Abstract
Short sleep/dark durations, due to late bedtimes or early wake times or both, are common in modern society. We have previously shown that a series of days with a late bedtime phase delays the human dim light melatonin rhythm, as compared to a series of days with an early bedtime, despite a fixed wake time. Here we compared the effect of an early versus late wake time with a fixed bedtime on the human dim light melatonin rhythm. Fourteen healthy subjects experienced 2 weeks of short 6 h nights with an early wake time fixed at their habitual weekday wake time and 2 weeks of long 9 h nights with a wake time that occurred 3 h later than the early wake time, in counterbalanced order. We found that after 2 weeks with the late wake time, the dim light melatonin onset delayed by 2.4 h and the dim light melatonin offset delayed by 2.6 h (both p < 0.001), as compared to after 2 weeks with the early wake time. These results highlight the substantial influence that wake time, likely via the associated morning light exposure, has on the timing of the human circadian clock. Furthermore, the results suggest that when people truncate their sleep by waking early their circadian clocks phase advance and when people wake late their circadian clocks phase delay.
Keywords: Circadian, Dark, Light, Melatonin, Night, Sleep deprivation
People in modern society are chronically sleep deprived. In a recent National Sleep Foundation poll of over 1000 Americans, 31% reported regularly sleeping 6 h or less per weeknight, and 17% reported doing so on the weekend [21]. In contrast, experimental subjects (with no work and social commitments), when free to choose their bed and wake times, sleep for approximately 9 h per night (e.g. [8,29]). The shorter sleep durations in modern society may be due to longer working hours [21].
Melatonin is synthesized and released from the pineal gland [19], but its secretion is suppressed by light [15]. The circadian rhythm of melatonin in dim light is a reliable marker of the phase of the circadian clock, and in animals the duration of the dim light melatonin profile (onset to offset of secretion) signals photoperiodic and thus seasonal information to the neuroendocrine-gonadal axis. Previous work has shown that the duration and phase of the human dim light melatonin profile can be influenced by prior sleep/dark times. In one study, subjects experienced 4 weeks with a very early bedtime (dark 18:00–8:00) and then 1 week with a normal bedtime (dark 24:00–8:00) [28]. The earlier bedtime, and thus longer night length, led to a 1.6 h increase in the duration of the dim light melatonin profile, mainly due to an advance in the onset of melatonin secretion. In a similar but shorter study, nine long nights with an even earlier bedtime (dark 16:00–8:00) also advanced the melatonin rhythm by 2.4 h, but the duration of melatonin secretion did not change [23].
We recently investigated the effects of realistically short nights (6 h) and long nights (9 h) on the dim light melatonin rhythm in normal length sleepers [2]. We found that 7–19 days with a late bedtime (01:00) resulted in ~0.5 h delay in the dim light melatonin rhythm (both onset and offset) versus 7–19 days with an early bedtime (22:00). In both conditions wake time was fixed (07:00). This result suggested that the human circadian system is sensitive to changes in bedtime, most likely because of the associated exposure to indoor evening light. However, we did not find any significant changes in the duration of the melatonin profile in response to these realistic changes in night length, as both the onset and offset phase delayed by about the same amount. Interestingly, the duration of the dim light melatonin profile in habitual short sleepers (<6 h/night) is significantly shorter than the duration in habitual long sleepers (>9 h/night) [1]. However, it remains unclear if this difference is an intrinsic characteristic of these groups or if the sleep/dark times they habitually adopt have produced the difference in their melatonin profiles.
Many people truncate their sleep by delaying their bedtime, but maintain a set wake time, because of daytime work commitments. However on recovery from a series of short nights many people “sleep in” by delaying their wake time. To date all of the studies that have investigated the effect of longer night lengths in humans who usually have normal sleep lengths (as reviewed above), have extended the sleep/dark episode into the evening by assigning earlier bedtimes. Here, for the first time, we investigated the effects of short versus long nights on the phase and duration of the human dim light melatonin profile by having subjects maintain a fixed bedtime and adhere to both early and late wake times. We also asked subjects to record their subjective symptoms during both conditions.
