Abstract
As an example of the burgeoning importance of stem cell therapy, this past month the California Institute for Regenerative Medicine (CIRM) has approved $70 million to create a new network of stem cell clinical trial centers. Much work in the last decade has been devoted to developing the use of autologous and allogeneic adult stem cell transplants to treat a number of conditions, including heart attack, dementia, wounds, and immune system-related diseases. The standard model teaches us that adult stem cells exists throughout most of the body and provide a means to regenerate and repair most tissues through replication and differentiation. Although we have often witnessed the medical cart placed in front of the scientific horse in the development of stem cell therapies outside of academic circles, great strides have been made, such as the use of purified stem cells1 instead of whole bone marrow transplants in cancer patients, where physicians avoid re-injecting the patients with their own cancer cells.2 We most often think of stem cell therapy acting to regenerate tissue through replication and then differentiation, but recent studies point to the dramatic effects adult stem cells exert in the repair of various tissues through the release of paracrine and autocrine substances, and not simply through differentiation. Indeed, up to 80% of the therapeutic effect of adult stem cells has been shown to be through paracrine mediated actions.3 That is, the collected types of molecules released by the stem cells, called the secretome, or stem cell released molecules (SRM), number in the 100s, including proteins, microRNA, growth factors, antioxidants, proteasomes, and exosomes, and target a multitude of biological pathways through paracrine actions. The composition of the different molecule types in SRM is state dependent, and varies with cell type and conditions such as age and environment.
Keywords: stem cells, SRM, paracrine, growth factors, transplants, systems therapeutic, antimicrobial
So what are some of the paracrine actions of the adult stem cell secretome?
1. Antimicrobial4
2. The switching of differentiated cells to back to progenitor cells5
3. Wnt signaling to control stem cell niche organization6
4. Building extracellular matrix (ECM) in most tissues7
5. Modulation of the immune system8
6. Wound healing9
7. Regeneration of bone10
8. Reversal of stress urinary incontinence11
9. Regenerate functional intestine12
10. Enhancing recovery from traumatic brain injury13
11. Exosome release, a multipotent therapeutic14
12. Proteasome release for clearing misfolded proteins15
Development of therapeutics using the SRM of adult stem cell types leads us to a very different kind of therapeutic development than those offered by the development of small molecules or biologics.
Indeed, as in physics, where reductionism is being replaced with a systematic view of particles as an integral part of a collective, predictive, and coherent electrodynamic universe described by Mead,16 biology and therapeutic development are being viewed at multiple levels beyond the genome, explained well by Noble,17 where even multiple somatic genes exists,18 using a systems approach.19 As Ernst Mach taught us, and what Einstein called Mach’s Principle,20 motion can only have meaning when what it is that's moving is moving relative to other matter in the universe. And in biology, one molecule or one structure can only have meaning when viewed in relation to the other parts of the biological system. Indeed tissue models such as tensegrity exemplify the importance of the system where one part of the system is physically and chemically connected to the most other parts of the system,21 and involved in many cellular functions including chromatin rearrangement,22 ion channel flux control,23 triggering the cell fate of stem cells,24 and the tensional tuning of stem cells to the adjacent soft tissue physical state.25
The systems biology approach is leading to the path of developing therapeutics that interact with the system, with reverse engineering of the many paracrine factors that stem cells release being instructive for the development of “systems therapeutics.”26 The development of SRM as a systems therapeutic is in contradistinction to the reductionist method of developing targeted small molecule drugs that interact with only one defined target. Further, because multiple types of stem cells are normally involved in tissue maintenance and repair, the SRM from different cell types is utilized to form a more potent version of the SRM.26 Using a reductionist approach where just one of the molecules in SRM, instead of the total mix, has proven ineffective in a number of trials for therapeutic development and has led to the downfall of at least one public company.27
The advantages of using the SRM for therapeutic development rather than the stem cells themselves are numerous. For example, stem cells may die or not home into the site of damaged tissue yielding an unknown dosing regimen, whereas the SRM can be dosed directly to the site of damage in a dosing regimen that can be controlled and defined in space and time. And the molecules can be optimally harvested under controlled laboratory conditions, producing the SRM from multiple cell types, to produce a set of state dependent molecules with the desired composition.
