Achieving traffic safety goals in the United States: Lessons from other nations. Washington, DC: Transportation Research Board; 2010. [Google Scholar]
- van Ryn M, Burgess D, Malat J, Griffin J. Physicians’ perceptions of patients’ social and behavioral characteristics and race disparities in treatment recommendations for men with coronary artery disease. American Journal of Public Health. 2006;96:351–357. doi: 10.2105/AJPH.2004.041806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virnig BA, Baxter NN, Habermann EB, Feldman RD, Bradley CJ. A matter of race: Early-versus late-stage cancer diagnosis. Health Affairs. 2009;28:160–168. doi: 10.1377/hlthaff.28.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerling R. “Avoidable” causes of death in Sweden 1974–85. Quality Assurance in Health Care. 1992;4:319–328. doi: 10.1093/oxfordjournals.intqhc.a036732. [DOI] [PubMed] [Google Scholar]
- Westerling R, Rosén M. ‘Avoidable’ mortality among immigrants in Sweden. European Journal of Public Health. 2002;12:279–286. doi: 10.1093/eurpub/12.4.279. [DOI] [PubMed] [Google Scholar]
- Wheller L, Baker A, Griffiths C, Rooney C. Tends in avoidable mortality in England and Wales, 1993-2005. Health Statistics Quarterly. 2007:6–25. [PubMed] [Google Scholar]
- Williams DR, Mohammed SA. Discrimination and racial disparities in health: Evidence and needed research. Journal of Behavioral Medicine. 2009;32:20–47. doi: 10.1007/s10865-008-9185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, Sternthal M. Understanding racial-ethnic disparities in health: Sociological contributions. Journal of Health and Social Behavior. 2010;51(Suppl):S15–S27. doi: 10.1177/0022146510383838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolhandler S, Himmelstein D, Silber R, Bader M, Harnly M, Jones A. Medical care and mortality: Racial differences in preventable deaths. International Journal of Health Services. 1985;15:1–11. doi: 10.2190/90P3-LEFF-WNU0-GLY6. [DOI] [PubMed] [Google Scholar]
- Xu JQ, Kochanek KD, Murphy SL, Tejada-Vera B. Deaths: Final data for 2007. National vital statistics reports. Hyattsville, MD: National Center for Health Statistics; 2010. [PubMed] [Google Scholar]