Fourteen healthy young adults (10 males, 4 females, mean age ± S.D. = 28.8 ± 5.2 years; BMI 23.1 ± 3.8 kg/m2) participated. They were nonsmokers, medication free, consumed only moderate caffeine doses (<300 mg/day) and reported no medical, psychiatric or sleep disorders. A urine drug screen confirmed that all subjects were free of common drugs of abuse. The menstrual phase of the female subjects during the study was not controlled, as menstrual phase does not affect the melatonin rhythm (e.g. [22]). No female subject was taking oral contraceptives. No subject had worked night shifts or traveled across more than two time zones in the previous month. No subject was color blind as determined from the Ishihara test. Morningness–eveningness was assessed [13] and there were five moderate morning, six neither, and three moderate evening types. The self-reported mean (±S.D.) habitual weekday sleep schedule of the subjects was 0:30 ± 1.2 h to 7:57 ± 1.0 h. The protocol was conducted in accordance with the Declaration of Helsinki and was approved by the Rush University Medical Center Institutional Review Board. All subjects participated with a thorough understanding of the study and gave written informed consent prior to their participation.
As part of a within subjects counterbalanced design, subjects participated in 2 weeks of short 6 h nights with an early wake time (waking at their habitual weekday wake time and going to bed 6 h earlier), and in 2 weeks of long 9 h nights with a late wake time (going to bed at the same time as in the short nights but waking 3 h later). Seven subjects completed the early wake time first and seven subjects completed the late wake time first. After each series of nights, we measured each subject’s dim light melatonin rhythm. In between each series of short and long nights, subjects experienced a 2–3 day phase shifting treatment and then another phase assessment (results reported in [3,4]). Subjects then had a 6–7 day washout period where they returned to their habitual sleep times before the next series of short or long nights. In order to avoid excessive sleep deprivation during the days with the early wake time (short nights), napping was permitted during a 3 h interval, centered 12 h from the midpoint of the short nights. Sleep/dark at this time should not alter circadian rhythms as neither afternoon sleep/dark episodes [7] nor afternoon bright light episodes [11] phase shift circadian rhythms.
All subjects slept at home and none had bed partners. During the study sunrise varied between 04:18 and 07:11 and sunset between 16:21 and 19:18 (central standard time). We covered each subject’s bedroom windows with black plastic to ensure light levels were <1 lx. During their scheduled sleep/dark times at home, subjects were instructed to lie in bed in the dark and try to sleep. They were not permitted to read, watch TV, listen to music or talk on the telephone at this time. To ensure compliance, subjects wore an actigraphy monitor (Actiwatch-L, Mini-Mitter, Bend, Oregon, USA) on their nondominant wrist during the entire study which recorded their activity every minute. Subjects called the lab voice mail (time and date of call was recorded) before turning out their lights at night and just after they turned on their lights each morning. They also completed a sleep log every day, noting lights off time before sleep, estimated sleep onset, any awakenings during the night (>5 min), final awakening, and lights on time in the morning.
To monitor light exposure, all subjects wore a photosensor around their neck as a medallion (Actiwatch-L, Mini-Mitter, Bend, Oregon, USA), except when they were in bed, or during showers and baths. The medallion recorded light intensity (lx) every minute. During sleep periods the light medallion was placed on their nightstand and during showers and baths it was placed on a bathroom surface. The subjects were required to receive a minimum of 10 min of outdoor light in the first 1.5 h after their scheduled wake time every morning, in order to mimic the light exposure that many people receive every day. Subjects were asked not to wear sunglasses during the morning light requirement (and were offered clear sunglasses that filtered out UV light to wear at this time), but could wear sunglasses at any other time if they so wished. Two subjects wore their own sunglasses during the study and noted their use on light logs. We measured the transmission of their individual sunglasses and corrected the light data for each minute that the sunglasses were worn.
Subjective symptoms were monitored by the “How Are You Feeling Right Now?” questionnaire, which subjects completed in the 15 min before bedtime and 15 min after scheduled wake time every day. This questionnaire consists of the Stanford Sleepiness Scale [12], where one of seven descriptors is selected to best describe the subjective level of sleepiness. There are six additional questions relating to physical fatigue, mental fatigue, sadness, anxiety, irritability and gastrointestinal problems. Subjects scored each of these six items by circling a number from 1 (very little) to 10 (very much). Subjects also completed the Columbia Scale questionnaire [26] in the 15 min before bedtime. This questionnaire assesses sleepiness, fatigue, daytime alertness, concentration, lethargy, light-headedness, weakness, clumsiness and memory across the whole day and yields a score with possible range of 0–36. Subjects also completed a daily event log which asked them to note the amount and time of day of caffeine and alcohol consumption.