Consider wound healing and cancer, their common mechanistic underpinnings of a disordered extracellular matrix (ECM) and described as “tumors: wounds that never heal,”28 are controlled significantly by SRM acting on the ECM. Then the observation that highly invasive fetal surgery produces no scarring,29 rather a perfectly reformed ECM, and is not mediated by conditions of the womb such as hypoxia, but instead is mediated by an intrinsic primordial state of the fetal tissue,29 and therefore presumably a primordial SRM state, leads us to believe that SRM, in a primordial state, may be developed as a therapeutic for indications where a deregulated ECM is evident. The system of molecules within this framework to build ECM in a more organized, scarless manner exists at a minimum of 2 levels where the SRM of mesenchymal stem cells establishes the instruction set to build the niche for the fibroblasts, and the SRM of the fibroblasts provides instructions to build and regulate the ECM niche with primordial, scar-less capabilities. Developing SRM-based therapeutics with the molecules providing instruction sets in a fetal, primordial manner may lead to a more perfect union of the ECM relevant to the many diseases and indications where a deregulated ECM is involved, such as cancer. (Fig. 1)
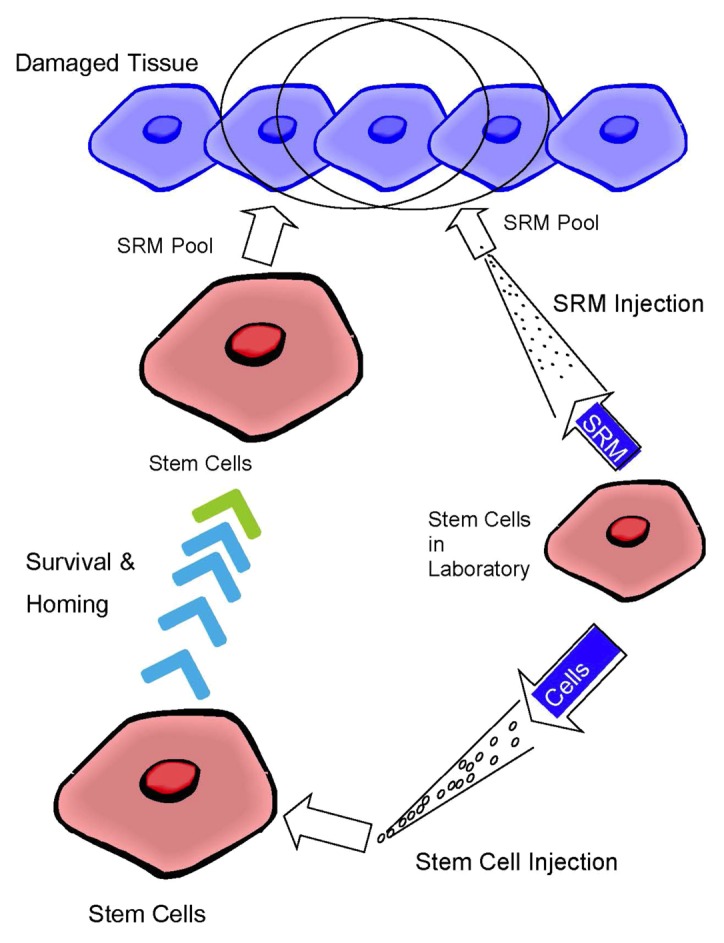
Figure 1. Molecules do the work. A model shows the injection of SRM (molecules) directly to the injured tissue, vs. the injection of cells that then indirectly release the SRM (molecules) to the injured tissue. Direct injection of the SRM to the tissue allows for a precise dosing schedule in space and time, whereas injection of cells into the tissue is highly variable.
Disclosure of Potential Conflicts of Interest
Maguire G is part owner of BioRegenerative Sciences, Inc.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/26631
References
- 1.Czechowicz A, Weissman IL. Purified hematopoietic stem cell transplantation: the next generation of blood and immune replacement. Immunol Allergy Clin North Am. 2010;30:159–71. doi: 10.1016/j.iac.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsao GJ, Allen JA, Logronio KA, Lazzeroni LC, Shizuru JA. Purified hematopoietic stem cell allografts reconstitute immunity superior to bone marrow. Proc Natl Acad Sci U S A. 2009;106:3288–93. doi: 10.1073/pnas.0813335106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chimenti I, Smith RR, Li TS, Gerstenblith G, Messina E, Giacomello A, Marbán E. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res. 2010;106:971–80. doi: 10.1161/CIRCRESAHA.109.210682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JW, Krasnodembskaya A, McKenna DH, Song Y, Abbott J, Matthay MA. Therapeutic effects of human mesenchymal stem cells in ex vivo human lungs injured with live bacteria. Am J Respir Crit Care Med. 2013;187:751–60. doi: 10.1164/rccm.201206-0990OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chien KR. Regenerative biology: heartbroken embryos heal. Nature. 2013;498:439–40. doi: 10.1038/nature12262. [DOI] [PubMed] [Google Scholar]
- 6.Sato T, Clevers H. Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science. 2013;340:1190–4. doi: 10.1126/science.1234852. [DOI] [PubMed] [Google Scholar]
- 7.Souders CA, Bowers SL, Baudino TA. Cardiac fibroblast: the renaissance cell. Circ Res. 2009;105:1164–76. doi: 10.1161/CIRCRESAHA.109.209809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scandling JD, Busque S, Dejbakhsh-Jones S, Benike C, Sarwal M, Millan MT, Shizuru JA, Lowsky R, Engleman EG, Strober S. Tolerance and withdrawal of immunosuppressive drugs in patients given kidney and hematopoietic cell transplants. Am J Transplant. 2012;12:1133–45. doi: 10.1111/j.1600-6143.2012.03992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warriner RA, Cardinal M. Human fibroblast-derived dermal substitute: results from a treatment investigational device exemption (TIDE) study in diabetic foot ulcers. Adv Skin Wound Care. 2011;24:306–11. doi: 10.1097/01.ASW.0000399647.80210.61. [DOI] [PubMed] [Google Scholar]