To ensure compliance to the study protocol, subjects came to the lab every 1–3 days so that the data from the wrist actigraph, photosensor, and sleep logs could be examined in their presence. Subjects could consume up to 300 mg of caffeine in the first 3 h after their scheduled wake time but could not consume caffeine at any other time. Two standard drinks of alcohol per day were allowed except on the last 2 days of each condition. Subjects chose their own meal times. Non-steriodal anti-inflammatory drugs were not permitted, as they suppress melatonin [20].
On the final day in each condition, each subject participated in a 20–22 h long phase assessment which began between 16:00 and 18:00. During the phase assessments, subjects remained awake and seated in dim light (<5 lx, at the level of the subjects’ eyes, in the direction of gaze, Minolta TL-1 light meter, Ramsey, NJ). Subjects gave a saliva sample every 30 min using Salivettes (Sarstedt, Newton, North Carolina, USA). The samples were later radioimmunoassayed for melatonin by Pharmasan Labs (Osceola, Wisconsin, USA). The sensitivity of the assay was 0.7 pg/ml, and intra- and interassay coefficients of variance were 12.1 and 13.2% respectively (based on standards 0.6, 3.5, 8, 14 and 105 pg/ml).
Two phase markers were derived from each melatonin profile, the dim light melatonin onset (DLMO) and dim light melatonin offset (DLMOff). For each subject’s melatonin profile, a threshold was calculated as the mean of three consecutive low daytime values plus twice the standard deviation [27]. Each subject’s DLMO was the point in time (as determined with linear interpolation) when the melatonin concentration exceeded the threshold. The DLMOff was the point in time when melatonin levels fell below the threshold. The mean (±S.D.) threshold was 1.6 ± 0.7 pg/ml. We also calculated area under the curve of each melatonin profile [25].
Fig. 1 and Table 1 show a phase delay in the melatonin rhythm following the late wake time, long nights, as compared to after the early wake time, short nights. A 2 × 2 MANOVA with within subjects factor wake time/night length and between subjects factor order of condition on the DLMO, DLMOff, DLMO-DLMOff duration and area under the curve was significant (p < 0.001). Subsequent univariate ANOVAs revealed a significant main effect of wake time/night length for the DLMO and DLMOff, but there were no significant main effects of order of condition nor any significant interactions (see Table 1). On average the DLMO occurred 2.4 ± 0.7 h later after the late wake time days compared to after the early wake time days (the delay in individuals ranged from 1.5 to 4.2 h). On average the DLMOff occurred 2.6 ± 1.0 h later after the late wake time compared to the early wake time days (delay ranged from 1.3 to 5.4 h). Although not significant for the entire group, four subjects had at least a 0.5 h increase in their DLMO-DLMOff duration during the long nights. There was no significant correlation between morningness–eveningness and the shift in the DLMO and DLMOff (both Pearson correlations p > 0.20), suggesting the results were not affected by circadian type.
Fig. 1.
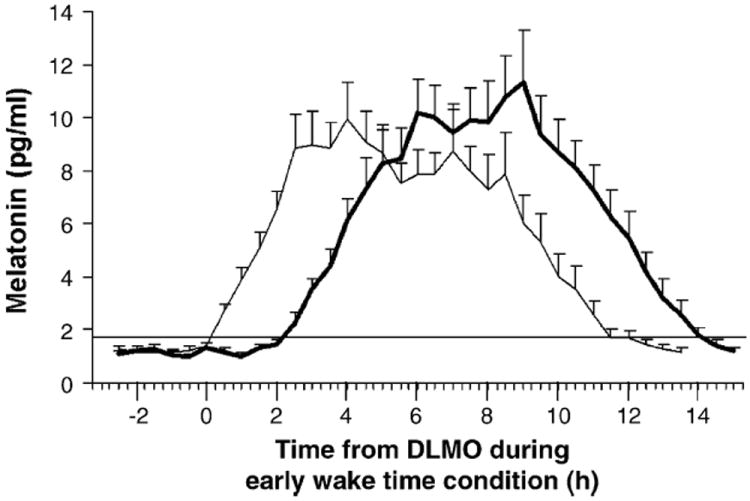
Mean dim light salivary melatonin profiles following 2 weeks with an early wake time (light line) and 2 weeks with a late wake time (dark line) with the same bedtime in both conditions. Error bars represent S.E.s. The horizontal line indicates the average threshold. The mean melatonin profiles were constructed by referencing each individual subject’s data to the time of their DLMO following the days with the early wake time.
Table 1.
The mean of the scheduled sleep times and dim light melatonin rhythm parameters (S.D. in parentheses) after the early wake time and late wake time days
| Early wake time (short nights) | Late wake time (long nights) | |
|---|---|---|
| Scheduled bed time | 1:56 (0.7) | 1:56 (0.7) |
| Scheduled wake time | 7:56 (0.7) | 10:56 (0.7) |
| DLMO | 21:27 (1.2) | 23:49* (1.2) |
| DLMOff | 8:29 (1.3) | 11:04* (1.6) |
| DLMO-DLMOff duration | 11.0 (0.9) | 11.3 (1.1) |
| Area under the curve | 172.1 (63.3) | 190.2 (77.0) |
Mean times are in hour:minute format, S.D.s are in hours.
Significantly different from early wake time condition (ANOVA, p < 0.001).
Total sleep time was determined from the actigraphy verified sleep logs as time from estimated sleep onset to final awakening minus any awakenings, plus any daytime naps. During early wake time, short night condition subjects slept on average 5.7 ± 0.2 h/day at night and 0.8 ± 0.4 h/day in naps. Thus in total, subjects slept an average of 6.5 ± 0.4 h/day during the early wake time, short night condition which was significantly less than the 8.1 ± 0.6 h/day during the late wake time, long night condition (paired t-test, p < 0.001).
A 2 × 2 MANOVA with within subjects factors wake time/night length and time of day (morning versus evening) on the seven items from the “How Are You Feeling Right Now?” was significant (p = 0.01). Subsequent univariate ANOVAs revealed significant results for the Stanford Sleepiness Scale and for the physical fatigue and mental fatigue items (see Table 2). These results indicate that subjects had greater levels of sleepiness, physical and mental fatigue during the early wake time, short nights versus late wake time, long nights and they also had greater levels in the evening versus in the morning (see Table 2). Furthermore, the increase in sleepiness and physical fatigue from morning to evening was less during the short nights than long nights. The Columbia score, which reflected symptoms throughout the day, was also significantly higher during the short nights (Table 2).
Table 2.
The mean of reported subjective symptoms (S.D. in parentheses) during the early wake time and late wake time days and associated statistics
| Early wake time (short nights)
|
Late wake time (long nights)
|
Results of ANOVAs
|
|||||
|---|---|---|---|---|---|---|---|
| Morning | Evening | Morning | Evening | Main effect of night length | Main effect of time of day | Interaction | |
| Stanford sleepiness scale | 3.8 (1.2) | 4.1 (1.1) | 2.4 (0.9) | 3.4 (1.1) | p < 0.001 | p = 0.033 | p = 0.035 |
| Physical fatigue | 4.2 (2.1) | 4.7 (2.1) | 2.8 (1.7) | 4.1 (1.8) | p = 0.01 | p = 0.013 | p = 0.008 |
| Mental fatigue | 4.0 (1.9) | 4.5 (1.9) | 2.6 (1.6) | 3.7 (1.7) | p = 0.004 | p = 0.012 | n.s. |
| Sadness | 1.5 (0.7) | 1.5 (0.8) | 1.3 (0.4) | 1.4 (0.5) | n.s. | n.s. | n.s. |
| Anxiety | 1.7 (1.0) | 1.5 (0.4) | 1.5 (0.6) | 1.4 (0.4) | n.s. | n.s. | n.s. |
| Irritability | 1.6 (0.6) | 1.8 (1.1) | 1.3 (0.3) | 1.4 (0.5) | n.s. | n.s. | n.s. |
| Gastrointestinal problems | 1.2 (0.5) | 1.1 (0.2) | 1.3 (0.6) | 1.2 (0.3) | n.s. | n.s. | n.s. |
| Columbia scale | – | 7.3 (4.7) | – | 3.6* (2.5) | – | – | – |
Note: Higher numbers indicate worse symptoms.