- 10.Katagiri W, Osugi M, Kawai T, Ueda M. Novel cell-free regeneration of bone using stem cell-derived growth factors. Int J Oral Maxillofac Implants. 2013;28:1009–16. doi: 10.11607/jomi.3036. [DOI] [PubMed] [Google Scholar]
- 11.Dissaranan C, Cruz MA, Kiedrowski MJ, Balog BM, Gill BC, Penn MS, Goldman HB, Damaser MS. Rat mesenchymal stem cell secretome promotes elastogenesis and facilitates recovery from simulated childbirth injury. Cell Transplant. 2013 doi: 10.3727/096368913X670921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agopian VG, Chen DC, Avansino JR, Stelzner M. Intestinal stem cell organoid transplantation generates neomucosa in dogs. J Gastrointest Surg. 2009;13:971–82. doi: 10.1007/s11605-009-0806-x. [DOI] [PubMed] [Google Scholar]
- 13.Menge T, Zhao Y, Zhao J, Wataha K, Gerber M, Zhang J, Letourneau P, Redell J, Shen L, Wang J, et al. Mesenchymal stem cells regulate blood-brain barrier integrity through TIMP3 release after traumatic brain injury. Sci Transl Med. 2012;4:161ra150. doi: 10.1126/scitranslmed.3004660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maguire G, Friedman P, McCarthy D, Friedman R, Maniotis AJ. Stem cell released molecules and exosomes in tissue engineering. Procedia Engineering. 2013;59:270–8. doi: 10.1016/j.proeng.2013.05.121. [DOI] [Google Scholar]
- 15.Lai RC, Yeo RW, Tan KH, Lim SK. Exosomes for drug delivery - a novel application for the mesenchymal stem cell. Biotechnol Adv. 2013;31:543–51. doi: 10.1016/j.biotechadv.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Mead CA. Collective Electrodynamics. MIT Press, 2000. [Google Scholar]
- 17.Noble D. The Music of Life. Oxford University Press, 2006. [Google Scholar]
- 18.Lupski JR. Genetics. Genome mosaicism--one human, multiple genomes. Science. 2013;341:358–9. doi: 10.1126/science.1239503. [DOI] [PubMed] [Google Scholar]
- 19.Maguire G. Using a systems-based approach to overcome reductionist strategies in the development of diagnostics. Expert Rev Mol Diagn. doi: 10.1586/14737159.2013.846828. [DOI] [PubMed] [Google Scholar]
- 20.Barbour J, Pfister H. Mach’s Principle: From Newton's Bucket to Quantum Gravity (Einstein Studies). Birkhauser, 1995. [Google Scholar]
- 21.Ainsworth C. Cell biology: Stretching the imagination. Nature. 2008;456:696–9. doi: 10.1038/456696a. [DOI] [PubMed] [Google Scholar]
- 22.Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci U S A. 1997;94:849–54. doi: 10.1073/pnas.94.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maguire G, Connaughton V, Prat AG, Jackson GR, Jr., Cantiello HF. Actin cytoskeleton regulates ion channel activity in retinal neurons. Neuroreport. 1998;9:665–70. doi: 10.1097/00001756-199803090-00019. [DOI] [PubMed] [Google Scholar]
- 24.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 25.Solon J, Levental I, Sengupta K, Georges PC, Janmey PA. Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys J. 2007;93:4453–61. doi: 10.1529/biophysj.106.101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maguire G, Friedman P. The Systems Biology of Stem Cell Released Molecules-Based Therapeutics. ISRN Stem Cells, 2013. [Google Scholar]
- 27.Kathju S, Gallo PH, Satish L. Scarless integumentary wound healing in the mammalian fetus: molecular basis and therapeutic implications. Birth Defects Res C Embryo Today. 2012;96:223–36. doi: 10.1002/bdrc.21015. [DOI] [PubMed] [Google Scholar]
- 28.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–9. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 29.Lorenz HP, Longaker MT, Perkocha LA, Jennings RW, Harrison MR, Adzick NS. Scarless wound repair: a human fetal skin model. Development. 1992;114:253–9. doi: 10.1242/dev.114.1.253. [DOI] [PubMed] [Google Scholar]