Significantly different from early wake time condition (paired t-test, p = 0.001).
Our results indicate that when wake time is shifted, the human circadian clock phase shifts closely in response. The later timing of the melatonin phase markers after the late wake time was likely because of the additional 3 h of darkness that subjects received every morning. Similarly, the earlier timing of the melatonin phase markers after the early wake time was probably because of the extra 3 h of morning light, some of it bright outdoor light, that subjects received every morning. Analysis of the light data from the medallion photosensor, corrected for sunglasses use, supports this (Fig. 2). During the early wake time condition subjects received significantly more light exposure in the first 3 h after the scheduled wake time (mean ± S.D., 32.2 ± 13.4 min of bright light >1000 lx), than during the late wake time condition.
Fig. 2.
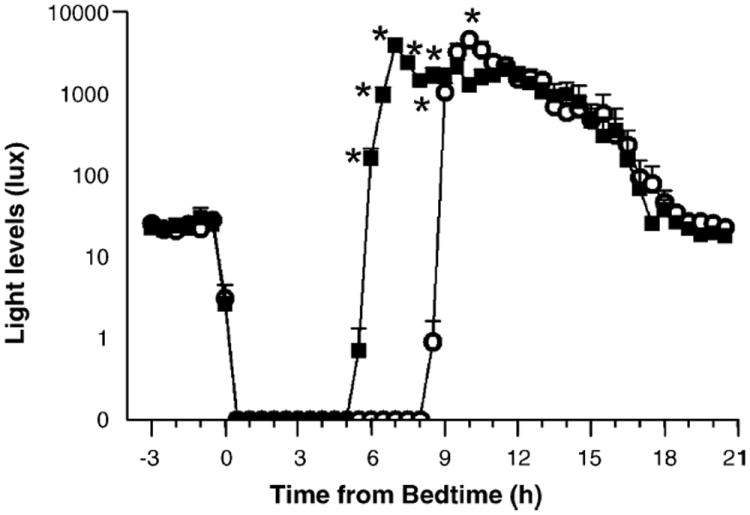
Mean light levels from medallion photosensor during the 2 weeks of short nights with the early wake time (filled squares) and 2 weeks of long nights with the late wake time (open circles), averaged into 30 min bins. Data are averaged according to time from bedtime and are corrected for sunglasses use. Error bars represent S.E.s. Asterisks indicate times when the light intensity was significantly different between the conditions (data log transformed, repeated measures ANOVA followed by simple main effect analysis, p < 0.001).
It is also possible that changes in the timing of nonphotic zeitgebers between conditions, such as activity, posture, food intake and the timing of sleep per se, may have led to the phase change in the melatonin rhythm. However, as such nonphotic zeitgebers are typically much less powerful than light (e.g. [10,18]), we believe that the difference in the timing of morning light exposure associated with the different wake times is the most likely cause of the phase shift. Indeed, even ordinary indoor light can suppress melatonin and can phase shift the circadian clock [31].
Our results highlight the substantial influence that wake time, likely via the associated morning light exposure, has on determining human circadian phase. In our previous study [2], when wake time was fixed, a 3 h delay in bedtime led to ~0.5 h phase delay in the melatonin rhythm. Here, when bedtime was fixed a 3 h delay in wake time led to ~2.5 h phase delay in the melatonin rhythm. Thus, the effect of altering wake time on the melatonin rhythm was about five times greater than the effect of altering bedtime. This finding supports the findings of many studies that have reported that the DLMO is more highly correlated with wake time than bedtime [5,6,9,16,30]. This is again probably due to the associated light exposure. Morning light, associated with wake time, is typically brighter than evening light, and may occur closer to the most sensitive portions of the human light phase response curve [14,17,24], at a time that produces phase advances.
As we found in our previous study which also changed night length by 3 h [2], we observed no change in the duration of the melatonin profile nor any change in the area under the curve. As changes in duration have been observed following 4 weeks of long 14 h nights compared to 1 week of 8 h nights [28], it may be that a 3 h difference in night length or only 2 weeks of each photoperiod (as in our study) is not great enough to induce a significant change in melatonin duration in many humans. Differences in melatonin duration have been observed in habitual short (<6 h/night) versus long sleepers (>9 h/night) [1], which supports the idea that 2 weeks is not a sufficient time period to observe a change in duration. Future studies are required to further explore human photoperiodic responses to various realistic night lengths.
Acknowledgments
This work was supported by NIH grant NHLBI R01 HL072408 and the American Sleep Medicine Foundation, a foundation of the American Academy of Sleep Medicine. We thank Young Cho, Erin Cullnan, Meredith Durkin, Valerie Ellios, Clifford Gazda, Cynthia Hiltz, Hyungsoo Kim, Clara Lee, Kathryn Lenz, Tom Molina, Courtney Pearson, Victoria Revell, Mark Smith and Jonathan Swisher for their assistance with data collection, Erin Cullnan for her assistance with Fig. 2 and Louis Fogg for his statistical advice.
References
- 1.Aeschbach D, Sher L, Postolache TT, Matthews JR, Jackson MA, Wehr TA. A longer biological night in long sleepers than in short sleepers. J Clin Endocrinol Metab. 2003;88:26–30. doi: 10.1210/jc.2002-020827. [DOI] [PubMed] [Google Scholar]
- 2.Burgess HJ, Eastman CI. Early versus late bedtimes phase shift the human dim light melatonin rhythm despite a fixed morning lights on time. Neurosci Lett. 2004;356:115–118. doi: 10.1016/j.neulet.2003.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burgess HJ, Eastman CI. Short nights attenuate light-induced circadian phase advances in humans. J Clin Endocrinol Metab. 2005;90:4437–4440. doi: 10.1210/jc.2005-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burgess HJ, Eastman CI. Short nights reduce light-induced circadian phase delays in humans. SLEEP. 2006;29(1) doi: 10.1093/sleep/29.1.25. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burgess HJ, Eastman CI. The dim light melatonin onset following fixed and free sleep schedules. J Sleep Res. 2005;14:229–237. doi: 10.1111/j.1365-2869.2005.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgess HJ, Savic N, Sletten T, Roach G, Gilbert SS, Dawson D. The relationship between the dim light melatonin onset and sleep on a regular schedule in young healthy adults. Beh Sleep Med. 2003;1:102–114. doi: 10.1207/S15402010BSM0102_3. [DOI] [PubMed] [Google Scholar]
- 7.Buxton OM, L’Hermite-Baleriaux M, Turek FW, Van Cauter E. Daytime naps in darkness phase shift the human circadian rhythms of melatonin and thyrotropin secretion. Am J Physiol. 2000;278:R373–R382. doi: 10.1152/ajpregu.2000.278.2.R373. [DOI] [PubMed] [Google Scholar]
- 8.Campbell SS, Zulley J. Ultradian components of human sleep/wake patterns during disentrainment. Exp Brain Res. 1985;12:234–255. [Google Scholar]
- 9.Crowley SJ, Acebo C, Carskadon MA. Predicting melatonin onset phase in adolescents on summer and school schedules. Sleep. 2004;27:A78. doi: 10.1093/sleep/29.12.1632. [DOI] [PubMed] [Google Scholar]
- 10.Danilenko KV, Cajochen C, Wirz-Justice A. Is sleep per se a zeitgeber in humans? J Biol Rhythms. 2003;18:170–178. doi: 10.1177/0748730403251732. [DOI] [PubMed] [Google Scholar]
- 11.Dumont M, Carrier J. Daytime sleep propensity after moderate circadian phase shifts induced with bright light exposure. Sleep. 1997;20:11–17. doi: 10.1093/sleep/20.1.11. [DOI] [PubMed] [Google Scholar]
- 12.Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: a new approach. Psychophysiology. 1973;10:431–436. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- 13.Horne JA, Östberg O. Self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 14.Khalsa SBS, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol. 2003;549(3):945–952. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewy AJ, Wehr TA, Goodwin FK, Newsome DA, Markey SP. Light suppresses melatonin secretion in humans. Science. 1980;210:1267–1269. doi: 10.1126/science.7434030. [DOI] [PubMed] [Google Scholar]
- 16.Martin SK, Eastman CI. Sleep logs of young adults with self-selected sleep times predict the dim light melatonin onset. Chronobiol Int. 2002;19:695–707. doi: 10.1081/cbi-120006080. [DOI] [PubMed] [Google Scholar]
- 17.Minors DS, Waterhouse JM, Wirz-Justice A. A human phase-response curve to light. Neurosci Lett. 1991;133:36–40. doi: 10.1016/0304-3940(91)90051-t. [DOI] [PubMed] [Google Scholar]
- 18.Mistlberger RE, Skene DJ. Nonphotic entrainment in humans? J Biol Rhythms. 2005;20:339–352. doi: 10.1177/0748730405277982. [DOI] [PubMed] [Google Scholar]
- 19.Moore RY. The innervation of the mammalian pineal gland. Prog Reprod Biol. 1978;4:1–29. [Google Scholar]
- 20.Murphy PJ, Myers BL, Badia P. Nonsteroidal anti-inflammatory drugs alter body temperature and suppress melatonin in humans. Physiol Behav. 1996;59:133–139. doi: 10.1016/0031-9384(95)02036-5. [DOI] [PubMed] [Google Scholar]
- 21.National Sleep Foundation. Less fun, less sleep, more work an American portrait, A National Sleep Foundation Poll. 2001 http://www.sleepfoundation.org.
- 22.Parry BL, Berga SL, Mostofi N, Klauber MR, Resnick A. Plasma melatonin circadian rhythms during the menstrual cycle and after light therapy in premenstrual dysphoric disorder and normal control subjects. J Biol Rhythms. 1997;12:47–64. doi: 10.1177/074873049701200107. [DOI] [PubMed] [Google Scholar]
- 23.Rajaratnam SMW, Dijk DJ, Middleton B, Stone BM, Arendt J. Melatonin phase-shifts human circadian rhythms with no evidence of changes in the duration of endogenous melatonin secretion or the 24-h production of reproductive hormones. J Clin Endocrinol Metab. 2003;88:4303–4309. doi: 10.1210/jc.2003-030460. [DOI] [PubMed] [Google Scholar]
- 24.Revell VL, Eastman CI. How to trick mother nature into letting you fly around or stay up all night. J Biol Rhythms. 2005;20:353–365. doi: 10.1177/0748730405277233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salas SL, Hille E. Calculus: One and Several Variables with Analytic Geometry. Wiley; New York: 1982. pp. 366–372. [Google Scholar]
- 26.Spitzer RL, Terman M, Williams JB, Terman JS, Malt UF, Singer F, Lewy AJ. Jet lag: clinical features, validation of a new syndrome-specific scale, and lack of response to melatonin in a randomized, double-blind trial. Am J Psychiatry. 1999;156:1392–1396. doi: 10.1176/ajp.156.9.1392. [DOI] [PubMed] [Google Scholar]
- 27.Voultsios A, Kennaway DJ, Dawson D. Salivary melatonin as a circadian phase marker: validation and comparison to plasma melatonin. J Biol Rhythms. 1997;12:457–466. doi: 10.1177/074873049701200507. [DOI] [PubMed] [Google Scholar]
- 28.Wehr TA, Moul DE, Barbato G, Giesen HA, Seidel JA, Barker C, Bender C. Conservation of photoperiod-responsive mechanisms in humans. Am J Physiol. 1993;265:R846–R857. doi: 10.1152/ajpregu.1993.265.4.R846. [DOI] [PubMed] [Google Scholar]
- 29.Wever RA. Properties of human sleep-wake cycles: parameters of internally synchronized free-running rhythms. Sleep. 1984;7:27–51. doi: 10.1093/sleep/7.1.27. [DOI] [PubMed] [Google Scholar]
- 30.Yoon IY, Kripke DF, Elliott JA, Youngstedt SD, Rex KM, Hauger RL. Age-related changes of circadian rhythms and sleep-wake cycles. J Am Geriatr Soc. 2003;51:1085–1091. doi: 10.1046/j.1532-5415.2003.51356.x. [DOI] [PubMed] [Google Scholar]
- 31.Zeitzer JM, Khalsa SBS, Boivin DB, Duffy JF, Shanahan TL, Kronauer RE, Czeisler CA. Temporal dynamics of late-night photic stimulation of the human circadian timing system. Am J Physiol. 2005;289:R839–R844. doi: 10.1152/ajpregu.00232.2005. [DOI] [PubMed] [Google Scholar]


